Optimization of High-Gravity Chelated Iron Process for Removing H2S Based on Response Surface Methodology
(Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering, North University of China, Taiyuan 030051)
Optimization of High-Gravity Chelated Iron Process for Removing H2S Based on Response Surface Methodology
Luo Ying; Zhang Zhongzhe; Qi Jibing; Li Gang; Qi Guisheng; Liu Youzhi
(Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering, North University of China, Taiyuan 030051)
By using a mixture of N2and H2S as the simulated APG (associated petroleum gas), the desulfurization experiment was performed in a cross-flow rotating packed bed (RPB) based on the chelated iron oxidation-reduction method. In order to determine the operating conditions of the system, the effects of the concentration of Fe3+ions (ranging from 0.1 to 0.2 mol/L), the liquid-gas volume ratio (ranging from 15 to 25 L/m3) and the high gravity factor (ranging from 36 to 126) on the removal of H2S were studied by means of the Box-Behnken design (BBD) under response surface methodology (RSM). The overall results have demonstrated that the BBD with an experimental design can be used effectively in the optimization of the desulfurization process. The optimal conditions based on both individualized and combined responses (at a Fe3+ion concentration of 0.16 mol/L, a liquid-gas volume ratio of 20.67 L/m3and a high gravity factor of 87) were found. Under this optimum condition, the desulfurization efficiency could reach 98.81% when the H2S concentration was 7 g/m3in APG. In this work, the sulfur product was analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and the energy dispersive X-ray spectrometer (EDX). The results of analysis show that the sulfur is made of the high-purity orthorhombic crystals, which are advantageous to environmental conservation.
hydrogen sulfide; chelated iron; high gravity technology; response surface methodology; Box-Behnken design
1 Introduction
Hydrogen sulfide (H2S) with high toxicity and corrosivity generally exists in the APG (associated petroleum gas) that is used as raw material or gaseous fuel in the chemical industry. Consequently, it is necessary to remove H2S from the APG before utilization[1]. Due to its high desulfurization efficiency and innoxiousness, the chelated iron method has been applied widely. The desulfurization process with chelated iron is based on the mechanism of redox reactions, which proceeds as follows[2]:
(a) Absorption of H2S by caustic (for example Na2CO3) solution:
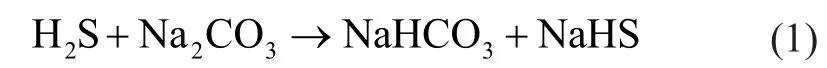
(b) Formation of elemental sulfur:

(c) Regeneration of desulfurization solution:

in which n denotes the charge of an organic ligand L.
The rotating packed bed is a new type of enhanced mass transfer equipment[3-4], which has been widely used in distillation, absorption, desulfurization and chemical synthesis thanks to its great advantages of high mass transfer efficiency, short residence time, small equipment size and low energy consumption[5]. Leng Jibin, et al.[6]and Cao Huibo, et al.[7]adopted an iron-chelated solution in the rotating packed bed for experimental study on the removal of hydrogen sulfide from the simulated natural gas and APG. In view of the fact that APG in offshore drilling platforms with space and bearing capability is far away from the coastline, therefore, the combination of high gravity technology and chelated iron method is very suitable for purification of combustible gas through desulfurization, which is in favor of transportation and operation of skid structure[8-9].
The response surface methodology (RSM) is an excellent tool for optimizing a process, and has been successfully used in testing the process parameters and their interactiveeffects[10-13]. In this work, desulfurization experiments by chelated iron method were performed in a rotating packed bed. The influence of the concentration of Fe3+ions, the liquid-gas volume ratio and the high gravity factor (while sometimes the three factors may interact on each other) on the desulfurization effect was studied with a Box-Behnken model under response surface methodology, and the regression relation between desulfurization efficiency and independent parameters of the desulfurization process was also analyzed. At the same time, the crystal structure and morphology of sulfur were studied by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive X-ray spectrometer (EDX).
2 Experimental
2.1 Experimental instruments and reagents
The instruments included: A D8 Advance X-ray diffractometer manufactured by the German Bruker AXS Ltd., a JSM-7001F scanning electron microscope made by the Japanese Electron Optics Laboratory Co., Ltd., a PHS-3C type pH-meter and a L5 type ultraviolet spectrophotometer made by the Shanghai Yidian Scientific Instruments Co., Ltd., a HZ85-2 magnetic stirrer made by the Beijing Zhongxing Weiye Instrument Co., Ltd., an AUY120 type electronic balance made by the Japanese Shimadzu Company, a GWA-UN type ultra-pure water device made by the Beijing Puxi General Instrument Co., Ltd., a LG10-2.4A type high-speed centrifuge made by the Beijing Jingli Centrifuge Co., Ltd., a CHI660E type electrochemical work station made by the Shanghai Chenhua Instrument Co., Ltd., and a M-40 type hydrogen sulfide detector made by the INSCO Group Inc. of USA.
Chemical reagents included: EDTA, NTA, and HEDTA, provided by the Tokyo Kasei Kogyo Co., Ltd.; (NH4)2SO4·Fe2(SO4)3·24H2O and (NH4)2SO4·FeSO4·6H2O, provided by the Tianjin Red Rock Reagent Plant; ascorbic acid and o-phenanthroline purchased from the Tianjin Hengxing Chemical Reagent Manufacturing Company; and glacial acetic acid, zinc acetate, and NaOH, made by the Tianjin Tianli Chemical Reagents Co., Ltd.
2.2 Equipment and process flow diagram
The experimental unit for removal of H2S by chelated iron in the cross-flow rotating packed bed is presented in Figure 1.
H2S and N2streams were mixed in a surge drum to form the simulated APG containing H2S (7 g/m3). The gas was measured by a rotameter, and entered the cross-flow rotating packed bed, in which the gas came into contact with the chelated iron solution pumped into the bed by a recycle pump from the circulating tank A. Under the conditions of high turbulence, large contact surface and highspeed renewal of interfaces between the gas and liquid, the hydrogen sulfide gas was readily absorbed by chelated iron solution and passed through an additional absorbing tank C filled with NaOH solution for further sulfur removal, and finally was vented into the atmosphere. The chelated iron solution containing the absorbed H2S was sent back to the liquid storage tank B for reuse.

Figure 1 Experimental unit for absorption of H2S in a cross-flow rotating packed bed
2.3 Experimental methods
The desulfurization efficiency Y is used to characterize the effect on removal of H2S[1], as defined by:

in which Y is H2S removal efficiency (%), and ρ1and ρ2denote the H2S concentration (g/m3) at the inlet and outlet of the rotating packed bed, respectively.
The high gravity factor β is defined as the ratio of the high gravitational (centrifugal) acceleration in the rotatingpacked bed to the gravitational acceleration:

where ω is the angular rotation speed (rad/s), r is the radius (m), and g is the gravitation acceleration. In the rotor, β varies with the radial direction r when ω is constant. The average high gravity factoris the magnitude of the centrifugal fields from the inner radius r1to the outer radius r2of the rotor, which is used to replace the high gravity factor β[3].

2.4 Box-Behnken experimental design and optimization by RSM
RSM is a statistical and mathematical technique which uses quantitative data obtained from experiments to confirm regression model equations and operating conditions. Therefore, it is useful for improving, developing and optimizing the desulfurization process. In this study, RSM was used to optimize and study the influence of independent variables such as the concentration of Fe3+ions, the liquid-gas volume ratio and the high gravity factor on the desulfurization efficiency (the selection of the three factors and levels are based on previous studies[1,14-15]). The experiments were established based on the Box-Behnken design with three factors and three levels. Each factor in the experiment was established and coded into levels -1, 0 and +1 as shown in Table 1.

