离子液体复合电解质与三元正极的相容性
王振峰,张小雪,李翠华,刘剑洪,张黔玲
深圳大学化学与化工学院,深圳市功能高分子重点实验室,深圳 518060

【材料科学 / Materials Science】
离子液体复合电解质与三元正极的相容性
王振峰,张小雪,李翠华,刘剑洪,张黔玲
深圳大学化学与化工学院,深圳市功能高分子重点实验室,深圳 518060
室温下合成离子液体N-甲基-N-丙基哌啶双(三氟甲烷磺酰)亚胺(N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide, PP13TFSI)和N-甲基-N-丙基吡咯烷双(三氟甲烷磺酰)亚胺(N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)imide, PYR13TFSI),并与有机溶剂和双(三氟甲烷磺酰)亚胺锂(lithium bis(trifluoromethanesulphoyl)imide, LiTFSI)混合制备成复合电解质, 研究该电解质的热稳定性和不燃性能. 发现将其与锂离子电池三元正极材料镍钴锰酸锂LiCo1/3Mn1/3Ni1/3O2和锂片组装成CR-2032纽扣电池(LiCo1/3Mn1/3Ni1/3O2/电解质/锂),在0.1 C倍率下首次放电比容量为154.5 mA·h/g、库伦效率达到85.5%. 该复合电解质具有宽的电化学窗口、高的热稳定性、不燃性和良好的充放电循环性能.
应用电化学;锂离子电池;电解质;离子液体;相容性;不燃性
锂离子电池具有比能量高、电压高以及放电电压平稳等优点,是混合动力汽车和纯电动汽车最有前景的蓄电电池之一[1-3].三元正极材料镍钴锰酸锂LiCo1/3Ni1/3Mn1/3O2具有较高的容量(160~215 mA·h/g)和高电压平台(2.5~4.6 V), 是一种具有钴、镍和锰三元协同效应的新型氧化物正极材料[4-5],但由于传统有机电解质在高电压范围内易发生电化学反应[6-7],能量较高的三元电池较难通过针刺和过充等安全性测试,安全问题是长期限制高能量的LiCo1/3Ni1/3Mn1/3O2大规模应用的一大难题.离子液体(ionic liquid, IL)具有宽的液程和电化学稳定窗口、蒸汽压低、不燃烧以及分解电压高等优点[8-10],能很好地满足基于LiCo1/3Ni1/3Mn1/3O2材料的高能量密度电池对电解质安全性的要求.本研究通过合成两种离子液体N-甲基-N-丙基哌啶双(三氟甲烷磺酰)亚胺(N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide, PP13TFSI)与N-甲基-N-丙基吡咯烷双(三氟甲烷磺酰)亚胺(N-methyl-N-propylpyrrolidinium bis(trifluorometh anesulfonyl)imide, PYR13TFSI), 并与有机溶剂和双三氟甲烷磺酰亚胺锂(lithium bis(trifluoromethanesulphoyl)imide, LiTFSI)组成离子液体复合电解质,研究其稳定性和不燃性,并通过充放电测试、热重分析(thermogravimetric analysis, TG)、循环伏安法(cyclic voltammetry, CV)和扫描电子显微镜(scanning electron microscope, SEM)等表征离子液体复合电解质与LiCo1/3Mn1/3Ni1/3O2三元材料的相容性和电化学性能,研发适用于具有高能量和高电压平台的正极三元材料的安全性电解质.
1 材料与方法
1.1 离子液体的合成
PP13TFSI和PYR13TFSI的合成方法如图1[11]所示,产率分别为85.0%和87.5%.

图1 PYR13TFSI和PP13TFSI离子液体的合成路线[11]Fig.1 Synthesis process of ionic liquids PYR13TFSI and PP13TFSI[11]
1.2 电解质稳定性测试
电解质的燃烧性能通过自熄时间法测试[12],电解质的稳定性由热重分析仪(TA Q50)测试.
