Ascl2与结肠癌发生发展的关系研究进展
陈曦,朱蓉,赵逵
(遵义医学院附属医院,贵州遵义563099)
·综述·
Ascl2与结肠癌发生发展的关系研究进展
陈曦,朱蓉,赵逵
(遵义医学院附属医院,贵州遵义563099)
Ascl2是一个碱性/螺旋—环—螺旋转录因子,是Wnt信号通路的靶分子,仅表达于胎盘及小肠、大肠隐窝基底的Lgr5阳性的肠隐窝基底柱细胞(CBC细胞)。近来研究证实Ascl2是CBC成体肠干细胞的一个重要标记物,在维持结肠癌干/前体细胞的干细胞性方面发挥重要作用。本文对Ascl2与结肠癌的关系进行了综述。
干性标志物;Ascl2;Wnt信号通路;结肠癌
据统计,结肠癌居全球肿瘤发病率的第3位[1]。近年来随着生活水平的提高和饮食习惯的改变,结肠癌发病率逐年升高,且趋于年轻化[2,3]。肿瘤干细胞理论的提出为治疗结肠癌带来了新希望,寻找理想的结肠癌干细胞特异性表面分子标志物是该领域目前最重要的研究方向之一。新近研究发现,Ascl2是肠隐窝基底柱细胞(CBC细胞)作为成体肠干细胞的一个重要标记物[4],是Wnt通路的直接靶基因[5];在结肠癌发生发展的各个时期,结肠癌组织中Ascl2均表达上调[6,7]。近年来有关Ascl2与结肠癌发生发展关系的研究有较大进展,现综述如下。
1 肿瘤干细胞理论
Dontu等[8]首次证实肿瘤干细胞存在于乳腺癌等实体瘤中,随后在脑肿瘤[9]、前列腺癌[10]、乳腺癌[11]、胰腺癌[12]、黑素瘤[13]及肺癌中也得到证实[14],近年来O′Brien等[15]和Ricci-Vitiani等[16]陆续发现结直肠癌中肿瘤干细胞的存在。肿瘤干细胞理论认为肿瘤发生的根源是肿瘤干细胞,肿瘤干细胞是根治肿瘤的靶点[17]。肿瘤干细胞具有自我更新和分化为祖细胞的能力,在所有肿瘤细胞中仅占极少数,其具有的不对称分裂和无限增殖能力导致恶性肿瘤难治疗、易转移和易复发[18]。Tang等[19]认为正常干细胞和肿瘤干细胞在维持组织稳态方面存在共性。Todaro等[20]认为正常干细胞通过不对称分裂成一个新细胞,而肿瘤干细胞对称分裂成两个细胞,并且只有一小部分细胞分化。除肿瘤干细胞以外,其余大部分肿瘤细胞不具有无限增殖能力,短暂的生存后即开始凋亡,放化疗时即可被杀死。传统的肿瘤“随机模型”认为所有肿瘤细胞均具有同等增殖、转移能力。肿瘤干细胞理论的提出颠覆了这一传统认识。肿瘤干细胞在整个肿瘤过程中起着至关重要的作用,是肿瘤发生、发展过程的起始细胞。从肿瘤干细胞角度分析结肠癌的生物学特性,可能为结肠癌提供新的治疗思路[21]。
肠黏膜上皮是人体组织中更新速度最快的,正常情况下每4~5 d更新一次,不断补充衰老脱落的上皮细胞,其细胞学基础是黏膜干细胞的自我更新和定向分化[17]。肠隐窝干细胞是位于肠隐窝基底部的成体干细胞,一部分沿“隐窝—绒毛轴”方向分化为四种类型细胞—杯状细胞、潘氏细胞、肠吸收细胞、肠内分泌细胞;另一部分细胞则向隐窝基底迁移分化为潘氏细胞,并在CBC细胞周围形成CBC细胞的干细胞壁龛[27,28],“干细胞壁龛”通过一系列信号通路(Notch、Wnt、BMP、Hedgehog和JAK/STAT等)调控肠干细胞的命运,并提供干细胞增殖与分化必要的物质基础,对细胞的增殖、分化起决定性的作用[29~32]。Zhang等[17]采用荧光标记发现,位于基底部肠隐窝的是活跃的肠干细胞,在+4位置的肠干细胞并不活跃。有学者通过研究小鼠的基因过表达和基因敲除模型发现,Ascl2是控制CBC成体肠干细胞命运非常重要的转录因子,是CBC成体肠干细胞的重要标记物,小鼠肠上皮中Ascl2转基因过表达可导致肠隐窝的过度增生以及肠绒毛上的异位隐窝出现,而Ascl2基因表达缺失可导致Lgr5阳性的CBC细胞消失。上述均表明Ascl2在干细胞维持中具有重要作用。
2 Ascl2与结直肠癌
Ascl基因属于一个保守的转录因子家族,被定义为基本的螺旋—环—螺旋结构域[22]。Ascl2基因定位于染色体11p15.5,是一个碱性/螺旋—环—螺旋转录因子,负责人类正常胎盘的滋养层细胞谱系的分化,其表达仅限于胎盘以及小肠、大肠隐窝基底的Lgr5阳性CBC细胞[4]。Ascl2(Mash2/HASH2)基因与果蝇的Achaete-scute复合体基因同源[23],编码一个碱性/螺旋—环—螺旋转录因子,主要表达于胚外组织,具有部位特异性[24]。Jubb等[22]通过原位杂交实验证实其在正常组织中仅表达于胎盘及小肠、大肠的隐窝基底部,在其他正常组织中几乎不表达。

