Experimental Study on the Characteristics of CO2Hydrate Formation in Porous Media below Freezing Point
Zhang Xuemin; Li Jinping; Wu Qingbai,; Wang Chunlong; Nan Junhu
(1. Western China Energy & Environment Research Center, Lanzhou University of Technology, Lanzhou 730050; 2. Cold and Arid Regions Environmental Engineering Research Institute, Chinese Academy of Sciences, Lanzhou 730000)
Experimental Study on the Characteristics of CO2Hydrate Formation in Porous Media below Freezing Point
Zhang Xuemin1; Li Jinping1; Wu Qingbai1,2; Wang Chunlong1; Nan Junhu1
(1. Western China Energy & Environment Research Center, Lanzhou University of Technology, Lanzhou 730050; 2. Cold and Arid Regions Environmental Engineering Research Institute, Chinese Academy of Sciences, Lanzhou 730000)
Porous medium has an obvious effect on the formation of carbon dioxide hydrate. In order to study the characteristics of CO2hydrate formation in porous medium below the freezing point, the experiment of CO2hydrate formation was conducted in a high-pressure 1.8-L cell in the presence of porous media with a particle size of 380 μm, 500 μm and 700 μm, respectively. The test results showed that the porous medium had an important influence on the process of CO2hydrate formation below the freezing point. Compared with porous media with a particle size of 500 μm and 700 μm, respectively, the average hydrate formation rate and gas storage capacity of carbon dioxide hydrate in the porous medium with a particle size of 380 μm attained 0.016 14 mol/h and 65.094 L/L, respectively. The results also indicated that, within a certain range of particle sizes, the smaller the particle size of porous medium was, the larger the average hydrate formation rate and the gas storage capacity of CO2hydrate during the process of hydrate formation would be.
CO2hydrate; formation rate; porous media; formation characteristics; gas storage capacity
1 Introduction
Naturally occurring gas hydrates in deposits have been found in many regions of the world such as in permafrost regions and in seafloor sediments where the existing pressures and temperatures allow for thermodynamic stability of the hydrate[1]. Because the gas hydrates contain a large amount of methane gas, hydrates in those regions have been considered to be a future energy resource having a potential prospect. So the methods for efficient and safe exploitation of natural gas hydrate have been attracting more attention of researchers. The natural occurrence conditions of the gas hydrate in sediments are always below the freezing point in permafrost regions. Based on the natural occurrence conditions of natural gas hydrate, several feasible schemes for producing methane from the hydrate reservoir have been put forward, such as: (1) depressurization, which decreases the pressure of hydrate reservoir under the condition of phase equilibrium; (2) thermal stimulation, which increases the temperature of reservoir above the phase equilibrium conditions through injecting hot water or steam; (3) inhibitor injection, which injects chemical additives such as methanol or glycol to shift the P-T equilibrium of hydrate; and (4) CO2replacement, which replaces the CH4molecules trapped in hydrate structure with CO2molecules. Most of the above-mentioned methods can change the temperature or pressure of hydrate reservoir below the equilibrium condition in order to bring about hydrate dissociation. However, each method has its own merits and demerits to some extent.
Moreover, it is considered that CO2replacement is a potential method for methane recovery from the gas hydrate reservoir. And the replacement process between CO2and CH4hydrate includes two critical processes of CH4hydrate dissociation and CO2hydrate formation simultaneously. Qi, et al.[2]experimentally investigated the processes of exchange between CO2and CH4hydrate using molecular dynamics simulation. The results have confirmed that CH4is released from hydrate and enter into the gas phase by the replacement with CO2and it will spend a long time without the dissociation of methane hydrate. Jung, et al.[3]experimentally studied the process for replacement of methane from the gas hydrate withCO2. The results showed that the replacement process of CO2and CH4hydrate occurred locally and gradually so that the overall hydrate mass remains solid and no stiffness loss should be expected in the sediment. Deusner, et al.[4]also studied the replacement of CH4-hydrate deposits through injecting CO2at different sediment temperatures in the high-pressure vessel. The result showed that the temperature and pressure had an obvious influence on the replacement of CO2and CH4hydrate in porous media. Furthermore, Yuan, et al.[5]investigated the characteristics of CO2hydrate formation in porous media during the process of replacement. Goel, et al.[6]found that CO2was preferentially sequestered over methane in the hydrate phase. Lee, et al.[7]experimentally studied the replacement process of CO2-CH4hydrate and found that new CO2hydrate formed on the surface of the CH4hydrate would wrap the CH4hydrate and prevent the replacement reaction[8-11]. However, the natural gas hydrate usually exists steadily in porous media at temperatures below the freezing point. And the formation of CO2hydrate and the dissociation of CH4hydrate are the two critical processes of replacement between CO2and CH4hydrate. Therefore, it is necessary to study the process of CO2hydrate formation in porous media at temperatures below the freezing point.