1.3 电池组装与性能测试
首先,将离子液体PP13TFSI和PYR13TFSI按体积比为1∶1配制混合离子液体.然后,将0.025 8 g LiTFSI、210 μL混合离子液体、75 μL碳酸二甲酯(dimethyl carbonate, DMC)和15 μL碳酸亚乙烯酯(vinylene carbonate, VC)制备成离子液体复合电解质Ⅰ(称为电解质Ⅰ),m(LiTFSI)∶m(混合离子液体)∶m(DMC)∶m(VC)=6∶71∶20∶3; 将210 μL混合离子液体用PYR13TFSI代替,制备成离子液体复合电解质Ⅱ(称为电解质Ⅱ),m(LiTFSI)∶m(PYR13TFSI)∶m(DMC)∶m(VC)=6∶71∶20∶3. 最后,分别用电解质Ⅰ、电解质Ⅱ和商用315电解质(深圳新宙邦科技股份有限公司生产)与正极LiCo1/3Ni1/3Mn1/3O2(深圳天骄科技开发有限公司生产)、 负极锂片组装成CR2023扣式电池, 由Land电池测试系统(CTA 2001A)和CV分析电池的充放电性能.
2 结果与讨论
2.1 可燃性测试
通过直接点燃、记录自熄时间观察电解质的可燃性能.将电解质滴加到表面皿上,使用火焰接触样品3 s,直接观察火焰判断其可燃性,记录燃烧时间.由表1和图2可见,燃烧时间随着离子液体含量增加而减小,PYR13TFSI体积分数为70%~100%时电解质不燃,表明含70%以上离子液体的电解质安全性高.
2.2 热稳定性分析
图3为4种离子液体和有机溶剂的TG曲线.从图3可见,当温度达到50 ℃时,有机溶剂碳酸乙烯酯(ethylene carbonate, EC)和DMC(质量比为1.3∶1.0)的质量损失为29.6%,该失重主要是由于DMC(沸点90 ℃)挥发;达到150 ℃时,有机溶剂基本完全挥发.离子液体PYR13TFSI、PP13TFSI 以及PYR13TFSI和PP13TFSI的混合离子液体(体积比为1∶1)在340 ℃前基本无失重,表明离子液体在此温度前无挥发、无分解,用离子液体作电解质溶剂,对提高锂离子电池安全性具有重要意义.图4为商用315电解质和电解质Ⅰ的热重分析曲线.由图4可知,商用315电解质在50 ℃和150 ℃前失重分别为35.5%和89.6%;而电解质Ⅰ在50 ℃和150 ℃前分别失重6.5%和9.5%,比有机溶剂实际质量分数23.0%分别低71.7%和58.7%,在150 ℃以下稳定,一半以上的有机溶剂不挥发.表明在离子液体复合电解质中,加入离子液体可显著提高电解质的安全性[13].该性能对研究高温型、大功率和大倍率的动力汽车锂离子电池电解质具有重要价值.

表1 不同体积分数PYR13TFSI的电解质的燃烧时间Table 1 Burning time of electrolytes with different concentrations of PYR13TFSI

图2不同体积分数PYR13TFSI的复合电解质的燃烧性能Fig.2 (Color online) Flammability of composite electrolytes with different concentrations of PYR13TFSI

图3 四种溶剂的热重曲线Fig.3 (Color online) TG curves of four solvents

图4 电解质Ⅰ和商用315电解质的热重曲线Fig.4 (Color online) TG curves of electrolyte Ⅰ and the commercial electrolyte 315
2.3 循环伏安测试
图5为LiCo1/3Ni1/3Mn1/3O2电极在电解质Ⅰ中的循环伏安图,扫描速率为0.10 mV/s,测试电压区间为2.75~4.50 V.从图5可见,扫描第1循环时,在4.00 V电压附近出现1个较尖锐的氧化峰,3.60 V出现1个还原峰;从第2循环开始氧化峰峰值有所降低,并移至3.98 V,这是由于在电极表明形成了一层钝化膜,被称为固体电解质界面膜(solid electrolyte interface, SEI);第2和3循环曲线基本无变化[14],且氧化峰面积与还原峰面积相当,说明锂离子的嵌脱锂可逆性较好[15],离子液体复合电解质与正极LiCo1/3Ni1/3Mn1/3O2相容性好.
