Synthesis of Biodiesel Using ZrO2Polycrystalline Ceramic Foam Catalyst in a Tubular Reactor
Wang Yunpu; Fan Liangliang; Dai Leilei; Liu Yuhuan; Ruan Roger; Liu Shitao; Zhang Xueqin; Wan Yiqin
(1. Nanchang University, State Key Laboratory of Food Science and Technology, Nanchang 330047; 2. Nanchang University, Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang 330047; 3. Nanchang University, School of Food Science Technology, Nanchang 330047)
Synthesis of Biodiesel Using ZrO2Polycrystalline Ceramic Foam Catalyst in a Tubular Reactor
Wang Yunpu1,2; Fan Liangliang1,2; Dai Leilei3; Liu Yuhuan1,2; Ruan Roger1,2; Liu Shitao1,2; Zhang Xueqin3; Wan Yiqin1,2
(1. Nanchang University, State Key Laboratory of Food Science and Technology, Nanchang 330047; 2. Nanchang University, Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang 330047; 3. Nanchang University, School of Food Science Technology, Nanchang 330047)
With the help of the ceramic foam research efforts and preparation techniques, the ZrO2polycrystalline ceramic foam catalyst was synthesized, and its characteristics, including the crystal structure, the phase composition, the acid–base properties, and the microstructure, were analyzed by XRD, SEM, Py-IR, and BET techniques. The performance of the ZrO2polycrystalline ceramic foam catalyst in a tubular reactor was investigated via biodiesel synthesis using S. wilsoniana oil and methanol. The effects of reaction conditions (i.e., reaction temperature, reaction pressure, and volume ratio of methanol to S. wilsoniana oil) on transesterification efficiency were investigated, and the reaction conditions were optimized using RSM. The optimum reaction temperature, reaction pressure, and volume ratio of methanol to S. wilsoniana oil were determined to be 290 ℃, 10 MPa, and 4:1, respectively. Under this condition, the FAME content in the product oil reached 98.38%. The performance of the ZrO2polycrystalline ceramic foam catalyst synthesized in this work for biodiesel synthesis from S. wilsoniana oil with a moisture content of 7.1% and an acid value of 130.697 mg KOH/g was examined, and the FAME content in the product oil was found to be 93% and 97.67%, respectively. The FAME content in the product oil exceeded 97% after five consecutive cycles (12 h per cycle of use) of the catalyst. The proposed catalyst represents a new type of solid catalyst with excellent acid resistance, water resistance, esterification efficiency, and catalytic stability.
ZrO2polycrystalline ceramic foam catalyst; tubular reactor; S. wilsoniana oil; biodiesel
1 Introduction
Given the worsening energy crisis, the search for new alternatives to clean energy is currently becoming an important research focus worldwide. Biodiesel in particular is a frequent research subject in the field of global environmental protection using renewable alternative energy sources. Biodiesel can be easily transported and stored because of its high flash point and it possesses a high cetane number to have good combustion properties. Low sulfur content, low pollution, and good lubrication performance are among the other advantages of biodiesel.[1]As such, this alternative fuel is considered as a new type of green and renewable energy[2-3].
Catalysts can aid in achieving expected effects in biodiesel production and research through chemical methods, since the catalytic performance imposes a direct influence on reaction progress. Easily separable products with high efficiency and good repeatability perform a key function in the industrial application of biodiesel preparation techniques. Commonly-used solid acid catalysts (e.g., molecular sieves[4-6], heteropoly acids[7-8], cation exchange resins[9-10]and solid superacids[11-12]) exhibit high activity. However, while these solid acid catalysts demonstrate good immunity against free acids and moisture in the raw material, their applications are limited because they require numerous operations, such as neutralization and washing, and they are easily susceptible to corrodibility by liquid acid, which consequently leads to catalyst loss and pollution. Solid-base catalysts, which mainly include supported solid bases (e.g., Al2O3[13-14]and molecular sieves[15-16]used as carriers) and non-supported solid bases(e.g., metallic oxides[17-18], hydrotalcite[19-20], hydrotalcitelike bases, and anion exchange resins[21-22]) carry out significantly improved reaction and provide good conversion rates. However, because their application requires higher raw material quality, pretreatment must be conducted beforehand to reduce free fatty acids and moisture in the materials and prevent saponification. Therefore, the development of new solid catalysts with good acid resistance, water resistance, high efficiency, and catalytic stability is a highly unequivocal research topic.