Many researchers have investigated the process of hydrate formation in porous media. Handa, Stupin, et al.[12]experimentally demonstrated that the disassociation pressure of CH4and C3H8hydrates in porous media with small pores was higher than those in bulk. Uchida, et al.[13]observed an equilibrium shift of CH4hydrate in porous glass with a pore radius of 100 Å, 300 Å, and 500 Å, respectively. Besides, many other researchers reported the equilibrium shift for hydrates in porous medium with a pore radius smaller than 600 Å[14-16]. Dicharry, et al.[17]experimentally studied the process of CO2hydrate formation in porous silica gel with pure water or surfactant solution. The results showed that, the smaller the nominal pore diameter of porous medium was, the lower the temperature and the higher the pressure for the shift of equilibrium conditions of CO2hydrate would be. Ruan, et al.[18]developed a numerical model to study the process of hydrate dissociation and gas production in porous media by depressurization. Their simulation results showed that the permeability characteristics had a significant impact on the hydrate dissociation and gas production behavior.
Although there have been many researches on the process of hydrate formation and dissociation in porous media, there are few focusing on the characteristics of hydrate formation in porous media at temperatures below the freezing point. In this paper, the experiment on CO2hydrate formation was conducted in porous media with different pore diameter at temperatures below the freezing point in a high-pressure cell with a volume of 1.8 L. These results are expected to provide some useful information on the kinetic behaviors and formation characteristics in porous media at temperatures below the freezing point, and can also provide an important theoretical guidance for the exploitation process of natural gas hydrate in permafrost regions.
2 Experimental
2.1 Apparatus and reagent
The schematic diagram of the experimental apparatus is shown in Figure1. The experimental apparatus consists of a thermostatic bath equipped with a low-temperature circulating cooler using alcohol as the cooling medium, a high-pressure cell with a volume of 1.8 L which is equipped with a 5.0-L buffer tank made of 316 L stainless steel the temperature of which is controlled by a lowtemperature circulating device, a gas cylinder, which supplies carbon dioxide and controls the system pressure, a vacuum pump for vacuumizing the high-pressure cell before charging carbon dioxide, and a data acquisition system including a temperature sensor (with a precision of ±0.05 K), a pressure transducer (with a precision of ±0.01 MPa), a data acquisition system operated by Agilent 34970A, and a data logger system. Inside the highpressure cell, two temperature sensors are installed and employed to measure the temperature of gaseous phase and sediment of porous media during the experiment. Carbon dioxide with a purity of 99.99% used in the experiments was purchased from the Lanzhou Lanheng Special Gas Co., Ltd. The quartz sand with a purity of 99.0% used in the experiment was provided by the Tianjin Yuanli Chemical Co., Ltd. Nitrogen with a purity of 99.9% was provided by the Zhejiang Tricyclic Chemical ReagentCo., Ltd. The water used in the experiment was deionized distilled water produced in the laboratory.