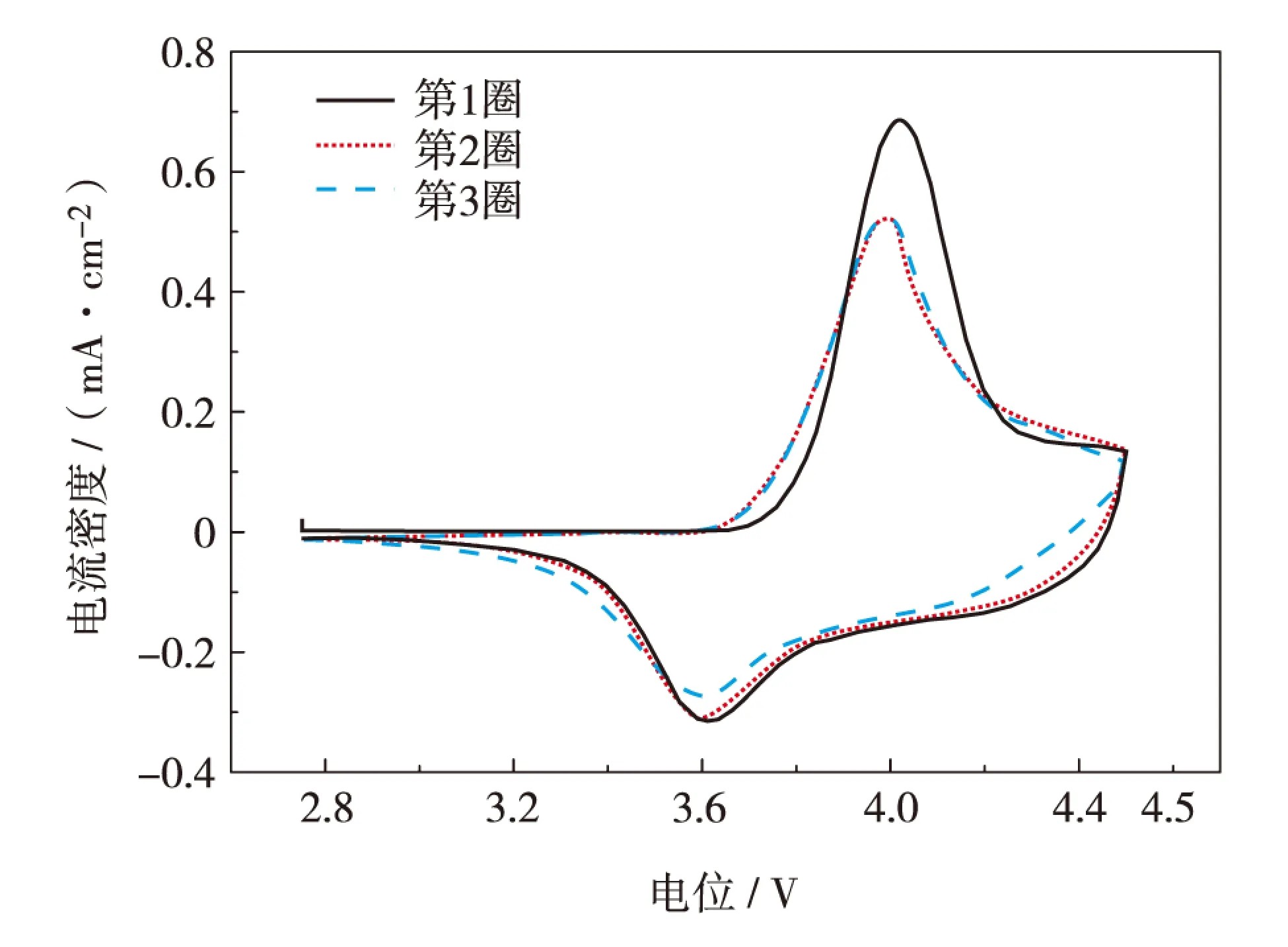
图5 电解质Ⅰ的循环伏安曲线 (扫描速率为0.10 mV/s)Fig.5 (Color online) Cyclic voltammograms of ionic liquid electrolyteⅠ(scanned at 0.10 mV/s)
2.4 电池性能测试
图6为电解质Ⅰ、电解质Ⅱ和商用315电解质分别在2.75~4.20 V电压区间和0.1 C倍率下的循环性能及库仑效率曲线;单一组分离子液体复合电解质循环30圈后,比容量达87.2 mA·h/g,库仑效率37.7%;双组分离子液体复合电解质比容量149.1 mA·h/g,库伦效率达到99.3%;商用315电解质比容量156.8 mA·h/g,库伦效率97.6%,双组分复合电解质与LiCo1/3Ni1/3Mn1/3O2正极材料相容性良好,常温时充放电性能与商用有机电解质相差不大,表明PP13TFSI与PYR13TFSI一起使用,可提高复合电解质对LiCo1/3Ni1/3Mn1/3O2电极的稳定性.这可能是PP13TFSI型离子液体在电极表面形成了稳定的钝化膜,阻止了PYR13TFSI型离子液体在电极表面的分解[16].

图6 0.1 C倍率下电解质Ⅰ、Ⅱ和商用315电解质的 循环性能和库仑效率曲线Fig.6 (Color online) Cycle performance and Coulombic efficiency at 0.1 C for cells with electrolyteⅠ,Ⅱand 315 electrolyte
图7为电解质Ⅰ和商用315电解质分别在2.75~4.20 V电压区间和1 C倍率条件下的循环性能及库仑效率曲线.双组分离子液体复合电解质循环30圈后,可逆比容量为124.3 mA·h/g,库伦效率为98.9%,商用315电解质为129.1 mA·h/g,库伦效率99.0%. 表明双组分离子液体复合电解质在正极LiCo1/3Ni1/3Mn1/3O2较大功率充放电时仍保持与商用有机电解质相近的可逆比容量和库伦效率.