Requirements of easy automation, recycling, and product separation under mild reaction conditions of solid catalysis, along with the development of theories, catalyst preparation techniques, and study on more problems in traditional homogeneous catalysis reactions, have drawn increased attention in recent years. ZrO2has become a research focus because of its excellent physicochemical properties[23-24]. Acidic and alkaline sites exist on the surface of ZrO2solid catalysts to provide them with properties of oxidizability and reducibility. The surface of ZrO2p-semiconductors can easily produce oxygen vacancies, which can facilitate the Lewis acid formation. Amorphous ZrO2exists in the monoclinic, square, and cubic crystal phase forms; its thermodynamic structure is relatively stable in the monoclinic crystal phase, which enables itself to exist at room temperature. The monoclinic phase is a common crystal type of ZrO2. ZrO2can hardly exist at room temperature in the form of square and cubic crystal phases, which are metastable structures. ZrO2possesses different physical and chemical properties among its various forms, and these properties exert influence on its catalytic reactions[25].
By using developments in ceramic foam research methods and preparation techniques, a ZrO2polycrystalline ceramic foam catalyst was synthesized in this work[26-27], and its characteristics, such as crystalline structure, phase composition, acid–base properties, and microstructure, were analyzed by XRD, SEM, Py-IR, and BET techniques. The application of the ZrO2polycrystalline ceramic foam catalyst in biodiesel synthesis in a tubular reactor was investigated using Swida wilsoniana oil and methanol, and the reaction conditions were optimized using the response surface methodology (RSM). Finally, the acidity resistance, water resistance, and catalytic stability of the catalyst were investigated.
2 Experimental
2.1 Materials
S. wilsoniana fruit oil was provided by the Jiangxi Academy of Forestry. ZrO2(AR), Al2O3(AR), TiO2(CP), NaHCO3(AR), and NaCl (AR) were provided by the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Catalyst preparation
A mixture of ZrO2, Al2O3, and TiO2was ball-milled for 24 h. Sodium chloride, NaHCO3powder, and acidic silica sol serving as the binder were added to the mixture, which was subsequently granulated after uniform mixing by a comminutor. The catalyst was dried at 120 ℃ for 12 h, cooled, and then kept at 500 ℃ for 1 h to be subject to volatilization. Low temperature pore-forming agents were removed in a muffle furnace. The high-temperature ore-forming agent was completely evaporated after rapid heating to a specified temperature. The mixture was finally cooled down to obtain the ZrO2polycrystalline ceramic foam catalyst.
2.3 Characterization of catalyst and product
The phase composition of the catalyst was identified through X-ray diffractometry (XRD) (Bede, DISYSTEM) using CuKα radiation. The microstructure of the catalyst was characterized by transmission electron microscopy (TEM by JEOL with JEM-3010). Scanning electron microscopic (SEM) photographs were obtained using an ASAP2010 scanning electron microscope. The fatty acid methyl ester (FAME) content and the biodiesel product purity were determined by proton nuclear magnetic resonance (1H NMR) technique.1H NMR analyses were performed using an AVANCEIII spectrometer (600 MHz) with CDCl3(99.8%, Aldrich) serving as the solvent[28].
2.4 Synthesis of biodiesel using catalysts in a tubular reactor and optimization of conditions
The catalyst was loaded into the tubular reactor. After preheating, a constant flow rate (20 mL/min) was specified by means of a constant flow pump. At different reaction temperatures and reaction pressures, methanol and oil were allowed to react steadily for a certain time, andsamples were collected at the bottom of the collection tank. The unreacted methanol was removed through evaporation. The upper layer consisted of FAME, whereas the lower layer consisted of glycerol. The FAME content was measured by NMR (1H-NMR)[28]. During RSM analysis, the reaction temperature, reaction time, and methanol-tooil ratio were selected as independent variables and the FAME content of the product oil was used as a response value to optimize reaction conditions.
2.5 Analysis of catalyst performance
The selected model compounds included S. wilsoniana oil samples with different moisture contents (0.1%, 1.1%, 3.1%, 5.1%, and 7.1%) and acid values (1.697, 11.697, 51.697, 91.697, and 130.697 mgKOH/g). Biodiesel produced under optimal conditions was analyzed, and the catalyst was reused five consecutive times with each operation lasting 12 h (with the original S. wilsoniana oil containing 0.1% of moisture and an acid value of 1.697 mgKOH/g). Finally, the catalyst performance was analyzed.
