Secondary Crystallization of Na2CO3-Modified HZSM-5 Zeolites with Tetrapropylammonium Hydroxide and Their Catalytic Performance in Thiophene Alkylation Reaction
Liu Dongmei; Kong Feifei; Zhai Yuchun; Wang Haiyan
(1. School of Material and Metallurgy, Northeast University, Shenyang 110004; 2. School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
Secondary Crystallization of Na2CO3-Modified HZSM-5 Zeolites with Tetrapropylammonium Hydroxide and Their Catalytic Performance in Thiophene Alkylation Reaction
Liu Dongmei1,2; Kong Feifei2; Zhai Yuchun1; Wang Haiyan2
(1. School of Material and Metallurgy, Northeast University, Shenyang 110004; 2. School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
The Na2CO3-modified HZSM-5 zeolites were further treated by tetrapropylammonium hydroxide (TPAOH) solution. The effect of TPAOH concentration on the secondary crystallization process was investigated. The resulting samples were characterized by a complementary combination of X-ray diffraction, N2adsorption/desorption, scanning electron microscopy, X-ray fluorescence spectroscopy, XPS,27Al and29Si magic-angle spinning nuclear magnetic resonance spectroscopy, BET and temperature-programmed desorption techniques. The results showed that the secondary crystallization of the HZSM-5 zeolite could result in migration of non-framework species from the internal channels to the zeolite surface and their transformation into framework species. The catalytic activity of these modified samples for thiophene alkylation was evaluated. Both the activity and stability of the catalysts were improved after secondary crystallization.
tetrapropylammonium hydroxide; sodium carbonate; HZSM-5; secondary crystallization
1 Introduction
The loss of octane number of gasoline fraction is quite significant in deep desulfurization by means of conventional gasoline hydrodesufurization method[1]. An effective way for removing thiophenic sulfur can be realized by alkylation over acidic catalysts[2-7]. The technique is based on the alkylation reaction that thiophene would react on 1-hexene in gasoline to produce high boiling thiophenic compounds. Therefore, light sulfur-free gasoline fraction is obtained, which can avoid the loss of octane number in the process of olefin saturation reaction when the light gasoline fraction is directly added into the hydrodesulfurization unit. According to the transition state theory, the conversion of thiophene and olefins to alkyl-thiophenes is in need of big intermediates formed in this process. These intermediates is large in size, and therefore the alkylationdesulfurization catalysts should provide such a space. The catalyst must also have sufficient acid strength, so that the carbocation can be obtained via the reaction between olefins and H+ions at elevated temperatures. Zhang, et al.[8]found that the Hβ catalyst showed good catalytic performance for thiophene alkylation, but the channels of the Hβ framework were small, leading to alkylation of high aromatics. Ke, et al.[9]used acidic ionic liquid [BMIM] and H2SO4as a catalyst complex system and conjugated diene as an alkylation agent to study alkylation/desulfurization of gasoline, which could overcome the problem related with narrow pores of the catalysts. The alkylation reaction between thiophene and isoprene exhibited higher selectivity as compared with the polymerization reaction of olefins, but there were several disadvantages such as waste acid emissions, environmental pollution and corrosion of equipment. Therefore, the environmentally friendly solid acid catalysts have become a research focus in recent years. Xu, et al.[10]investigated the activity of thiophene alkylation on HZSM-5, HY, Hβ, and SAPO-11 catalysts. They found out that HZSM-5 zeolite showed a much higher activity than other molecular sieves under the same reaction conditions. It is well known that the ZSM-5 zeolites are stronger acid that plays an important role in the acid-catalyzed reactions. Nevertheless, they show poor activity in macromolecular reactions due totheir smaller pore size[11-19]. Recently, desilication by treatment in an alkaline medium was a very suitable and reproducible method to obtain the mesoporous ZSM-5 zeolites with promoted diffusivity and catalytic activity, and had attracted a growing interest in zeolite-catalyzed macromolecular reactions. Ogural, et al.[20]found that the activity (for conversion of benzene to cumene) of the HZSM-5 catalyst was significantly improved after treatment with NaOH solution. The results were attributed to the increase in diffusion of reactants. Song, et al.[21]prepared a micro-mesoporous HZSM-5 catalyst by NaOH treatment, resulting in significant improvements in the selectivity of catalytic cracking of octane. Currently[20-22], the inorganic NaOH solution was usually used as an alkaline medium. However, a problem was identified in this method, viz.: there was a significant decrease in the crystallinity and acidity of HZSM-5 catalyst due to the strong damage of channels, leading to lower activity and stability of HZSM-5 catalyst. In our previous study, it was found out that in comparison with NaOH treatment, Na2CO3treatment was more moderate and the process could be more easily controlled. Therefore, there was a slight decrease in the crystallinity and acidity after treatment by Na2CO3. However, the shortcoming of Na2CO3modification was similar to that of NaOH modification. The non-framework species removed by modification could accumulate in the channels, leading to decreased crystallinity and acidity and resulting in the decrease of catalytic performance of HZSM-5 catalyst.