图7 1 C倍率下电解质Ⅰ和商用315电解质的 循环性能和库仑效率曲线Fig.7 (Color online) Cycle performance and Coulombic efficiency at 1 C for cells with electrolyte Ⅰ and 315 electrolyte
2.5 SEM表征
图8(a)为原始电极LiCo1/3Ni1/3Mn1/3O2材料,乙炔黑和聚偏氟乙烯(polyvinylidene fluoride, PVDF)的SEM图.由图8(a)可以看出,电极具有较好的球形或近似球形形貌,且颗粒均匀,分散较好;亦有部分颗粒团聚,形成不规则形状颗粒[17].图8(b)为电极在电解质Ⅰ, 以0.1 C倍率循环30圈后的SEM图,由图8(b)可知,循环后电极形态变化微小,整体保持原来形貌.说明在充放电过程中,复合离子液体电解质与正极LiCo1/3Ni1/3Mn1/3O2相容性好.
结 语
探究了PYR13TFSI和PP13TFSI类离子液体复合电解质的热稳定性、不燃性及其与LiCo1/3Ni1/3Mn1/3O2三元正极材料的相容性.研究结果表明,添加离子液体可以有效抑制有机溶剂的挥发,离子液体体积分数为70%以上的复合电解质不燃烧,说明离子液体对提高锂离子电池的安全性具有重要意义.同时发现双组分离子液体复合电解质与高容量LiCo1/3Ni1/3Mn1/3O2电极材料相容性比单一组分离子液体电解质高,PP13TFSI的加入在电极表面可形成稳定的钝化膜,阻止了PYR13TFSI在电极表面的分解,提高了电解质与LiCo1/3Ni1/3Mn1/3O2电极的稳定性.随着研究的逐步深入,有机溶剂与PYR13TFSI/PP13TFSI离子液体的复合物有望成为新的锂离子电池电解质.