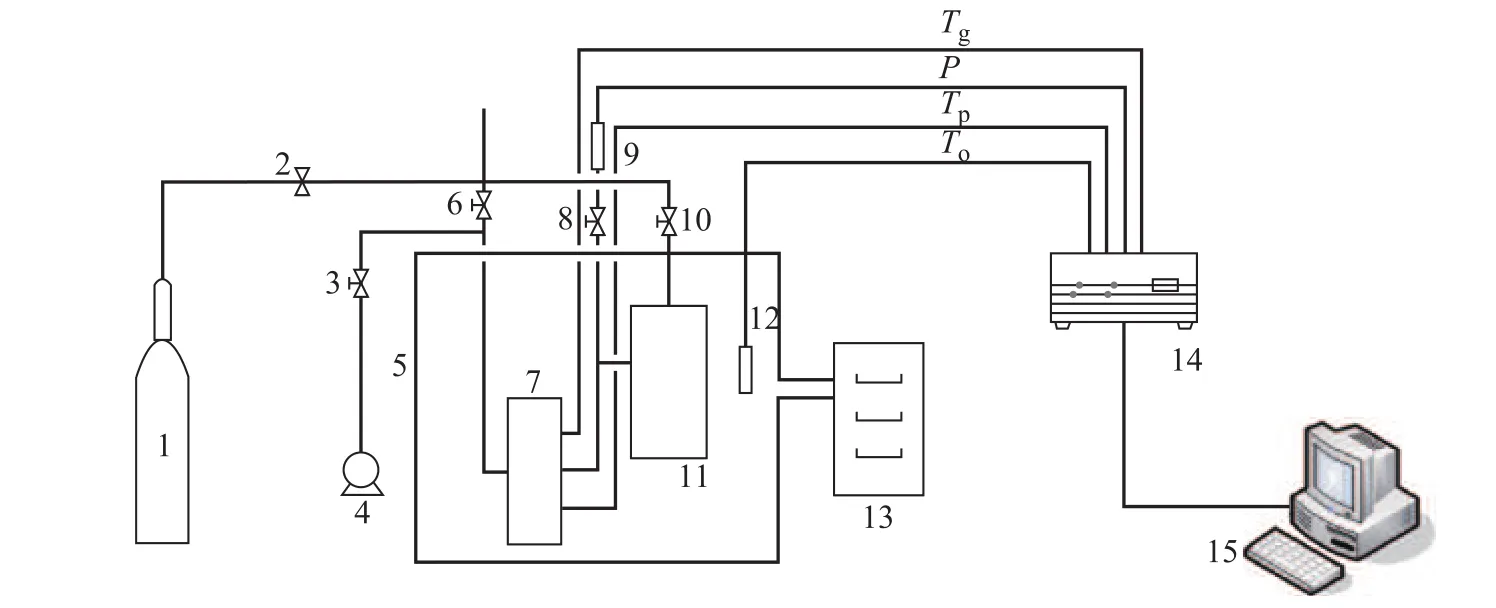
Figure 1 Schematic diagram of experimental apparatus
2.2 Experimental procedures
In the experiment, 450 mL of deionized water were added into 1 L of porous medium made of clean quartz sand with a particle size of 700 μm so that the deionized water was distributed homogeneously in the quartz sand. The mixture was subject to freezing immediately and adequately, while the water would become ice at the same time. Then ice was crushed into powder with a certain particle size under the protection of liquid nitrogen. After that, the ice powder was added into the high-pressure cell which was immersed in the alcohol bath, and the whole system was tested for air tightness under vacuum and then at a certain pressure, respectively. If the pressure variation in the high-pressure cell was less than 0.000 2 MPa/h, the device was considered to be in compliance with good air tightness. After the air tightness testing, the high-pressure cell was purged with carbon dioxide and the procedure was repeated three times at least. Then CO2was injected into the high-pressure cell until its initial pressure reached 3.6 MPa and then the temperature of the thermostatic bath was set at 273.15 K. When the temperature was stabilized at 273.15 K, it was regulated to 271.15 K and the reaction system began to cool down. Then the variation in temperature and pressure was observed and recorded through the data acquisition device during the process of CO2hydrate formation. When the temperature and pressure changes were negligible, it was considered that the process of CO2hydrate formation was completed. Then the cooling process in the thermostatic bath was terminated and the experiment was finished at the same time.