Table 1 Factors and levels for BBD
2.5 Characterization techniques
Different characterization methods involving XRD, EDX and SEM were used to identify the sulfur product. The structural characterization studies on the sulfur species were carried out by X-ray diffraction (Bruker D8 Advance, with Co-Kα radiation, step size=0.02 deg/sec, λ=1.540 6 Å at a tube voltage of 40 kV and a tube current of 40 mA). Surface characteristics of the sulfur species were studied by using scanning electron microscopy (JSM-7001F), in the process of which gold was used as the conductive material for sample coating.
3 Results and Discussion
3.1 Box-Behnken statistical analysis
In this study, experiments were planned by using the Box-Behnken design of RSM to achieve a quadratic model requiring 17 experimental runs. The Box-Behnken design matrix of the three variables in natural units and corresponding response values are given in Table 2, which includes experimental and predicted values of desulfurization efficiency.
The quadratic equation was generated by the RSM design, which was used to predict the optimum point. Basedon the RSM analysis, the second order quadratic equation of coded units for the desulfurization efficiency (Y) is presented in Equation (8):

Table 2 Box-Behnken design matrix of three variables in natural units and response values

in which Y is the desulfurization efficiency (%), A is the concentration of Fe3+ions (mol/L), B is the liquid-gas volume ratio (L/m3), and C is the high gravity factor.
Statistical analysis of variance (ANOVA) includes the main and interacting effects of the variables on the desulfurization process. The ANOVA based on data from experiments are shown in Table 3.
It can be seen from Table 3 that the small F-value of 3.59 obtained for the Lack-of-Fit is found to be non-significant (a non-significant Lack-of-Fit is good), and there is a 12.46% chance that a Lack-of-Fit value (F-value) of this size could occur due to noise, which further verifies that the quadratic model is statistically valid. The value of the determination coefficient (R2=0.995 2) indicates that the model could explain 99.52% of the variability in the desulfurization process. The predicted R2of 0.942 0 is in reasonable agreement with the adjusted R2of 0.989 0. The high value of the adjusted R2supports a high correlationbetween the experimental and the predicted values. The“adequate precision” measures the signal to noise ratio, and a ratio greater than 4 is desirable in this study. The ratio of 35.523 indicates an adequate signal, so this model can be used to navigate the design space.

Table 3 Analysis of variance (ANOVA) for response surface quadratic model
Figure 2 shows the predicted values versus the actual ones for the desulfurization process. According to this figure, the data points are well distributed to be close to a straight line with a R2value of 0.995 2, indicating an excellent relationship between the predicted and actual values of the response for the desulfurization process. The results also confirm that the model is appropriate in assuming the response variables for the experimental data.

Figure 2 Comparison between predicted and actual values of desulfurization efficiency
3.2 Effect of interactive variables in the desulfurization process
In order to clarify the interactive effect of the concentration of Fe3+ions, the liquid-gas volume ratio, and the high gravity factor, three-dimensional (3D) response surface plots and contour plots are presented in Figure 3(a)—(f), depicting the interaction of each of the two variables by keeping the other at its central level for desulfurization.
The degree of enhancement of desulfurization on the interaction between the concentration of Fe3+ions and the liquid-gas volume ratio can be explained from the 3D response surface plots and contour plots (Figure 3(a) and (b)), which shows that the desulfurization efficiency is directly related to the concentration of Fe3+ions that are higher than 0.16 mol/L and the high liquid-gas volume ratio. Moreover, increasing the concentration of Fe3+ions would reduce the desulfurization efficiency. The contour plots display elliptical lines. The maximum desulfuriza-tion efficiency occurs at a concentration of Fe3+ions ranging between 0.16 mol/L and 0.20 mol/L and a liquid-gas volume ratio ranging between 17 L/m3and 23 L/m3in the area confined to the smallest ellipse. This interaction implies that the maximum desulfurization efficiency has reached 98.81%.