3 Results and Discussion
3.1 Characterization of ZrO2polycrystalline ceramic foam catalyst
Figure 1(A) shows the SEM image of the ZrO2polycrystalline foamed ceramic catalyst. The loose voids can be found within hollow shapes on the catalyst surface after addition of the pore-forming agent. Voids with large diameters can retain reactants inside the catalyst, thereby prolonging the contact time of the catalyst with reactants and improving catalytic efficiency. Most of the voids are of deep-hole structure, and the pore interior contains folds that increase the surface area of the catalyst, as evidenced by the conversion rate of the reactants. The specific surface area (0.365 7 m²/g), pore volume (0.001 542 cm³/g), and pore diameter (20.790 1 nm) of the ZrO2polycrystalline ceramic foam catalyst were determined by BET technique. The pore diameter of the catalyst was approximately 20 nm, and the grease molecules with sizes in the nanometer scale could easily pass through these pores to improve the utilization rate of active sites inside the catalyst. This finding is consistent with the SEM characterization results.
Figure 1(B) shows the XRD patterns obtained after calcination of the ZrO2mixture with the co-solvent and stabilizer at 1 550 ℃. The comparison with the XRD standard card revealed that the co-solvent Al2O3(JCPDS No. 10173) was of the α-Al2O3form while the stabilizer TiO2(JCPDS No. 211276) was in the rutile phase. Comparison of the locations of the XRD diffraction peaks also shows that Al2O3, TiO2, and ZrO2formed a substitution solid solution during calcination. The Al2O3peak intensity became significantly weaker at 2θ = 25.45°, 35.09°, 37.80°, 57.58°, 61.31°, and 66.53° and it completely disappeared at 2θ = 43.50°, 52.60°, 68.5°, and 77.01°. The TiO2diffraction peak became significantly weaker at 2θ = 36.12°, 39.17°, 41.19°, 54.38°, 56.58°, and 64.01°, while it completely disappeared at 2θ = 27.5°, 44.05°, 62.83°, 68.92°, and 76.70°. These results indicated the formation of a solid solution through displacement of Zr4+by Al3+and Ti4+. This solid solution was created through the formation of a mutually permeable structure because of the similar coordination numbers and bond lengths of Al-O and Zr-O bonds by spreading at high temperature. If a cation with a radius smaller than 12% of the Zr4+radius was present, the cation could displace Zr4+to form the solid solution[30]. The radius of A13+in Al2O3, which was 0.50 Å in this experiment, was smaller than that of Zr4+. Thus, during calcination, Al3+ions migrated to the ZrO2lattice. The crystal boundary potential formed from the potential difference between A13+and Zr4+prevented their diffusion, which stabilized the tetragonal ZrO2in the catalyst. Addition of TiO2could expand the crystal particles, thereby accelerating the spread of tetragonal ZrO2species. Mingling Ti ions could increase the c parameter and reduce volume mainly because two oxygen atoms mutually penetrated the tetrahedron inside the square ZrO2crystal to form two-cation O bonds. If the shorter bonds began to reduce in length when the successive fixed bonds were present in the metallic ions, the longer bonds would increase to compensate for the shorter ones. This configuration increased the migration of oxygen atoms with an increasing TiO2content. The intensity of the diffraction peaks of the calcined catalyst weakened in comparison with that of ZrO2, which might be related to the formation of the solid solution[29-30].

Figure 1 (A)SEM image of ZrO2polycrystalline ceramic foam catalyst; (B)XRD patterns of the calcined catalyst and materials; (C) Infrared spectra of pyridine adsorbed on the ZrO2polycrystalline foam ceramic catalyst
Figure 1(C) shows the infrared spectra of pyridine adsorbed by the ZrO2polycrystalline foam ceramic catalyst. The adsorption peak of the ZrO2catalyst appeared at 1 445 cm–1, and the characteristic peak at 1 609 cm–1corresponded to pyridine, which implied the presence of certain Lewis acid sites in the sample. A weak pyridine adsorption peak could be observed at 1 488 cm–1. As no characteristic Brönsted acid center peak could be observed at 1 545 cm–1, weak Brönsted acid sites in the sample were nonexistent.[31]
3.2 Influence of reaction conditions on FAME content
Synthesis of biodiesel from S. wilsoniana oil and methanol was performed in a tubular reactor using the ZrO2polycrystalline ceramic foam catalyst, and the effects of reaction conditions (i.e., reaction temperature, reaction pressure, and volume ratio of methanol to S. wilsoniana oil) on esterification efficiency were investigated.