He, et al.[23]worked on the secondary crystallization of HZSM-5 after treating with tetrapropylammonium hydroxide (TPAOH) solution. The results indicated that the relative crystallinity of the zeolite increased after modification. Thus, the modified sample showed a significant improvement in catalytic performance for the conversion of methanol to gasoline. Huang, et al.[24]also observed secondary crystallization after modifying TS-1 zeolite by TPAOH solution, and the crystal grains became more regular and some new crystal grains appeared after modification. These results indicated that the occurrence of secondary crystallization of the zeolite could result in the migration of non-framework species to zeolite surface coupled with their transformation into framework species. Herein, in this study, the Na2CO3-modified HZSM-5 zeolites were further treated by TPAOH solution and their catalytic performance was evaluated in thiophene alkylation reaction.
2 Experimental
2.1 Catalyst preparation
The Na-form ZSM-5 zeolite powder with a SiO2/Al2O3molar ratio of 50 was calcined in a muffle furnace at 550 ℃for 4 h to remove the templating agent. After the alkalitreatment, the calcined sample was treated with Na2CO3solution. The ZSM-5 zeolite was treated with an aqueous solution containing 4 mol/L of Na2CO3. Meanwhile, the solution was kept at 90 ℃ for 2 h under vigorous stirring. The sample labeled as HZSM-5(4) was divided into three portions, added into the TPAOH solution at a different rate respectively (at a molar ratio of n(TPAOH):n(SiO2) = 0.1—0.3, respectively), mixed uniformly, and then crystallized at 170 ℃ for 24 h. After being subject to filtration and washing, the samples were dried at 120 ℃ for 12 h, and the NaZSM-5 zeolite samples were obtained after crystallization. After drying, the samples were calcined at 550 ℃ for 2 h to be converted to the H-form ZSM zeolite. Finally, these extrudates were impregnated with an 1.0 mol/L ammonium nitrate solution and washed with deionized water at 80 ℃, repeatedly. The samples after crystallization were denoted as HZSM-5(4) or HZSM-5 (4-y) (with y = n(TPAOH): n(SiO2), in molar ratio).
2.2 Catalyst characterization
The X-ray diffraction (XRD) patterns were used to identify the phase and assess the crystallinity of the powder samples. The XRD patterns were obtained with a Rigaku D/max-RB X-ray diffractometer, using CuKα (λ=0.154 06 nm) radiation at room temperature and an instrumental setting of 40 kV and 100 mA. The diffraction patterns were recorded with a goniometer radius of 185 mm, and a diaphragm system of DS=SS=1. The form of body phase of zeolite samples was analyzed by a Bruker AXS S4 X-ray fluorescence spectrometer (XRF). The temperature-programmed desorption of ammonia (NH3-TPD) was performed with a CHMBET-3000 chemisorption analyzer (Quantachrome, USA) to determine the acid strength and the content of acidic sites. The NH3-TPD was carried out as follows. Each sample was loaded into a stain-less U-shaped microreactor and pretreated. The pretreated sample was cooled down to 120 ℃ for 160 min until the specified temperature was stabilized. Then, NH3-TPD was carried out under a constant flow of He (at a rate of 20 mL/min) at a heating rate of 10 ℃/min. The concentration of ammonia in the exit gas was continuously detected by a gas chromatograph equipped with a TCD. The X-ray photoelectron spectra (XPS) were obtained with the Kα photoelectron spectroscopy (VG, UK) using a monochromatized