/ References:
[1] Goodenough J B, Kim Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materials, 2009, 22(3): 587-603.
[2] An Yongxin, Zuo Pengjian, Du Chunyu, et al. Effects of VC-LiBOB binary additives on SEI formation in ionic liquid-organic composite electrolyte[J]. RSC Advances, 2012, 2(10): 4097-4102.
[3] Li Huifeng, Pang Jing, Yin Yanping, et al. Application of a nonflammable electrolyte containing PP13TFSI ionic liquid for lithium-ion batteries using the high capacity cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2[J]. RSC Advances, 2013, 3(33): 13907-13914.
[4] Wu Haiyan, Wang Jianhua, Li Bin, et al. Progress in cath-ode materials LiCo1/3Ni1/3Mn1/3O2for Li-ion batteries[J]. Materials Review, 2007, 21(F11): 299-302.(in Chinese) 吴海燕, 王剑华, 李 斌, 等. 锂离子电池正极材料LiCo1/3Ni1/3Mn1/3O2的研究进展[J]. 材料导报, 2007, 21(F11): 299-302.
[5] Ren Xiangzhong, Liu Tao, Zhang Peixin, et al. Progress of LiNi1/3Co1/3Mn1/3O2cathode materials for lithium-ion batteries[J]. Journal of Shenzhen University Science and Engineering, 2014, 31(3): 239-248.(in Chinese) 任祥忠, 刘 涛, 张培新, 等. 锂离子电池正极材料LiNi1/3Co1/3Mn1/3O2研究进展[J]. 深圳大学学报理工版, 2014 , 31(3): 239-248.
[6] Balakrishnan P G, Ramesh R, Prem Kumar T. Safety mechanisms in lithium-ion batteries[J]. Journal of Power Sources, 2006, 155(2): 401-414.
[7] Wang Maofeng, Shan Zhongqiang, Tian Jianhua, et al. Mixtures of unsaturated imidazolium based ionic liquid and organic carbonate as electrolyte for Li-ion batteries[J]. Electrochimica Acta, 2013, 95: 301-307.
[8] Armand M, Endres F, MacFarlane D R, et al. Ionic-liquid materials for the electrochemical challenges of the future[J]. Nature materials, 2009, 8(8): 621-629.

[10] Jin Yide, Fang Shaohua, Chai Ming, et al. Properties and application of ether-functionalized trialkylimidazolium ionic liquid electrolytes for lithium battery[J]. Journal of Power Sources, 2013, 226: 210-218.
[11] Reiter J, Nádherná M, Dominko R. Graphite and LiNi1/3Co1/3Mn1/3O2electrodes with piperidinium ionic liquid and lithium bis(fluorosulfonyl)imide for Li-ion Batteries[J]. Journal of Power Sources, 2012, 205: 402-407.
[12] Yang Binbin, Li Cuihua, Zhou Junhui. Pyrrolidinium- based ionic liquid electrolyte with organic additive and LiTFSI for high-safety lithium-ion batteries[J]. Electrochimica Acta, 2014, 148: 39-40.
[13] Zheng Jianming, Zhu Derong, Yang Yong, et al. The effects of N-methyl-N-butylpyrrolidinium bis(trifluorom- ethylsulfonyl)imide-based electrolyte on the electroc- hemical performance of high capacity cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2[J]. Electrochimica Acta, 2012, 59: 14-22.
[14] Yu Xiaoyuan, Yu Shixi, Hu Guorong, et al. Properties characerization of LiNi1/3Co1/3Mn1/3O2cathode materials for Li-ion Batteries[J]. Chinese Journal of Power Sources, 2008, 32(10): 655-658.(in Chinese) 禹筱元, 余仕僖, 胡国荣, 等. 锂离子蓄电池 LiNi1/3Co1/3Mn1/3O2正极材料的性能表征[J]. 电源技术, 2008, 32(10): 655-658.