3.2.1 Influence of reaction temperature on fatty acid methyl ester content
At a reaction pressure of 8.0 MPa and a methanol-to-oil volume ratio of 1:1, the influence of reaction temperature on FAME content was investigated, with the relevant results shown in Figure 2(A).
As the reaction temperature increased, the FAME content in the product also increased gradually and reached a maximum value at 285 ℃. A further increase in temperature resulted in a slight decline in FAME content, which could be attributed to cracking of the product. FAME was cracked and vaporized at temperatures above 300 ℃. Therefore, the optimal reaction temperature was selected as 285 ℃.
3.2.2 Influence of reaction pressure on FAME content
At a reaction temperature of 285 ℃ and a methanol-tooil volume ratio of 1:1, the influence of reaction pressure on the FAME content was investigated, with the results shown in Figure 2(B). With an increasing pressure, the FAME content of the product gradually increased and reached a maximum value at 12 MPa. At a certain methanol to oil volume ratio, the residence time of the material in the reactor increased because of the pressure increase, while the FAME content in the product also increased. However, at the equilibrium condition, the product was mostly composed of FAME. Hereby, the ester content did not increase because of the long residence time; and instead, the FAME content decreased because of cracking of the product. Thus, the optimal reaction pressure was selected as 12 MPa.
3.2.3 Influence of methanol-to-oil volume ratio on FAME content
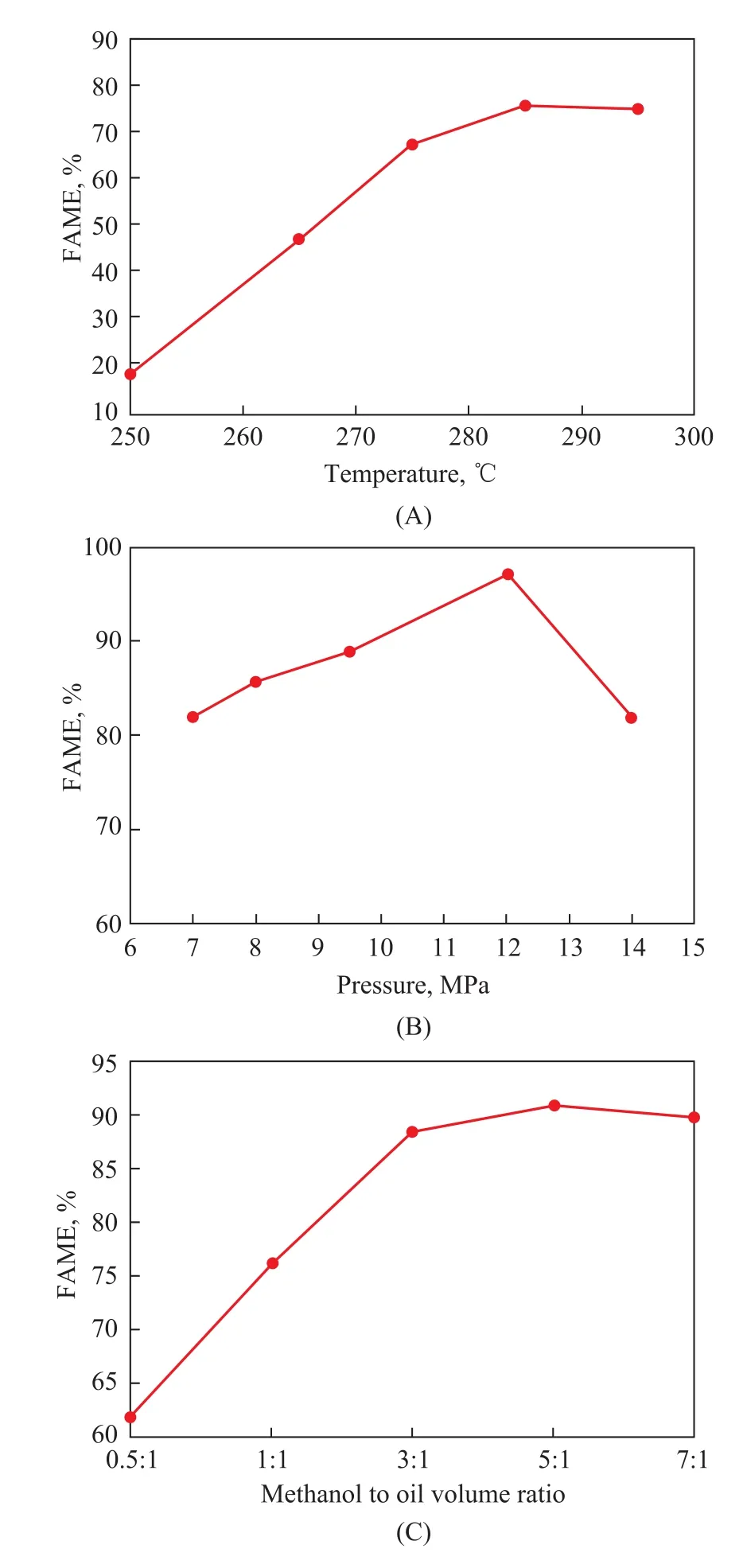
Figure 2 (A)Influence of reaction temperature on fatty acid methyl ester (FAME) content; (B)Influence of reaction pressure on fatty acid methyl ester (FAME) content; (C) Influence of alcohol-to-oil ratio on FAME content
At a reaction temperature of 285 ℃ and a reaction pressure of 12 MPa, the influence of methanol-to-oil volume ratio on FAME content was investigated, with the results presented in Figure 2(C).
Given that the catalyst contains a number of active sites, the catalyst shows only partial catalytic ability when the number of reactant molecules is less than the number of active sites. Figure 2(C) shows that the FAME content increased with an increasing alcohol to oil volume ratio. The inflection point appeared near the alcohol-to-oil volume ratio of 5:1, and the number of reactant molecules and active sites decreased slowly after the inflection point. This response was attributed to the increase in alcohol to oil volume ratio, which could result in a decreased contact time between the reactant material and active sites under a constant pressure. Thus, a decrease in FAME content occurred. Some of the FAME species in the product were cracked under the present condition. Thus, the optimal alcohol to oil volume ratio was selected as 5:1.
3.2.4 Optimization of biodiesel synthesis using ZrO2polycrystalline ceramic foam catalyst