aluminum Al Kα source (E=1 486.6 eV) and C1s (284.6 eV) was used to calibrate the nuclear effect as the internal standard. The Si coordination state on the zeolite samples was confirmed by29Si MAS NMR, and was recorded on an Avancell 600 nuclear magnetic resonance spectrometer (Bruker, Germany) at a frequency of 119.2 MHz with a 4 mm MAS probe. The27Al spectra were recorded using the 4 μs RF pulse (π/2 flipping angle) with an acquisition delay of 4 s. Chemical shifts were referenced to an external standard of (NH4)Al(SO4)2·12H2O. The scanning electron microscopy (SEM) was performed with a S4800 transmitting and scanning electron microscope (Hitachi) to observe the morphology of samples at an accelerating voltage of 5 kV. The transmission electron microscopy (TEM) images were obtained from a JEOL JSM-3010 instrument (Japan) working at an accelerating voltage of 100—300 kV. The N2adsorption and desorption experiments were performed in liquid nitrogen at 77 K on an ASAP 2010 automatic physical adsorption instrument (Micromeritics Instrument Co, USA). The high purity nitrogen was used as an adsorption medium, with the liquid nitrogen serving as the cold trap. The relative pressure P/P0was 0 to 0.995, with the pore size ranging from 1.7 nm to 300 nm. The total surface area was calculated according to the BET isothermal equation, and the micropore volume and external surface area of the catalyst were evaluated by the t-plot method. The mesopore distribution was obtained according to the BJH method. The pyridine adsorption-IR was carried out on a Bruker Vector 22 Fourier transform infrared spectrometer (Bruker, Germany). Self-supporting wafers (10 mm in diameter) were made of fine zeolite particles with size ranging from 10 mesh to 20 mesh. The IR cell could hold the wafers of HZSM-5 zeolite. The pretreatment of the fresh samples was conducted as follows: The cell containing the zeolite wafer was evacuated while slowly increasing the temperature to 450 ℃ under high vacuum for 0.5 h. After that, the sample was saturated with pyridine and evacuated at 400 ℃ for 0.5 h. Subsequently, the IR spectra were measured (after cooling down to room temperature).
2.3 Catalytic tests and analytical procedures
The performance evaluation of thiophene alkylation catalyst was conducted in a continuous fixed bed reactor. In each run 5 mL of the catalyst was charged in the reactor (made of a stainless steel tube, 80 cm in length, and 1.0 cm in inner diameter). The space above and below the catalyst particles layer was filled with quartz beads. Thiophene, 1-hexene, xylene and n-hexane (at a volume ratio=1.6:12.8:4.9:500) were employed as the feedstock for alkylation reaction testing. The condition for evaluation of catalyst covered a temperature of 623 K, a reaction pressure of 1.0 MPa, and a weight hourly space velocity of 1.0 h-1. The main products were determined by GC-MS (7890A, Agilent) and analyzed by a gas chromatograph that was equipped with a capillary HP-5 column (30 m×320 μm×0.25 μm) connected to a sulfur chemiluminescence detector (SCD). The hydrocarbons in the products were analyzed with a 1002 type gas chromatograph (China) equipped with a flame ionization detector (FID). The conversion of different thiophenic compounds is calculated by the following formula:
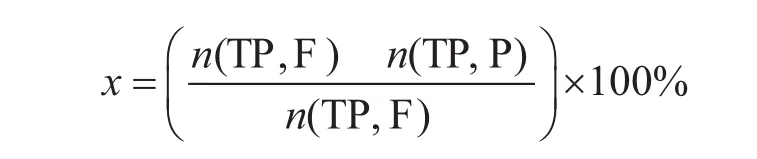
in which x denotes the conversion rate of thiophene, n (TP, F) and n(TP, P) denote the mass amount of thiophene in the reactants and products, respectively.