Figure 3 Response surface plots and contour for H2S removal as a function of concentration of Fe3+vs. liquid-gas volume ratio (a, b), concentration of Fe3+vs. high gravity factor (c, d) and liquid-gas volume ratio vs. high gravity factor (e, f)
The effect of the interaction between the concentration of Fe3+ions and the high gravity factor is presented in Figure 3(c) and (d). According to the F and P-value depicted in Table 3, the interaction between the concentration of Fe3+ions and the high gravity factor (F=9.96 and P=0.016 0) has a significant interaction influence on changing the desulfurization efficiency. In particular, the contour plots (Figure 3(d)) exhibited an elliptic feature with the longaxis of the elliptic running along the respective concentration of the Fe3+axis, indicating that the rising concentration of Fe3+ions and the high gravity factor raise the desulfurization efficiency to its highest level. As shown in Figure 3(e) and (f), the liquid-gas volume ratio and the high gravity factor have the least significant interaction effect, depicting that the desulfurization efficiency increases with an increasing liquid-gas volume ratio and high gravity factor.
3.3 Characterization of sulfur product
Figure 4 presents an XRD pattern of the sulfur product. As shown in Figure 4, the obtained sulfur product is highly crystallized, and there are clear characteristic diffraction peaks at 2θ of 23.078°, 25.823° and 27.719°, respectively, ascribed to amorphous phase sulfur species. This shows that the sulfur product is rhombic and aggregated easily, which can facilitate the separation of solids from the liquid phase.

Figure 4 XRD pattern of sulfur product
The SEM micrographs under different magnifications (15 000 and 10 000 times) are presented in Figure 5. These photos also show that the granular sulfur has a rough surface, with the particle size generally ranging between 0.30 μm and 3.68 μm, and many sulfur particles get together to form large aggregates, which are beneficial to sulfur coagulation and can facilitate the separation of precipitates.
In order to understand the composition and purity of the sulfur product, the EDX analysis of a spot on the surface of sulfur was carried out with the results shown in Figure 6. The peaks of the elemental sulfur are very strong, attesting to the relatively high purity sulfur. In addition, a small amount of C and O elements are present in the sulfur product, which may be caused by background interference.