By using the same method, the process of CO2hydrate formation was conducted in a porous medium with a particle size of 380 μm. During the experiment, 420 mL of deionized water were added into 1 L of the clean porous medium with a particle size of 380 μm in the high-pressure cell. And other procedures were similar to those used in the experimental process of CO2hydrate formation using the porous medium with a particle size of 700 μm.
3 Results and Discussion
3.1 Effects of porous media on process of CO2hydrate formation
In order to investigate the influence of porous media on the process of CO2hydrate formation below freezing point, the formation experiment was conducted in porous media made of quartz sand with different particle size. The variation in pressure and temperature in the course of CO2hydrate formation is shown in Figure 2, Figure 3 and Figure 4, respectively.
In Figures 2—4, the variation in pressure and temperature shows the typical characteristics of carbon dioxide hydrate formation in porous media. The temperature and pressure decreased in the high-pressure cell with the continuous cooling of the bath. After a period of induction time, the carbon dioxide hydrate would form and grow in porous media and the amount of CO2would decrease with time. The process of CO2hydrate formation was an exothermic reaction and the heat of formation was released when CO2hydrate was formed in porous media. And thenit can be clearly seen that the temperature fluctuated from the two curves of the temperature and pressure variations during the hydrate formation process. Meanwhile, the pressure of the system gradually decreased as the temperature dropped with time. The amount of CO2gas was super-saturated through adsorption and the pressure of the reaction system was affected by the amount of component in the gaseous phase. As the process continued to take place, the pressure decreased gradually because of the continued consumption of CO2gas in the hydrate formation process. And the variation in temperature also fell off with a decreasing amount of formation heat released thereof. Finally, a phase equilibrium of CO2hydrate formation was reached under the experimental conditions of specified temperature and pressure.

Figure 2 The curves of temperature and pressure variations during the process of CO2hydrate formation in porous medium with a particle size of 700 μm

Figure 3 The curves of temperature and pressure variations during the process of CO2hydrate formation in porous medium with a particle size of 500 μm

Figure 4 The curves of temperature and pressure variations during the process of CO2hydrate formation in porous medium with a particle size of 380 μm
It can be seen from Figures 2—4 that the pressure of the system was influenced by the temperature variation during the process of CO2hydrate formation in porous media. Because the hydrate formation process was an exothermic reaction, the heat of formation was released, whereas the temperature would fluctuate in the course of CO2hydrate formation. Therefore, the pressure drop in the system would slow down. It can be seen from Figures 2—4 that the curves of temperature variation showed irregular fluctuations over the time of reaction. When the CO2hydrate formation process continued to go on, the variation in temperature weakened gradually with time. Compared with the process of CO2hydrate formation in pure water, the gas-liquid contact between CO2and water was quite sufficient and the diffusion process of CO2gas was strengthened in the porous medium. Sun and Li, et al.[19-20]drew up the similar conclusions during investigating the process of natural gas hydrate formation. Furthermore, this phenomenon could help to overcome the mass transfer limitations usually observed during the experiments on hydrate formation in porous media.
3.2 Effects of particle size of porous media on rate of CO2hydrate formation
The experiment was conducted under the condition of aconstant volume adopted in the process of CO2hydrate formation. The rate of CO2hydrate formation is defined as the consumption rate of CO2. And it can be characterized by changes in the number of moles of CO2gas in the reaction system. Because the pressure variation is influenced by the temperature of reaction system, it is very difficult to describe the changes in moles of gaseous phase in the reaction system. So the gas storage volume and gas storage rate of CO2are characterized by the ratio of moles of CO2converted into the CO2hydrate versus the moles of water. Thus the calculated value of the average formation rate of CO2was based on the consumption of CO2gas during the experiment. And CO2gas exists in two forms in the reaction system, namely: the free CO2in gaseous phase and the CO2converted into the hydrate. Hereby an assumption states that the number of moles are expressed as ngand nh, respectively. And n stands for the total number of moles of CO2gas in the reaction system. However, the CO2gas at first could diffuse into the porous medium gradually and then it was converted into CO2hydrate when it reached a certain concentration suitable for nucleation of the hydrate. The reaction system was in a flow state and CO2gas was surplus in the hydrate formation system during the experiment. Since water was present in a state of ice in the porous medium, there were two forms of CO2,viz.: the free CO2and the CO2converted into hydrate in the reaction system.