3 Results and Discussion
3.1 The XRD characterization of HZSM-5, HZSM-5(4) and HZSM-5(4-y)
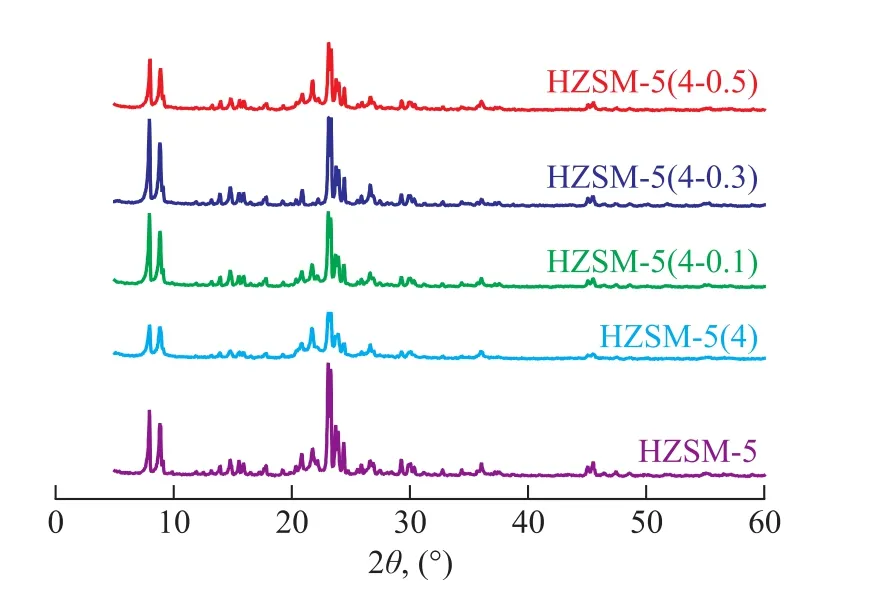
Figure 1 The XRD patterns of the HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
Figure 1 shows the XRD patterns of the HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples. The number of diffraction peaks and the peak positions (2θ=7.8°, 8.7°, 22.9°, 23.6° and 24.3°) did not change after Na2CO3or Na2CO3/TPAOH treatment, indicating that the TPAOH treatment did not adversely affect the crystal structure of HZSM-5 zeolite. Table 1 shows the relative crystallinity and n(SiO2)/n(Al2O3) of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples. It can be seen that the relative crystallinity and n(SiO2)/n(Al2O3) ratio decreased after Na2CO3treatment, which occurred because there were crystal defects on the framework of HZSM-5 zeolite after the alkaline treatment. However, a further treatment by TPAOH could stabilize the zeolite structure. It is known that TPAOH solution not only could act as the lye to remove framework silicon and aluminum, but also could act as a template which might lead to secondary crystallization of amorphous species and non-framework silicon and aluminum on the zeolite surface. Therefore, the relative crystallinity and n(SiO2)/n(Al2O3) ratio increased after TPAOH modification. In addition, the concentration of TPAOH solution had an influence on the relative crystallinity and n(SiO2)/n(Al2O3) ratio, and it was found out that the appropriate concentration was 0.5 mol/L.
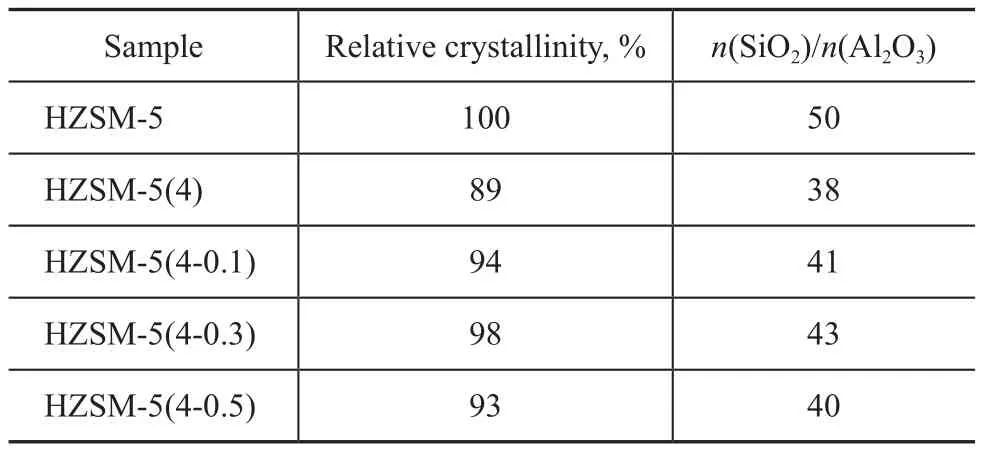
Table 1 Relative crystallinity and SiO2/Al2O3molar ratio of ZSM-5 samples treated with different alkaline
3.2 The SEM characterization of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
Figure 2 shows the SEM images of the HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples. It can be seen that the crystal morphology of unmodified HZSM-5 was regular. After Na2CO3treatment, the zeolite angles disappeared, and the edges and surfaces became rougher, which was similar to the results of NaOH modification[25]. It was worthy of note that a secondary crystallization occurred when HZSM-5 was treated by TPAOH solution, and the crystal grains became more regular and some new crystal grains appeared after treatment.