Based on the results of single-factor experiments, the reaction temperature, reaction pressure, and methanol to oil volume ratio were selected as the variables for further optimization. Biodiesel synthesis using the ZrO2polycrystalline ceramic foam catalyst in a tubular reactor was planned using a three-level, three-factor Box–Behnken design. The experimental factors and levels are presented in Table 1.Independent variables included the temperature (X1), the pressure (X2), and the volume ratio of methanol to oil (X3), and the corresponding high, medium, and low levels were represented as coded values of 1, 0, and -1, respectively. The FAME content of the product was considered as the response value and was represented by Y. Table 2 shows the matrix for the Box–Behnken experimental design together with the experimental results.

Table 1 The Box-Behnken design factors and levels
Details of the reaction were clarified through RSM, with the results shown in Tables 3 and 4. The 3D maps of the response surface are presented in Figure 3.
Experimental values obtained from the Box–Behnken experimental design are regressed using a quadratic polynomial equation, and the regression equation, which is expressed in terms of the coded factors defined in Table 3, is given as Eq. (1):

Table 2 The Box-Behnken method of experimental design and results

The results of significance tests for each regression coefficient of the established equation are shown in Table 3, and the results of analysis of variance for the model are presented in Table 4. Table 3 demonstrates that the P-value is extremely low (P<0.000 1), which implies the significance of this model (P-values <0.05 are generally considered to indicate that the model terms are significant).
The P-value of the “lack of fit” is 0.8895; here, P>0.05 suggests that the lack of fit to this regression equation is not significant. The adjusted R-squared value (R2adj) is 0.945 0, which indicates that the model can explain 89.60% of the response. Moreover, R2is 0.9759, which implies that the actual values are very close to the predicted values. Thus, the model is reliable for predicting and analyzing the FAME content of the product.
The optimum levels of the factors investigated can be deduced from Eq. (1); under optimal conditions, the maximum FAME content of the product is 99.494 9%. The model predicts that the maximum FAME content can be obtained at a reaction temperature of 292.52 ℃, a reaction pressure of 10.44 MPa, and a methanol to oil volumeratio of 4.33:1. To simplify operations, the following conditions were considered: a reaction temperature of 290 ℃, a reaction pressure of 10 MPa, and a volume ratio of methanol to oil of 4:1. The resulting FAME content of the product at these conditions is 98.38%, which is very close to the predicted value. This result clearly confirms the validity of the present model.