Before the CO2hydrate formation, the CO2gas existed mainly in the form of free CO2in gaseous phase. After the CO2hydrate formation, there were two forms of CO2in the system including the free CO2gas and the CO2that was converted into the hydrate. In this case the following equation applies:

At a certain temperature, ngis calculated according to the equation of state (EOS) as shown below:
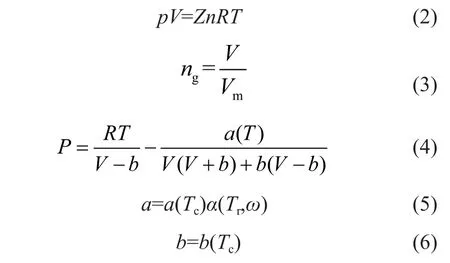

The amount of CO2in the experiment was calculated by the equation of state. In this work, it has been shown that the particle size of porous medium has an obvious influence on the rate of hydrate formation to some extent. According to the experiment on CO2hydrate formation in porous media, the calculated average rate of CO2hydrate formation in the porous medium with a particle size of 380 μm was 0.016 14 mol/h, while it was 0.014 82 mol/h and 0.014 03 mol/h in porous medium with a particle size of 500 μm and 700 μm, respectively. The rate of CO2hydrate formation in the porous medium with a smaller particle size was obviously greater than that of porous media with a larger pore size of 500 μm and 700 μm, respectively. The results showed that the smaller the particle size was, the larger the average rate of CO2hydrate formation would be.
Compared with the pure water system, the gas-liquid contact area between CO2gas and water was even sufficient and the diffusion process of CO2gas was strengthened in the porous medium, which could help to overcome the mass transfer limitations usually observed in experiments with porous media. Furthermore, it could provide larger specific surface area in the porous medium. And the smaller the particle size of porous medium, the larger the specific surface area, which could have greater gas adsorption capacity of CO2in the process of hydrate formation. Meanwhile, it could afford a greater driving force for the hydrate nucleation and growth process, which was consistent with Yang’s view on methane hydrate formation characteristics in porous media. So it is demonstrated that the average rate of CO2hydrate formation in porous medium with a particle size of 380 μm was greater than that in porous media with a particle size of 500 μm and 700 μm in the experiment, respectively.
3.3 Effects of particle size of porous media on gas storage capacity of CO2hydrate
In order to clarify the influence of porous medium on the gas storage capacity of CO2hydrate below the freez-ing point, the experiment of CO2hydrate formation was conducted in a porous medium made of quartz sand with different particle size. In this experiment, the hydration reaction was conducted under the condition of constant volume in the porous medium during the process of CO2hydrate formation. The volume of CO2gas remained constant in the high-pressure cell. Gas consumption in a unit time and the total amount of CO2gas were calculated by the equation of state of gas.

Figure 5 The curves of remaining amount of CO2in the process of CO2hydrate formation in porous medium with a particle size of 380 μm and 700 μm, respectively
The gas storage capacity of CO2was characterized by the consumed gas volume at the end of experiment. Figure 5 shows the remaining amount of CO2gas measured in the course of CO2hydrate formation in the porous medium with different particle size.