Figure 2 SEM images of HZSM-5, HZSM-5(4) and HZSM-5(4-y)
3.3 XPS characterization of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
The surface Si/Al ratios of these samples obtained from the XPS spectra are shown in Table 2. In the case of HZSM-5 sample, the surface Si/Al ratio was 9.4; it was 10.5 for HZSM-5(4) and the surface Si/Al ratio was equal to 16.7, 17.8 and 18.6 for HZSM-5(4-0.1), HZSM-5(4-0.3) and HZSM-5(4-0.5), respectively. The results indicated that there existed the phenomenon of migration of non-framework silicon from the HZSM-5 surface after modification, resulting in an increase in the Si/Al ratio on the zeolite surfaces. It is worthy to denote that the surface Si/Al ratios of HZSM-5(4-y) samples were obviously higher than that of HZSM-5(4) sample, indicating that asecondary crystallization of the zeolites occurred, making non-framework silicon migrate to the surfaces. The degree of the secondary crystallization increased with the increase in concentration of TPAOH solution.

Table 2 Surface Si/Al ratios of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
3.429Si MAS NMR characterization of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
Figure 3 shows the29Si MAS NMR spectra of the HZSM-5(4) and HZSM-5(4-y) samples. It can be seen that the signals at the chemical shift of -113 and -106 were observed on all the samples, which was attributed to framework Si(OSi)4(O4) and Si(OSi)3(OAl) (SiAl), respectively. The framework Si/Al ratio of these samples was further estimated. For the HZSM-5(4-y) sample, the framework Si/Al ratio increased slightly from 7.1 to 7.3—7.4 as compared to the HZSM-5(4) sample. The results indicated that a secondary crystallization of the zeolite occurred after the TPAOH treatment, resulting in the migration of non-framework silicon to the surface and the transformation into framework silicon, which was in agreement with the results of SEM and XPS (Figure 2 and Table 2).

Figure 329Si MAS NMR spectra of HZSM-5(4) and HZSM-5(4-y)
3.5 Pore structure of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
The nitrogen adsorption/desorption isotherms for HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples are shown in Figure 4. The pore properties of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples are listed in Table 3. It can be seen from Figure 4 that in comparison with HZSM-5(4) the hysteresis loops of HZSM-5(4-y) after TPAOH treatment in the isotherms were more pronounced and tended to migrate upwards. The mesopore area and average pore size of HZSM-5(4-y) all increased, which was mainly ascribed to the conversion of framework species to amorphous species during the secondary crystallization process. These results agreed well with the observation results in Figure 4. Although the mesopore areas and average pore size of HZSM-5(4-y) zeolite were less than those of HZSM-5(4-0.3) zeolite, the trends were in accordance with the above results. Therefore, the increase in concentration of TPAOH solution was disadvantageous to the secondary crystallization process.

Figure 4 Nitrogen adsorption-desorption isotherms of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
3.6 Surface acidity of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples
The NH3-TPD curves of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples are shown in Figure 5. It can be seen from Figure 5 that two main desorption peaks at 280 ℃ and 550 ℃ could be seen on HZSM-5—HZSM-5(4-0.5) samples, which corresponded to the desorption of NH3molecules from weak and strong acid sites[26]. In comparison with HZSM-5(4) zeolite, although the low-temperature peaks of the HZSM-5(4-0.1) and HZSM-5(4-0.3) samplesafter TPAOH treatment shifted towards higher temperatures and the high-temperature peaks shifted towards lower temperatures, the peak areas actually increased. The relative acidity amounts of weak acid and strong acid increased after TPAOH treatment within its lower concentration range (0.1 mol/L and 0.3 mol/L), indicating to a decrease in the weak acid strength and an increase in the strong acid strength. Compared with HZSM-5(4-0.3) zeolite, the low- and high-temperature peaks shifted towards lower temperatures after TPAOH treatment, showing a reduction in desorption area peak. The results indicated that both the acid strength and the relative acidity amount decreased with a further increase in concentration of TPAOH solution.