目前鉴别和分离出的结肠癌干/前体细胞表面标志物有许多,例如CD133[15,16]、CD44[33]、CD24[34]、和Lgr5[35]等,但尚无公认的非常特异的表面标志物,Ziskin[5]等研究认为Ascl2和Lgr5在结肠癌中的表达分别为85%和74%,二者表达呈正相关;且大部分腺癌来源于Lgr5+/ Ascl2+隐窝干细胞,依赖于Wnt/β-catenin信号通路[36]。隐窝干细胞与大肠癌的形成密切相关,对隐窝干细胞的生物学研究有助于深入了解大肠癌的生物学特性。因此,肠隐窝干细胞的准确识别很重要[37]。Neal等[38]认为每个隐窝通常含有约6个独立的干细胞和活跃的肠干细胞(a-iscs)、沉默的肠干细胞(q-iscs)两大派系,肠隐窝干细胞与损伤后的再生密切相关,参与结肠癌的发生。Papailiou等[39]也认为结肠癌干细胞可能起源于正常成体肠隐窝干细胞。结肠癌的发生、发展是一个渐进的过程,常因数年累积的突变导致,正常结肠隐窝干细胞突变可导致肠上皮组织结构和功能异常,造成内环境的稳定失调,促使结肠癌发生[40]。有学者通过基因芯片筛选发现Ascl2可以调控Lgr5、Olfm4、Sox9、EphB3等肠干细胞相关的分子。并且,Ascl2在肠道隐窝基底仅表达在Lgr5+的CBC细胞,而Lgr5是目前被公众认可度较高的正常成体肠干细胞标志物之一,因此认为Ascl2也应是肠隐窝干细胞标志物[4]。Barker等[41]对小鼠小肠隐窝基底部的干细胞标记Lgr5,证实Lgr5标记的细胞能够分化为上皮细胞谱系;且ascl2表达与Lgr5同步化,这提示Lgr5与Ascl2可能是肠道干细胞的标记物。我们前期研究亦证实Ascl2与结肠癌干细胞相关[28]。
2.2 Ascl2与Wnt信号通路 目前已知的结肠癌干细胞相关信号调节通路有Wnt、Notch、BMP通路等[42]。Wnt是一个富含半胱氨酸的分泌型配体家族。Wnt信号通路对于正常肠隐窝结构和稳态的维持非常重要,整个肠黏膜隐窝—绒毛结构都受到Wnt信号通路的调控;Wnt信号通路在正常细胞增殖以及干细胞的维持方面也发挥了重要作用[17]。其机制是胞质内游离的β-catenin积累,进入核内与TCF-4结合形成复合物,激活下游靶基因转录,启动肿瘤的生长程序[43]。Kuhnert等[44]在在体实验中使用Wnt拮抗剂Dkkl抑制该信号通路,破坏了正常肠隐窝的结构和稳态;而该信号通路的异常则与结肠癌的发生、发展有直接或间接关系。Radtke等[45]发现,抑癌基因Apc的截短将导致正常肠隐窝稳态的破坏,这与Wnt/β-catenin信号通路的表达增强密切相关。近来Sousa等[46]研究发现,Wnt信号通路在结肠癌干细胞中活性明显增高,而在已分化的结肠癌细胞中则明显降低,说明Wnt信号通路与结肠癌干细胞关系密切。有学者研究了在基底部表达的17个干细胞标记物,发现与Wnt信号关系最紧密的是Lgr5和Ascl2。Ascl2在肠道黏膜上皮组织中的表达是Wnt通路依赖的,是Wnt信号的一个靶分子[20,47,48]。Sansom等[49]报道当APC被截短,Ascl2的表达与对照组相比上调了22.2倍。Jubb等[6]发现,Ascl2在人类结直肠肿瘤组织中高表达,Ascl2过度表达是早期肠肿瘤Wnt信号异常调节的一个结果,Wnt信号在正常肠道和肠道肿瘤中均调节Ascl2的转录。
3 结语
靶向治疗是目前热门研究方向,而靶向治疗CSC还处于实验阶段[50]。Ascl2在肠道的表达仅限于隐窝基底,是维持结肠癌干细胞"干性"的重要转录因子,为靶向治疗结肠癌带来新的希望,但具体功能和机制尚待进一步研究。
[1] Grizzi F, Celesti G, Basso G, et al. Tumor budding as a potential histopathological biomarker in colorectal cancer: hype or hope[J]. World J Gastroenterol, 2012,18(5):6532-6536.