Table 3 Quadratic response surface regression equation coefficient significance
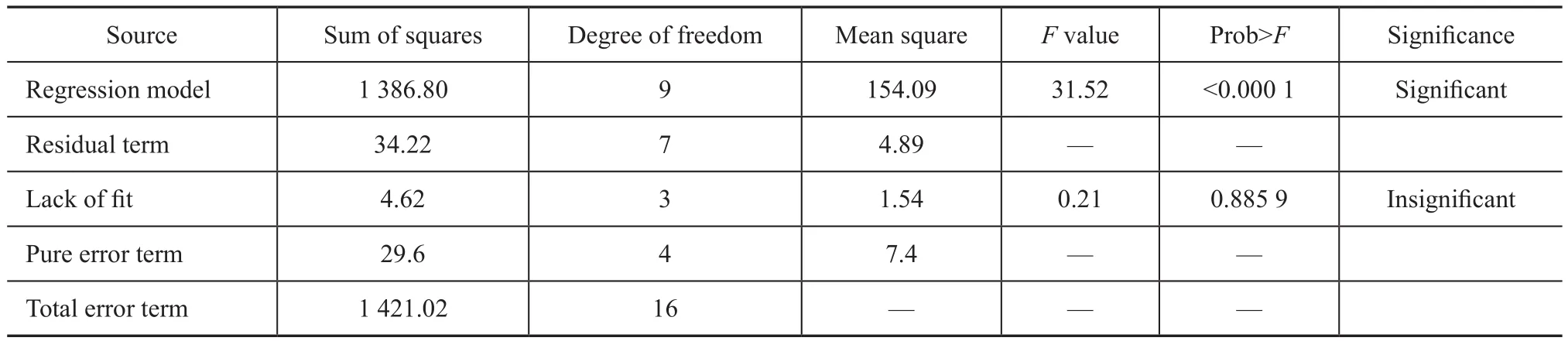
Table 4 Regression analysis of the variance table

Figure 3 (A) Influence of reaction temperature and pressure on FAME content; (B) influence of reaction temperature and alcohol-to-oil ratio on FAME content; (C) influence of reaction pressure and alcohol-to-oil ratio on FAME content
3.3 Catalytic performance of the ZrO2polycrystalline ceramic foam catalyst
The performance of catalysts directly affects their application in semi-continuous and automated production processes conducted in biodiesel tubular reactors. Catalysts with high operational stability invariably exhibit excellent reusability, thereby reducing the cost of biodiesel production. The acid resistance, water resistance, and operational stability of the ZrO2polycrystalline ceramic foam catalyst synthesized in this work was investigated via biodiesel synthesis from S. wilsoniana oil and methanol as a model reaction under the optimized conditions described above. The advantages of the catalyst, which include good heat stability, high conversion rate, good reusability, acid resistance, and alkali resistance can significantly reduce the production cost, improve operating conditions, and expand the range of raw materials. We studied the acid resistance, water resistance, and reusability of the ZrO2polycrystalline ceramic foam catalyst with the relevant results presented in Figure 4(a) and Figure 4(b), in which the performance of ZrO2polycrystalline ceramic foam catalyst for biodiesel synthesis from S. wilsoniana oil with a moisture content of 7.1% and an acid value of 130.697 mg KOH/g was examined, and the FAME content in the product oil was 93% and 97.67%, respectively. It is evident from Figure 4(c) that the FAME content in the product oil remained above 97% even after the catalyst was reused five consecutive times (12 h per cycle of use). Thus, the proposed catalyst presents a new type of solid catalyst with excellent acid resistance, water resistance, esterification efficiency, and catalytic stability.

Figure 4 (A) Influence of water on FAME content;( B) Influence of acid value on FAME content; (C) Influence of number of cycles on FAME content
4 Conclusions
We synthesized a ZrO2polycrystalline ceramic foam catalyst and analyzed its characteristics, including the crystal structure, the phase composition, the acid–base properties, and the microstructure of the catalyst by XRD, SEM, Py-IR, and BET techniques. The performance of the ZrO2polycrystalline ceramic foam catalyst in a tubular reactor was investigated via biodiesel synthesis using S. wilsoniana oil and methanol as the starting materials. The effects of reaction conditions (i.e., reaction temperature, reaction pressure, and methanol to S. wilsoniana oil volume ratio) on esterification efficiency were investigated, and the reaction conditions were optimized using RSM.