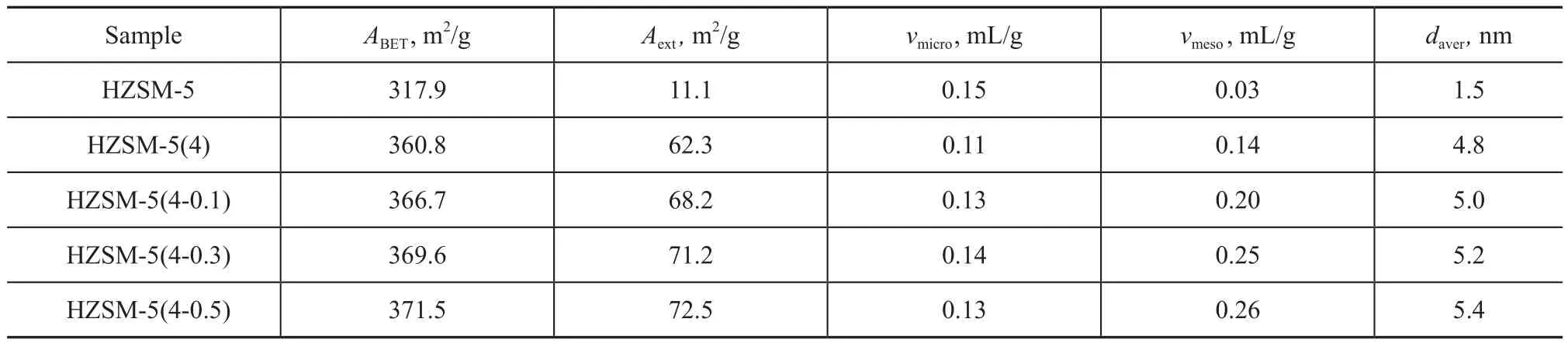
Table 3 The pore structure properties of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samp
The infrared spectra of adsorbed pyridine for HZSM-5(4) and HZSM-5(4-y) samples at 400 ℃ are shown Figure 6. The characteristic infrared bands near l 453 cm-1were attributed to pyridine species on the Lewis acid sites, which were related to the content of no-framework Al species. The bands near 1 544 cm−1corresponded to pyridine bonded to Brönsted acid centers, which was related to the content of framework Al species. As shown in Figure 6 for HZSM-5(4-0.5) zeolite, the areas of Lewis acid and Brönsted acid decreased compared with HZSM-5(4-0.3) zeolite. The contents of Brönsted acid and Lewis acid for the zeolite decreased during a higher-concentration TPAOH solution treatment, which was in agreement with the results of NH3-TPD analysis.

Figure 5 NH3-TPD profiles of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples

Figure 6 Py-IR spectras of HZSM-5(4) and HZSM-5(4-y)
3.7 Performance of olefin alkylation of thiophenic sulfur over HZSM-5, HZSM-5(4) and HZSM-5(4-y) zeolites
The OATS reactions were used as probe reactions to investigate the catalytic behavior of these catalyst samples, with the results shown in Figure 7. It is well-known that the OATS reactions proceeded at Brönsted acid centers. In the case of alkylation with olefin, the presence of an acidic catalyst was necessary to form this carbocation[27]. Although HZSM-5 zeolite was more acidic, the average aperture of the parent HZSM-5 zeolite was equivalent to the size of thiophene. Therefore, the zeolite catalyst was not very effective in the macromolecular reactions.
The thiophene conversion over HZSM-5 zeolite became much slower with time, as shown in Figure 7. In the course of reaction within 48 h, the catalyst deactivation phenomenon was very obvious and the catalyst became black within 96 h. The results indicated that the surfaceactive sites and internal channels were blocked by coke deposits and product molecules. After Na2CO3treatment, the BET surface area and apertures of the HZSM-5(4) zeolite increased, which were beneficial to promotion of alkylation of olefins with thiophenic sulfur. The increase of the thiophene conversion was mainly ascribed to the mesopores induced by Na2CO3treatment. With an increasing reaction time, the thiophene conversion fell down rapidly. Even though the increase of aperture of HZSM-5(4) zeolite was caused by the Na2CO3treatment, a large amount of non-framework species extracted from the zeolite could not be removed which would occupy the channels. Therefore, the activity and the diffusivity rate decreased but the carbon deposition rate increased. The effect of the TPAOH concentration on secondary crystallization in the HZSM-5(4) zeolite was investigated. The results indicated that with the increase in the concentration (0.1—0.3 mol/L) of tetrapropylammonium hydroxide (TPAOH) solution, the channels of the zeolite were cleared. Therefore, the number of active sites in HZSM-5(4-0.1)—HZSM-5(4-0.3) samples increased. This was beneficial to the increase in the conversion of thiophene. Therefore, the activity of the HZSM-5(4-0.3) zeolite became more stable with reaction time and the thiophene conversion decreased to 90% after 72 h. Moreover, the diffusion capacity improved and the formation of coke deposits reduced, which was attributed to the unblocked channels. Upon being subject to treatment with a higherconcentration tetrapropylammonium hydroxide (TPAOH) solution (0.5 mol/L), the initial activity of HZSM-5(4-0.5) zeolite was similar to that of HZSM-5(4-0.3) zeolite. With an increasing reaction time, the thiophene conversion of HZSM-5(4-0.5) zeolite decreased. These results further indicated that the increase of the concentration of tetrapropylammonium hydroxide (TPAOH) solution was disadvantageous to secondary crystallization of the zeolite.