In this study, we found out that the particle size of quartz sand used as the porous medium had an insignificant influence on the gas storage capacity of CO2hydrate in the porous medium. Then we can calculate the gas storage volume of CO2in the porous media with different particle size. It is indicated that the gas storage capacity of hydrate is related to the particle size of porous media to some extent. According to the experiment of CO2hydrate formation in porous media below the freezing point, the gas storage capacity of CO2hydrate was 65.094 L/L in the porous medium with a particle size of 380 μm, while it was 59.854 L/L and 56.605 L/L in the porous medium with a particle size of 500 μm and 700 μm, respectively. The results showed that the smaller the particle size, the smaller the pore diameter of the porous medium and the larger the gas storage capacity of CO2hydrate in the porous medium, which was largely dependent on the physical parameters of porous medium. Since the porous medium with different particle size had different specific surface area, so it would result in different adsorption capacity of CO2gas. In addition, the smaller the particle size of porous medium was, the bigger the specific surface area would be, which was beneficial to the process of gas adsorption in the system of hydrate formation. And more amount of CO2gas could penetrate into quartz sand used as the porous medium during the process of hydrate formation. In Figure 5, the gas consumption of CO2in the porous medium with a particle size of 380 μm was greater than that in porous media with a particle size of 500 μm and 700 μm, respectively. Furthermore, this phenomenon could accelerate the process of mass transfer in the porous media and provide favorable conditions for realizing the process of hydrate nucleation and growth. So the gas storage capacity of CO2in the porous medium with a particle size of 380 μm was greater than that in porous media with a particle size of 500 μm and 700 μm, respectively. Therefore, it was concluded that, within a certain particle size, the smaller the particle size of the porous media was, the larger the gas storage capacity of CO2hydrate in porous media would be.
4 Conclusions
In this communication, the characteristics of CO2hydrate formation has been investigated in porous media with a particle size of 380 μm, 500 μm and 700 μm, respectively, in an 1.8-L high-pressure cell operated at a temperature of below the freezing point. Based on the experimental results, the following conclusions were drawn up:
(1) In the porous medium with a particle size of 380 μm, the gas storage capacity and the average hydrate formation rate were the maximum among the three kinds of porous media, and each volume of CO2hydrate might contain 65.094 volumes of CO2gas under the standard condition, with the average hydrate formation rate reaching up to 0.01614 mol/h.
(2) In the porous medium with a particle size of 700 μm, the gas storage capacity and average hydrate formation rate were the minimum among the three kinds of porous media, and each volume of CO2hydrate might contain 56.605 volumes of CO2gas under the standard condition,with the average formation rate reaching 0.014 03 mol/h. (3) Within a certain range of particle size between 380 μm and 700 μm, the smaller the particle size of porous medium was, the faster the average rate of hydrate formation and the larger the gas storage capacity of CO2in the hydrate would be.
Acknowledgements: This work was financially supported by the Natural Science Foundation of China (No. 51266005) and the Science and Technology Research Key Project of the Ministry of Education (No. 1106ZBB007), the Hongliu Outstanding Talent Program of LUT (No. Q201101) and the Open Fund of Natural Gas Hydrate Key Laboratory, Chinese Academy of Sciences (No. y007s3).
[1] Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd Ed. CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008
[2] Qi Y, Ota M, Zhang H. Molecular dynamics simulation of replacement of CH4in hydrate with CO2[J]. Energy Conversion and Management, 2011, 52(7): 2682-2687
[3] Jung J W, Santamarina J C. CH4-CO2replacement in hydrate-bearing sediments: A pore-scale study[J]. Geochemistry, Geophysics, Geosystems, 2010, 11(12): 2-8