Figure 5 SEM micrographs of sulfur under different magnifications

Figure 6 EDX spectrum of a spot on the surface of sulfur
4 Conclusions
For constituting and acquiring the optimal conditions of H2S removal in the APG with chelated iron by high gravity technology, both the response surface methodology and the Box-Behnken design were adopted. Based on the analysis of the experimental results, all three parameters, viz.: the concentration of Fe3+, the liquid-gas volume ratio and the high gravity factor, were conducive to the H2S removal. The main effect of each parameter was more significant than the respective interactive effects, imply-ing the direct effect of variables on H2S removal. The combination of concentration of Fe3+ions and liquid-gas volume ratio was the most effective interaction with a positive effect on the desulfurization process.
Meanwhile, the crystal structure and morphology of sulfur were studied by X-ray diffraction and scanning electron microscopy. The analysis of samples by XRD techniques shows that the sulfur crystal type was rhombic. Furthermore, the analysis of SEM shows that the sulfur particle size was in the range of between 0.30 μm—3.68 μm. The characterization images indicate that the sulfur particles agglomeration did take place, which was conductive to coagulating and separating of the precipitates from the liquid phase.
Acknowledgements: This work was financially supported by the National Science Foundation of China (No. 21376229) and the Science and Technology Development Plan of Shanxi Province (No. 20130321035-02).
[1] Luo Ying, Liu Youzhi, Qi Guisheng, et al. Selection of new Fe(III)/Fe(II) chelating agents as catalysts for the oxidation of hydrogen sulfide [J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(2): 50-58
[2] Wubs H J, Beenackers A A C M. Kinetics of the oxidation of ferrous chelates of EDTA and HEDTA in aqueous solution [J]. Industrial & Engineering Chemistry Research, 1993, 32(11): 2580-2594
[3] Liu Youzhi. Chemical Engineering Process and Technology in High Gravity[M]. National Defence Industry Press, Beijing, 2009 (in Chinese)
[4] Chen Jianfeng. High Gravity Technology and Application[M]. Chemical Industry Press, Beijing, 2003 (in Chinese)
[5] Yu Yong, Liu Youzhi, Qi Guisheng, et al. H2S removal by chelated iron method with counter-current rotating packed bed[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 6(29): 1003-1008 (in Chinese)
[6] Leng Jibin, Yu Zhaoyang, Li Zhenhu, et al. Exploratory study on hydrogen sulfide removal from natural gas in a rotating packed bed (RPB) by oxidation reduction method[J]. Chemical Industry and Engineering Progress, 2007, 26(7): 1023-1027 (in Chinese)
[7] Cao Huibo, Li Zhenhu, Hao Guojun, et al. Pilot plant study on removal of hydrogen sulfide from associated gas by high gravity chelated-iron method[J]. Petrochemical Technology, 2009, 38(9): 971-974 (in Chinese)
[8] Chu Guanwen, Luo Yong, Zou Haikui, et al. Reaction process intensification: Applications of acidic tail-gas treatment by high gravity technology[J]. Chemical Reaction Engineering and Technology, 2013, 29(3): 193-198 (in Chinese)
[9] Li Wenming, Li Zhenhu, Hao Guojun, et al. Industrial application of rotating packed bed (RPB) on H2S removal from associated gas by iron-redox process[J]. Journal of Chemical Industry & Engineering, 2014, 35(2): 26-30 (in Chinese)
[10] Momene Moradi, Jafar Towfighi Daryan, Ali Mohamadalizadeh. Response surface modeling of H2S conversion by catalytic oxidation reaction over catalysts based on SiC nanoparticles using Box−Behnken experimental design[J]. Fuel Processing Technology, 2013, 109: 163-171
[11] Gabriela John Swamy, A Sangamithra , V Chandrasekar. Response surface modeling and process optimization of aqueous extraction of natural pigments from beta vulgaris using Box-Behnken design of experiments[J]. Dyes and Pigments, 2014, 111: 64-74
[12] Narges Esfandiar, Bahram Nasernejad, Taghi Ebadi. Removal of Mn (II) from groundwater by sugarcane bagasse and activated carbon (a comparative study): Application of response surface methodology (RSM)[J]. Journal of Industrial and Engineering Chemistry, 2014, 20: 3726-3736
[13] Han Xiuli, He Yuyuan, Zhao Haohao, et al. Optimization of preparation conditions of activated carbon from the residue of desilicated rice husk using response surface methodology[J]. Korean J. Chem. Eng, 2014, 31(10): 1810-1817
[14] Luo Ying, Zhu Zhenfeng, Liu Youzhi, et al. Hydrogen sulfide removal with ferric chelates of NTA and HEDTA solutions in cross-flow rotating packed bed[J]. Modern Chemical Industry, 2014, 34(7): 130-134(in Chinese)
[15] Zhu Zhenfeng, Liu Youzhi, Luo Ying, et al. Study on desulfurization performance with ferric chelates of EDTA and HEDTA system[J]. Chemistry, 2014, 77(5): 436-440(in Chinese)
date: 2015-02-15; Accepted date: 2015-04-19.
Dr. Luo Ying, Telephone: +86-351-3921986; E-mail: yingluozbdx@163.com.
- 中国炼油与石油化工的其它文章
- Catalytic Hydrogenation of Methanol-Containing Effluent from Epoxidation of Propylene
- Studies on the Hydrogenation of Acetonitrile over Fresh Mo2C/γ-Al2O3Catalyst by In-situ IR Spectroscopy
- Synthesis of Biodiesel Using ZrO2Polycrystalline Ceramic Foam Catalyst in a Tubular Reactor
- Investigation of Swelling and Dissolution Process of Natural Rubber in Aromatic Oil
- Secondary Crystallization of Na2CO3-Modified HZSM-5 Zeolites with Tetrapropylammonium Hydroxide and Their Catalytic Performance in Thiophene Alkylation Reaction
- Lubricant Biodegradation Enhancers: Designed Chemistry and Engineered Technology