Figure 7 Thiophene conversion of HZSM-5, HZSM-5(4) and HZSM-5(4-y) samples at different reaction time
4 Conclusions
The secondary crystallization of the HZSM-5(4) zeolite occurred after treatment by different concentrations of TPAOH solution. The modification of zeolite upon being treated with low concentration of TPAOH solution resulted in the migration of non-framework silicon to the zeolite surface and the remigration to the zeolite framework. The relative crystallinity of the zeolite was increased to facilitate the physical transport in the channels, which could result in the improvement of diffusion capacity. The activity and stability of the HZSM-5(4-y) catalyst samples could be improved during the OATS process. Especially for the HZSM-5(4-0.3) sample, the thiophene conversion reached a maximum value and the stability of the catalyst was significantly improved.
Acknowledgements: The authors are pleased to acknowledge the financial support by the Natural Science Foundation of Liaoning Province of China (Grant No.201202126), and the National Natural Science Foundation of China (Grant Nos. 21276253 and 21401093).
[1] Shen Z B, Ke M, Liu J Y. Research on the catalytic properties of thioetherification reaction on the mercaptans and isoprene with Ni/Al2O3[J]. Petroleum Processing and Petrochemicals, 2010, 41(11): 37-41 (in Chinese)
[2] Alexander B D, Huff G A, Pradhan V R, et al. Multiple stage sulfur removal process: The United States, US, 6059962[P]. 2000-05-09
[3] Richard F, Célérier S, Vilette M, et al. Inorganic hydroxide fluorides as solid catalysts for acylation of 2-methylfuran by acetic anhydride[J]. Appl Catal B: Environ, 2015, 168-169: 551-523
[4] Zhang Z K, Liu S L, Zhu X X, et al. Modification of Hβ zeolite by fluorine and its influence on olefin alkylation of thiophenic sulfur in gasoline[J]. J Fuel Process Technol, 2008, 89(1): 103-110 (in Chinese)
[5] Bellière V, Geantet C, Vriant M, et al. Alkylation of3-methyl-thiophene with 2-methyl-2-butene over a zeolitic catalyst[J]. Energy Fuels, 2004, 18(6): 1806-1813
[6] Dupuy B, Laforge S, Morais C, et al. Alkylation of 3-methylthiophene by 2-methyl-1-pentene over HY, HBEA and HMCM-22 acidic zeolites[J]. Appl Catal A: Gen, 2012, 31 (413/414): 192-204
[7] Dupuy B, Laforge S, Bachmann C, et al. Desulfurization of model FCC feedstocks by alkylation: Transformation of thiophenic compounds in presence of 2-methyl-1-pentene over acidic zeolite[J]. J Mol Catal A: Chem, 2012, 363-364: 273-282
[8] Zhang Z K, Niu X L, Liu S L, et al. The performance of HMCM-22 zeolite catalyst on the olefin alkylation of thiophenic sulfur in gasoline[J]. Catal Commun, 2008, 9(1): 60-64
[9] Ke M, Tang Y T, Cao W Z, et al. Study on alkylation reaction of diolefin with thiophene [J]. Journal of Xi,an Shiyou University (Natural Science Edition ), 2010, 32(3): 145-149 (in Chinese)
[10] Xu X, Luo G H, Tong Z M, et al. Alkylation of thiophenic sulfur compounds with olefin over zeolite catalysts[J]. Chemical Reaction Engineering and Technology, 2005, 21(2): 132-137 (in Chinese)
[11] Wang R, Wan J B, Li Y H, et al. An improvement of MCM-41 supported phosphoric acid catalyst for alkylation desulfurization of FCC gasoline[J]. Fuel, 2015, 143: 504-511
[12] Gong Jianhong, Xu Youhao, Long Jun, et al. Synergetic effect of Y zeolite and ZSM-5 zeolite ratios on cracking, oligomerization and hydrogen transfer reactions[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(3): 1-9