[4] Deusner C, Bigalke N, Kossel E, et al. Methane Production from Gas Hydrate Deposits through Injection of Supercritical CO2[J]. Energies, 2012, 5(7): 2112-2140
[5] Yuan Q, Sun C Y, Yang X. Recovery of methane from hydrate reservoir with gaseous carbon dioxide using a three-dimensional middle-size reactor[J]. Energy, 2012, 40(1): 47-58
[6] Goel N. In situ methane hydrate dissociation with carbon dioxide sequestration: Current knowledge and issues[J]. Journal of Petroleum Science and Engineering, 2006, 51(3): 169-184
[7] Lee H, Seo Y, Seo Y T. Recovering methane from solid methane hydrate with carbon dioxide[J]. Angewandte Chemie International Edition, 2003, 42(41): 5048-5051
[8] Yoon J H, Kawamura T, Yamamoto Y. Transformation of methane hydrate to carbon dioxide hydrate: in situ Raman spectroscopic observations[J]. The Journal of Physical Chemistry A, 2004, 108(23): 5057-5059
[9] Mc Grail B P, Zhu T, Hunter R B. A new method for enhanced production of gas hydrates with CO2[J]. Gas hydrates: energy resource potential and associated geologic hazards, 2004: 12-16
[10] Uchida T, Takeya S, Ebinuma T. Replacing methane with CO2in clathrate hydrate: observations using Raman spectroscopy[C]. Proceedings of the Fifth International Conference on Greenhouse Gas Control Technologies. CSIRO Publishing: Collingwood, Australia, 2001: 523-527
[11] Bai D, Zhang X, Chen G. Replacement mechanism of methane hydrate with carbon dioxide from microsecond molecular dynamics simulations[J]. Energy & Environmental Science, 2012, 5(5): 7033-7041
[12] Handa Y P, Stupin D Y. Thermodynamic properties and dissociation characteristics of methane and propane hydrates in 70-Å-radius silica gel pores[J]. The Journal of Physical Chemistry, 1992, 96(21): 8599-8603
[13] Uchida T, Ebinuma T, Ishizaki T. Dissociation condition measurements of methane hydrate in confined small pores of porous glass[J]. The Journal of Physical Chemistry B, 1999, 103(18): 3659-3662
[14] Anderson R, Burgass R W, Tohidi B. Experimental measurement of gas hydrate stability in porous media[C]. 63rd EAGE Conference and Exhibition, Amsterdam: The Netherlands, 2001: 11-15
[15] Seshadri K, Wilder J W, Smith D H. Measurements of equilibrium pressures and temperatures for propane hydrate in silica gels with different pore-size distributions[J]. The Journal of Physical Chemistry B, 2001, 105(13): 2627
[16] Turner D J, Cherry R S, Sloan E D. Sensitivity of methane hydrate phase equilibria to sediment pore size [J]. Fluid Phase Equilib., 2005, 228: 505-510
[17] Dicharry C, Duchateau C, Asbai H. Carbon dioxide gas hydrate crystallization in porous silica gel particles partially saturated with a surfactant solution[J]. Chemical Engineering Science, 2013, 98: 88-97
[18] Ruan X, Song Y, Zhao J. Numerical simulation of methane production from hydrates induced by different depressurizing approaches[J]. Energies, 2012, 5: 438-458
[19] Sun Shicai, Ye Yuguang, Liu Changling. Research on the formation process of methane hydrate in quartz sand[J]. Chemical Engineering of Oil & Gas, 2011, 10 (2): 123-128 (in Chinese)
[20] Li M C, Fan S S, Zhao J F. Experimental study on formation of gas hydrate in porous medium[J]. Natural Gas Industry, 2006, 26 (5): 27-28
[21] Yang M J, Song Y C, Liu Y. Effects of porous media and salinity on phase equilibrium of methane hydrates[J]. Journal of Dalian University of Technology, 2011, 51 (1): 31-35 (in Chinese)
date: 2015-05-09; Accepted date: 2015-08-24.
Prof. Li Jinping, Telephone: +86-931-2976332; E-mail: lijinping77@163.com.
- 中国炼油与石油化工的其它文章
- Catalytic Hydrogenation of Methanol-Containing Effluent from Epoxidation of Propylene
- Synthesis of Biodiesel Using ZrO2Polycrystalline Ceramic Foam Catalyst in a Tubular Reactor
- Investigation of Swelling and Dissolution Process of Natural Rubber in Aromatic Oil
- Optimization of High-Gravity Chelated Iron Process for Removing H2S Based on Response Surface Methodology
- Studies on the Hydrogenation of Acetonitrile over Fresh Mo2C/γ-Al2O3Catalyst by In-situ IR Spectroscopy
- Lubricant Biodegradation Enhancers: Designed Chemistry and Engineered Technology