[15] Wei Nini, Lai Qiongyu, Gao Yuan, et.al. Synthesis and electrochemical characterization of layered LiNi1/3Co1/3Mn1/3O2cathode material[J]. Journal of Inorganic Chemistry, 2005, 21(7): 999-1003.(in Chinese) 韦旎妮, 赖琼钰, 高 媛, 等. 层状 LiNi1/3Co1/3Mn1/3O2正极材料的合成及电化学性能研究[J]. 无机化学学报, 2005, 21(7): 999-1003.
[16] An Yongxin. Electrochemical performance of ionic liquid electrolyte for Lithium-ion Batteries[D]. Harbin: Harbin Institute of Technology, 2011.(in Chinese) 安永昕. 锂离子电池用离子液体电解质的电化学性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2011.
[17] Yu Xiaoyuan, Hu Guorong, Peng Zhongdong, et al. Synthesis and electrochemical characterization of layered LiNi1/3Co1/3Mn1/3O2cathode materials for Li-ion batteries[J]. Chinese Journal of Power Sources, 2006, 29(10): 641-643.(in Chinese) 禹筱元, 胡国荣, 彭忠东, 等. 层状 LiNi1/3Co1/3Mn1/3O2正极材料合成及电化学性能[J]. 电源技术, 2006, 29(10): 641-643.
【中文责编:晨 兮;英文责编:新 谷】
2014-05-27;Revised:2015-01-31;Accepted:2015-02-06
Compatibility of ternary cathode with ionic liquid-based composite electrolyte
Wang Zhenfeng, Zhang Xiaoxue, Li Cuihua†,Liu Jianhong, and Zhang Qianling
College of Chemistry and Chemical Engineering, Shenzhen University, Shenzhen Key Laboratory of Functional Polymer,Shenzhen 518060, P.R.China
N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide (PP13TFSI) and N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYR13TFSI) were synthesized at room temperature and mixed with organic solvents and lithium bis(trifluoromethanesulphonyl)imide (LiTFSI) to prepare the composite electrolyte. The thermal stability and non-flammability of the composite electrolyte were investigated. The electrolyte, LiCo1/3Mn1/3Ni1/3O2cathode and lithium metal anode were encapsulated into CR-2032 coin cell (LiCo1/3Mn1/3Ni1/3O2/electrolyte/Li). The initial discharge capacity of the coin cell reaches 154.5 mA·h/g at the rate of 0.1 C, and the coulombic efficiency is 85.5%. The results show that the composite electrolytes possess characteristics including a wide electrochemical stability window, high thermal stability, non-flammability, and better cycle performance.
applied electrochemistry; lithium-ion batteries; electrolyte; ionic liquid; compatibility; non-flammability
:Wang Zhenfeng, Zhang Xiaoxue, Li Cuihua, et al. Compatibility of ternary cathode with ionic liquid-based composite electrolyte[J]. Journal of Shenzhen University Science and Engineering, 2015, 32(3): 251-256.(in Chinese)
O 69
A
10.3724/SP.J.1249.2015.03251
国家重点基础研究发展计划资助项目(613142020201); 深圳市科学技术研究与发展基金资助项目(JCYJ20140418193546111)
王振峰(1984—),男 (汉族),广东省恩平市人,深圳大学硕士研究生,E-mail:supperfeng@163.com
Foundation:Major State Basic Research Development Program of China (613142020201); Scientific and Technological Research and Development Foundation of Shenzhen City (JCYJ20140418193546111)
† Corresponding author:Professor Li Cuihua. E-mail: licuihuasz@163.com
引 文:王振峰,张小雪,李翠华,等.离子液体复合电解质与三元正极的相容性[J]. 深圳大学学报理工版,2015,32(2):251-256.
——徐小林