[2] Markowitz SD, Bertagnolli MM.Molecular origins of cancer:Molecular basis of colorectal cancer[J]. N Engl J Med, 2009,361(25):2449-2460.
[3] Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer[J]. Lancet, 2010,375(9719):1030-1047.
[4] Gijn ME, Hatzis PE, Cers H, et al. ription factor achaete scute-like 2 controls intestinal stem cell fate[J], Cell, 2009,136(5):903-912.
[5] Ziskin JL, Dunlap D, Yaylaoglu M, In situ validation of an intestinal stem cell signature in colorectal cancer[J]. Gut, 2013,62(7):1012-1023.
[6]Jubb AM, Hoeflich KP, Haverty PM, et al. Ascl2 and 11p15.5 amplification incolorectalcancer[J]. Gut, 201l,60(11):1606-1607.
[7] Stange DE, Engel F, Longerich T, et al. Expression of an ASCL2 related stem cell signature and IGF2 in colorectal cancer liver metastases with 11p15.5 gain[J]. Gut, 2010,59(9):1236-1244.
[8] Dontu G, Al-Hajj M, Abdallah WM, et al. Stem cells in normal breast development and breast cancer[J]. Cell Prolif, 2003,36(1):59-72.
[9] Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells[J]. Nature, 2004, 432(7015):396-401.
[10] Collins AT,Berry PA,Hyde C,et al.Prospective identification of tumorigenic prostate cancer stem cells[J]. Cancer Res, 2005,65(23):10946-10951.
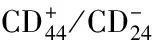
[12] Hermann PC, Huber SL,Herrler T,et al.Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer[J]. Cell Stem Cell, 2007,1(3):313-323.
[13] Fang D, Leishear K, Nguyen TK, et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells[J].Stem Cells,2006 ,24(7):1668-1677.
[14] Kim CF, Jackson EL,Woolfenden AE,et al.Identification of bronchioalveolar stem cells in normal lung and lung cancer[J]. Cell,2005,121(6):823-835.
[15] O'Brien CA, Pollett A, Gallinger S, et al, A human colon cancer cell capable of initiating tumour growth in immunodeficient mice[J]. Nature, 2007,445(7123):106-110.
[16] Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells[J]. Nature, 2007,445(7123):111-115.
[17] Zhang Z, Huang J. Intestinal stem cells-types and markers[J]. Cell Biol Int, 2013,37(5):406-414.
[18] Clevers H.The cancer stem cell:premises,promises and challenges[J]. Nat Med, 2011,17(3):313-319.
[19] Tang DG. Understanding cancer stem cell heterogeneity and plasticity[J]. Cell Res, 2012,22(3):457-472.
[20] Todaro M, Francipane MG,Medema JP,et al.Colon cancer stem cells: promise of targeted therapy[J].Gastroenterology,2010,138(6):2151-2162.
[21] Fujii M, Sato T. Culturing intestinal stem cells: applications for colorectal cancer research[J]. Front Genet, 2014,5(14):169.
[22] Jubb AM, Chalasani S, Frantz GD, et al. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia[J]. Oncogene, 2006,25(24):3445-3457.