The optimum reaction temperature, reaction pressure, and volume ratio of methanol to S. wilsoniana oil were determined to be 290 ℃, 10 MPa, and 4:1, respectively. Under these conditions, the FAME content in the product oil was 98.38%.
The performance of the ZrO2polycrystalline ceramic foam catalyst synthesized in this work for biodiesel synthesis from S. wilsoniana oil with a moisture content of 7.1% and an acid value of 130.697 mg KOH/g was examined, and the FAME content in the product oil was found to be 93% and 97.67%, respectively. The FAME content in the product oil exceeded 97% after five consecutive cycles (12 h per cycle of use) of the catalyst. The proposed catalyst represents a new type of solid catalyst with excellent acid resistance, water resistance, esterification efficiency, and catalytic stability.
Acknowledgements: We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21266022, No. 21466022), the National High Technology Research and Development Program 863 (2014AA022002, 2012AA101800-03, 2012AA021205-6, 2012AA021704), the Key Programs of the National Laboratory (No. SKLFZZB-201312), and the International Science & Technology Cooperation Program of China (2014DFA61040).
[1] Min E Z, Zhang L X. Development of Biodiesel Industry Chain-Biodiesel Refinery and Chemical Plant[M]. Beijing: China Petrochemical Press, 2006: 191
[2] Zhang X, Ma Q, Cheng B, et al. Research on KOH/La-Ba-Al2O3catalysts for biodiesel production via transesterification from microalgae oil[J]. J Nat Gas Chem, 2012, 21(6): 774-779
[3] Intarapong P, Iangthanarat S, Phanthong P, et al. Activity and basic properties of KOH/mordenite for transesterification of palm oil[J]. J Energy Chem, 2013, 22(5): 690-700
[4] Melero J A, Bautista L F, Morales G, et al. Biodiesel production from crude palm oil using sulfonic acid-modified mesostructured catalysts[J]. Chem Eng J, 2010, 161(3): 323-331
[5] Carmo A C, de Souza L K C, da Costa C E F, et al. Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41[J]. Fuel, 2009, 88(3): 461-468
[6] Jiménez-Morales I, Santamaría-González J, Maireles-Torres P, et al. Zirconium doped MCM-41 supported WO3solid acid catalysts for the esterification of oleic acid with methanol[J]. Appl Catal B-Environ,, 2010, 379(1): 61-68
[7] Srilatha K, Kumar C R, Devi B L A P, et al. Efficient solid acid catalysts for esterification of free fatty acids with methanol for the production of biodiesel[J]. Catal Sci Technol, 2011, 1(4): 662-668
[8] Shin H Y, An S H, Sheikh R, et al. Transesterification of used vegetable oils with a Cs-doped heteropolyacid catalyst in supercritical methanol[J]. Fuel, 2012, 96(6): 572-578
[9] Feng Y, He B, Cao Y, et al. Biodiesel production using cation-exchange resin as heterogeneous catalyst[J]. Bioresource Technol, 2010, 101(5): 1518-1521
[10] Son S M, Kimura H, Kusakabe K. Esterification of oleic acid in a three-phase, fixed-bed reactor packed with a cation exchange resin catalyst[J]. Bioresource Technol, 2011, 102(2): 2130-2132
[11] Zexue D U, Zhong T, Haijing W, et al. Research and development of a sub-critical methanol alcoholysis process for producing biodiesel using waste oils and fats[J]. Chin J Catal, 2013, 34(1): 101-115 (in Chinese)
[12] Komintarachat C, Chuepeng S. Solid acid catalyst for biodiesel production from waste used cooking oils[J]. Ind Eng Chem Res, 2009, 48(20): 9350-9353
[13] Pasupulety N, Gunda K, Liu Y, et al. Production of biodiesel from soybean oil on CaO/Al2O3solid base catalysts[J]. Appl Catal A-Gen, 2013, 452(3): 189-202
[14] Boz N, Degirmenbasi N, Kalyon D M. Conversion of biomass to fuel: transesterification of vegetable oil to biodiesel using KF loaded nano-γ-Al2O3as catalyst[J]. Appl Catal B-Environ, 2009, 89(3): 590-596
