Phenolics from grapefruit peels inhibit HMG-CoA reductase and angiotensin-I converting enzyme and show antioxidative properties in endothelial EA.Hy 926 cells
Ayokunle O.Ademosun,Ganiyu Ooh,Saina Passamonti,Federia Tramer,Lovro Zierna,Aline Augusti Boligon,Margareth Linde Athayde
a Functional Foods and Nutraceuticals Unit,Department of Biochemistry,Federal University of Technology,Akure,Nigeria
b Department of Life Sciences,University of Trieste,via L.Giorgieri 1,34127 Trieste,Italy
c Program of Post-Graduation in Pharmaceutical Sciences,Federal University of Santa Maria,Campus Camobi,Santa Maria,RS 97105-900,Brazil
Abstract
Keywords:Endothelial cells;Antioxidant;Angiotensin-I converting enzyme;Grapefruit peels;HMG-CoA reductase
1.Introduction
The link between oxidative stress,endothelial dysfunction and cardiovascular diseases(CVDs)has been well established[1].Clinical studies have shown that there is increased production of reactive oxygen species in CVD patients as demonstrated by clinical studies[2].The increase in reactive oxygen species induces oxidative stress,which then initiates atherosclerosis at the endothelium.Endothelial dysfunction is the first step in artherosclerosis and a major signal of cardiovascular complications[3].Lee et al.[4]reported that oxidative stress is not only linked to endothelial dysfunction,but to all the processes involved in atherogenesis up to myocardial infarctions.Furthermore,hypercholesterolemia has been shown to induce oxidative stress[5]thereby causing tissue damage[6].Lowering cholesterol levels has been shown to reduce the risk of myocardial infarctions and also increased endothelium dependent vasodilation[7].A vasoconstrictor that has been linked to cardiovascular complications is angiotensin II,which is produced by the renin–angiotensin system and has also been linked to increased oxidative stress[8].
Therefore,the therapeutic targets for the management of cardiovascular complications include angiotensin-I converting enzyme(catalysing the conversion of angiotensin-I to angiotensin-II),3-hydroxy-3-methylglutaryl-CoA(HMG-CoA)reductase(catalysing the conversion of HMG-CoA to mevalonate,which is the rate-limiting step in the synthesis of cholesterol and other isoprenoids)and the use of radical scavengers.The burden of cardiovascular diseases,which is a leading cause of death and disability in the world[9]is further increased by the cost of therapy which is beyond the reach of many in developing countries and the search for affordable therapy has led to natural alternatives.Grapefruits,like other citrus fruits have a smaller edible portion compared to the amount of nonedible portions such as peels and seeds.However,it has been shown that there are more bioactive compounds in the peels of citrus fruits than in the juices[10].Grapefruit peels are domestically processed into candies and consumed by some Americans homes,while the peels are taken as infusion drinks in Asia.The bioactive compounds present in the peels,especially the phenolic compounds have been shown to have some medicinal properties[11].However,these bioactive compounds in the peels have been of little benefit to humans as they are grossly underutilized in many countries.This study sought to investigate the effect of phenolic extracts from grapefruit peels on key enzymes relevant to the management of cardiovascular diseases and also the cellular antioxidant activities of the extracts in endothelial cells.
2.Materials and methods
2.1.Sample collection and preparation
Grapefruits(Citrusparadisii)were purchased from the Main Market,Akure,South West,Nigeria[7.2500◦N,5.1950◦E].The fruits were peeled and the peels were air dried and ground to fine powder using Warring Commercial heavy Duty Blender(Model 37BL18;24ØCB6).The water used was glass distilled,while the chemicals were of analytical grade.
2.2.Extraction of free soluble phenolics
The extraction of free soluble phenolics was carried out according to the method reported by Chu et al.[12].10 g of the ground peels was extracted with 80% acetone(1:5,w/v)and filtered(filter paper Whatman no.2)under vacuum.The filtrate was then evaporated using a rotary evaporator under vacuum at 45℃ until about 90% of the filtrate had been evaporated.The phenolic extracts were frozen at−40℃,while the residues were kept for the extraction of bound phenolics.
2.3.Extraction of bound phenolics
The residue from free soluble extraction above was flushed with nitrogen and hydrolysed with about 20 mL of 4 M NaOH solution at room temperature for 1 h with shaking.Then,the pH of the mixture adjusted to pH 2 with concentrated HCl and the bound phytochemicals were extracted with ethyl acetate and repeated five times.The ethyl acetate fractions were then evaporated at 45℃[12].
2.4.Cell cultures
The human endothelial cell line(EA.Hy 926)was purchased from the American Type Culture Collection(Rockville,MD,USA).EA.Hy 926 cells were cultured in complete DMEM(i.e.,supplemented with 10% foetal bovine serum,1 mM l-glutamine,and 1 mM penicillin–streptomycin).Cells were grown in an incubator at 37℃ in a humidified atmosphere(95% air and 5% carbon dioxide).