[23] Johnson JE,Birren SJ,Anderson DJ.Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors[J].Nature,1990 ,346(6287):858-861.
[24] Guillemot F,Nagy A,Auerbach A,et al.Essential role of Mash一2 in extraembryonic development[J].Nature,1994,371(6495):333-336.
[25] Budak M,Korpinar MA,Kalkan T,et al.Mutation detection in the promoter region of survivin gene on N-methyl-N-nitrosourea induced colon tumor model in experiment[J].Bratisl Lek Listy, 2014,115(9):554-556.
[26] Zhu R, Yang Y, Tian Y, et al. Ascl2 knockdown results in tumor growth arrest by miRNA-302b-related inhibition of colon cancer progenitor cells[J].PLoS One, 2012,7(2):32170.
[27] Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts[J]. Nature, 2011,469(7330):415-418.
[28] Barker N, van Es JH, Kuipers J,et al. Identification of stem cells in small intestine and colon by marker gene Lgr5[J]. Nature, 2007,449(7165):1003-1007.
[29] Kang H, Shibata D. Direct measurements of human colon crypt stem cell niche genetic fidelity: the role of chance in non-darwinian mutation selection[J]. Front Oncol, 201,3:264.
[30] Fre S, Hannezo E, Sale S, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice[J]. PLoS One, 2011,6(10): 25785.
[31] Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche[J]. Nature, 2009,459(7244):262-265.
[32] Yan KS, Chia LA, Li X,et al.The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations[J]. Proc Natl Acad Sci USA, 2012 ,109(2):466-471.
[33] Chu P, Clanton DJ, Snipas TS, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties[J]. Int J Cancer,2009 ,124(6):1312-1321.
[34] Vermeulen L, Todaro M, de Sousa Mello F, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity[J]. Proc Natl Acad Sci USA, 2008,105(36):13427-13432.
[35] Barker N, Ridgway RA, van Es JH,et al.Crypt stem cells as the cells-of-origin of intestinal cancer[J].Nature,2009,457(7229):608-611.
[36] Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus[J].Cold Spring Harb Perspect Biol,2012 ,4(11):55.
[37] Zhang Z, Huang J. Intestinal stem cells- types and markers[J]. Cell Biol Int,2013,37(5):406-414.
[38] Neal MD, Richardson WM, Sodhi CP, et al. Intestinal stem cells and their roles during mucosal injury and repair[J]. J Surg Res,2011 ,167(1):1-8.
[39] Papailiou J, Bramis KJ, Gazouli M, et al. Stem cells in colon cancer. A new era in cancer theory begins[J]. Int J Colorectal Dis, 2011 ,26(1):1-11.
[40] Odoux C, Fohrer H, Hoppo T, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer[J].Cancer?Res,2008 ,68(17):6932-6941.
[41] Barker?N,van Oudenaarden A,Clevers H.Identifying the stem cell of the intestinal crypt: strategies and pitfalls[J].Cell Stem Cell,2012 ,11(4):452-460.
[42] Le PN, McDermott JD, Jimeno A.Targeting the Wnt pathway in human cancers:Therapeutic targeting with a focus on OMP-54F28[J]. Pharmacol Ther, 2014 ,14:162-164.
[43] Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer[J]. Nat Rev Cancer, 2008,8(5):387-398.
[44] Kuhnert F, Davis CR, Wang HT, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviralexpression of Dickkopf-1[J]. Proc Natl Acad Sci U S A, 2004 ,101(1):266-271.
[45] Radtke F, Clevers H, Riccio O. From gut homeostasis to cancer.Curr Mol Med[J]. 2006,6(3):275-289.
[46] Sousa EM, Vermeulen L, Richel D, et al. Targeting Wnt signaling in colon cancer stem cells[J]. Clin Cancer Res, 2011, 17(4):647-653.
[47] Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration[J].Genes Dev,2004,18(12):1385-1390.
[48] Oving I, Haegebarth A, De Palo M, et al. The Intestinal Wnt/TCF Signature[J]. Gastroenterology, 2007,132(2):628-632.
[49] Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation,and migration[J]. Genes Dev, 2004,18(12):1385-1390.
[50] Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening[J]. Cell, 2009,138(4):645-659.
贵州省高层次人才科研条件特助基金资助项目(TZJF-2011-32)。
赵逵
10.3969/j.issn.1002-266X.2015.06.036
R735.3
A
1002-266X(2015)06-0091-04
2014-11-15)