[13] Zhang Baozhong, Liu Xiaopeng. Catalytic performance of MFI/MFI core-shell zeolites in benzene methylation[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 94-99
[14] Zheng X D, Dong H J, Wang X, et al. Study on olefin alkylation of thiophenic sulfur in FCC gasoline using La2O3-modified HY zeolite[J]. Catal Lett, 2009, 127(1/2): 70-74
[15] Xu Y, Long J, Zhang J S. Study on alkylation reaction of thiophenic compounds for desulfurization of FCC gasoline on zeolite catalysts[J]. Prepr – Am Chem Soc Div Fuel Chem, 2005, 50(2): 235-238
[16] Liu Z C, Hu J R, Gao J S. FCC Naphtha desulfurization via alkylation process over ionic liquid catalyst[J]. Petroleum Processing and Petrochemicals, 2006, 37(10): 22-26 (in Chinese)
[17] Zhang Z K, Jiang H, Liu S L, et al. Alkylation performance of thiophene and its derivatives during olefinic alkylation of thiophenic sulfur in gasoline[J]. Chinese Journal of Catalysis, 2006, 27(4): 309-313
[18] Hu L Y, Zhang Z K, Xie S J, et al. Effect of grain size of zeolite Y on its catalytic performance in olefin alkylation of thiophenic sulfur process[J]. Catal Commun, 2009, 10(6): 900-904
[19] García-Martínez J, Cazorla-Amorós D, Linares-Solano A, et al. Synthesis and characterization of MFI-type zeolites supported on carbon materials[J]. Micropor Mesopor Mater, 2001, 42 (2): 255-268
[20] Ogura M, Shinomiya S Y, Tateno J, et al. Alkali-treatment technique—New method for modification of structural and acid-catalytic properties of ZSM-5 zeolites[J]. Appl Catal A: Gen, 2001, 219(1/2): 33-34
[21] Song Y Q, Zhu X X, Song Y, et al. An effective method to enhance the stability on-stream of butene aromatization: Post-treatment of ZSM-5 by alkali solution of sodium hydroxide[J]. App Catal A: Gen, 2006, 302(1): 69-77
[22] Groen J C, Peffer L A, Moulijn J A, et al. Mesoporosity development in ZSM-5 zeolite upon optimized desilication conditions in alkaline medium[J]. Colloids Surf A: Physicochem Eng, 2004, 214: 53-58
[23] He Y P, Liu M, Dai C Y, et al. Modification of nanocrystalline HZSM-5 zeolite with tetrapropylammonium hydroxide and its catalytic performance in methanol to gasoline reaction[J], Chinese Journal of Catalysis, 2013, 34(6): 1149-1155
[24] Huang X L. Study on secondary crystallization method modified TS-1 and catalytic ammoxidation of cyclohexanone[D]. Xiangtan: Xiangtan University, 2008 (in Chinese)
[25] He Y P, Liu M, Dai C Y, et al. TPAOH modified nano HZSM-5 zeolite and the catalytic properties in methanol making gasoline[J]. Chinese Journal of Catalysis, 2013, 34(6): 1148-1158
[26] Li S, Li Y P, Di C Y, et al. Modification and catalyst performance of ZSM-5 zeolite by treatment with TPAOH/ NaOH mixed alkali[J]. J Fuel Chem Technol, 2012, 40 (3): 583-588 (in Chinese)
[27] Xu D O. Preparation and investigation on properties of hierarchical porous nano-molecular sieves[D]. Jilin: Jilin University, 2011 (in Chinese)
date: 2015-03-22; Accepted date: 2015-05-24.
Zhai Yuchun, Telephone: +86-13304048096; E-mail: zhaiyc@smm.neu.edu.cn.
- 中国炼油与石油化工的其它文章
- Catalytic Hydrogenation of Methanol-Containing Effluent from Epoxidation of Propylene
- Synthesis of Biodiesel Using ZrO2Polycrystalline Ceramic Foam Catalyst in a Tubular Reactor
- Investigation of Swelling and Dissolution Process of Natural Rubber in Aromatic Oil
- Optimization of High-Gravity Chelated Iron Process for Removing H2S Based on Response Surface Methodology
- Studies on the Hydrogenation of Acetonitrile over Fresh Mo2C/γ-Al2O3Catalyst by In-situ IR Spectroscopy
- Lubricant Biodegradation Enhancers: Designed Chemistry and Engineered Technology