[15] Babajide O, Musyoka N, Petrik L, et al. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production[J]. Catal Today, 2012, 190(1): 54-60
[16] Wu H, Zhang J, Wei Q, et al. Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalysts[J]. Fuel Process Technol, 2013, 109(5): 13-18
[17] Rubio-Caballero J M, Santamaría-González J, Mérida-Robles J, et al. Calcium zincate derived heterogeneous catalyst for biodiesel production by ethanolysis[J]. Fuel, 2013, 105(3): 518-522
[18] Dehkordi A M, Ghasemi M. Transesterification of waste cooking oil to biodiesel using Ca and Zr mixed oxides as heterogeneous base catalysts[J]. Fuel Process Technol, 2012, 97(5): 45-51
[19] Di Serio M, Mallardo S, Carotenuto G, et al. Mg/Al hydrotalcite catalyst for biodiesel production in continuous packed bed reactors[J]. Catal Today, 2012, 195(1): 54-58
[20] Wang Y B, Jehng J M. Hydrotalcite-like compounds containing transition metals as solid base catalysts for transesterification[J]. Chem Eng J, 2011, 175(21): 548-554
[21] Shibasaki-Kitakawa N, Honda H, Kuribayashi H, et al. Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst[J]. Bioresource Technol, 2007, 98(2): 416-421
[22] Li J, Fu Y J, Qu X J, et al. Biodiesel production from yellow horn (Xanthoceras sorbifolia Bunge.) seed oil using ion exchange resin as heterogeneous catalyst[J]. Bioresource Technol, 2012, 108(3): 112-118
[23] Takase M, Zhang M, Feng W, et al. Application of zirconia modified with KOH as heterogeneous solid base catalyst to new non-edible oil for biodiesel[J]. Energ Convers and Manage, 2014, 80(4): 117-125
[24] Li Y, Ye B, Shen J, et al. Optimization of biodiesel production process from soybean oil using the sodium potassium tartrate doped zirconia catalyst in microwave chemical reactor[J]. Bioresource Technol, 2013, 137(6): 220-225
[25] Patel A, Brahmkhatri V, Singh N. Biodiesel production by esterification of free fatty acid over sulfated zirconia[J]. Renew Energ, 2013, 51(3): 227-233
[26] Thompson C R, Marín P, Díez F V, et al. Evaluation of the use of ceramic foams as catalyst supports for reverse-flow combustors[J]. Chem Eng J, 2013, 221(4): 44-54
[27] Chen X, Lu A, Qu G. Preparation and characterization of foam ceramics from red mud and fly ash using sodium silicate as foaming agent[J]. Ceram Int, 2013, 39(2): 1923-1929
[28] Gelbard G, Bres O, Vargas R M, et al.1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol[J]. Am Oil Chem Soc, 1995, 72(10): 1239-1241
[29] Ali M M, Raj V. Synthesis and surface characterization of alumina–silica–zirconia nanocomposite ceramic fibers on aluminium at room temperature[J]. Appl Surf Sci, 2010, 256(12): 3841-3855
[30] Soisuwan S, Panpranot J, Trimm D L, et al. A study of alumina–zirconia mixed oxides prepared by the modified Pechini method as Co catalyst supports in CO hydrogenation[J]. Appl Catal A-Gen, 2006, 303(2): 268-272
[31] Do T O, Nossov A, Springuel-Huet M A, et al. Zeolite nanoclusters coated onto the mesopore walls of SBA-15[J]. J Am Chem Soc, 2004, 126(44): 14324-14325
Recieved date: 2015-01-13; Accepted date: 2015-05-26.
Professor Liu Yuhuan, Telephone: +86-13755621329; E-mail: liuyuhuan@ncu.edu.cn.
- 中国炼油与石油化工的其它文章
- Catalytic Hydrogenation of Methanol-Containing Effluent from Epoxidation of Propylene
- Studies on the Hydrogenation of Acetonitrile over Fresh Mo2C/γ-Al2O3Catalyst by In-situ IR Spectroscopy
- Investigation of Swelling and Dissolution Process of Natural Rubber in Aromatic Oil
- Optimization of High-Gravity Chelated Iron Process for Removing H2S Based on Response Surface Methodology
- Secondary Crystallization of Na2CO3-Modified HZSM-5 Zeolites with Tetrapropylammonium Hydroxide and Their Catalytic Performance in Thiophene Alkylation Reaction
- Lubricant Biodegradation Enhancers: Designed Chemistry and Engineered Technology