2.5.HMG-CoA reductase activity
The commercially available HMG-CoA reductase assay kit from Sigma–Aldrich(Steinheim,Germany)was used to screen the phenolic extracts according to the manufacturer’s instructions.A 0.2 mL reaction mixture containing 1L of appropriate dilutions of the extracts or pravastatin solution(control),4L of reconstituted NADPH,12L of HMG-CoA solution and 2L HMG-CoA reductase was vigorously shaken mechanically in the plate reader for 10 s,and the rates of NADPH consumed were monitored every 20 s for 600 s by scanning spectrophotometrically the decrease in absorbance at 340 nm,using Infinite M200 Pro plate reader(Tecan,Männedorf,Switzerland).The blank experiment did not contain HMG-CoA Reductase or any of the studied substances.The Area Under Curve(AUC)of the curves obtained were calculated using GraphPad Prism software(version 5.00,GraphPad Software,San Diego,USA).The obtained AUC values were divided by the AUC of the control experiments(no inhibitors)to obtain the percentage inhibition.All results were expressed as a mean±SD of three repetitions,and IC50,i.e.,the half maximal inhibition concentration,were calculated using GraphPad Prism software.
2.6.Angiotensin I-converting enzyme (ACE) inhibition assay
Appropriate dilution of the phenolic extracts(50 mL)and ACE solution(50 mL,4 mU)was incubated at 37℃ for 15 min.The enzymatic reaction was initiated by adding 150 mL of 8.33 mmol/L of the substrate Bz–Gly–His–Leu in 125 mmol/L Tris–HCl buffer(pH 8.3)to the mixture.After incubation for 30 min at 37℃,the reaction was arrested by adding 250 mL of 1 mol/L HCl.The Gly–His bond was then cleaved and the Bz–Gly produced by the reaction was extracted with 1.5 mL ethyl acetate.Thereafter the mixture was centrifuged to separate the ethyl acetate layer;then 1 mL of the ethyl acetate layer was transferred to a clean test tube and evaporated.The residue was re-dissolved in distilled water and its absorbance was measured at 228 nm.The ACE inhibitory activity was expressed as percentage inhibition[13].
2.7.Cellular antioxidant activity (CAA) assay
The intracellular formation of peroxyl radical was detected by the method of Wolfe and Liu[14].EA.Hy 926 cells were seeded at a density of 1×104cells/well on a 96-well microplate in 100L of growth medium/well.The outside wells of the plate were not used.Twenty-four hours after seeding,the growth medium was removed,the wells were washed with PBS,and the cells were treated for 1 h with the extracts(0.1g/L,1g/L,10g/L,100g/L)plus 25mol/L DCFH-DA dissolved in treatment medium.After the indicated period,cells were washed and 600mol/L ABAP dissolved in HBSS was added to the cells.Fluorescence was measured(λex=485 nm,λem=538 nm)every 5 min for 30 min at 37℃ on a microplate reader(Plate Chameleon,HIDEX).Each concentration of each substance was repeated in six wells.Each plate also included six control and six blank wells:control wells contained cells treated with the dye(DCFH-DA)and the oxidant(ABAP);blank wells contained cells treated with DCFH-DA and HBSS without the oxidant.
2.7.1.QuantificationofCAA
After blank subtraction from fluorescence readings,the area under the curve of fluorescence versus time was integrated to calculate the CAA value at each concentration of extract as follows:CAA unit=SA/CA×100,whereSA is the integrated area under the sample fluorescence versus time curve andCA is the integrated area from the control curve[14].
2.8.DPPH free radical scavenging ability
The free radical scavenging ability of the extracts against DPPH(1,1-diphenyl-2 picrylhydrazyl)free radical was evaluated as described by Gyamfi et al.[15].Briefly,appropriate dilution of the extracts(1 mL)was mixed with 1 mL,0.4 mmol/L methanolic solution containing DPPH radicals,the mixture was left in the dark for 30 min and the absorbance was taken at 516 nm.The DPPH free radical scavenging ability was subsequently calculated.
2.9.2,2 -Azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)(ABTS) radical scavenging ability
The ABTS*scavenging ability of the essential oil was determined according to the method described by Re et al.[16].The ABTS*was generated by the reaction of an(7 mmol/L)ABTS aqueous solution with K2S2O8(2.45 mmol/L,final concentration)in the dark for 16 h and adjusting the Abs 734 nm to 0.700 with ethanol.Samples of 0.2 mL of the extract were added to 2.0 mL ABTS*solution and the absorbance was measured at 734 nm after 15 min.Trolox was used as a reference compound.The results were expressed as Trolox equivalent antioxidant capacity(TEAC)values.
2.10.Quantification of compounds by HPLC–DAD
Reverse phase chromatographic analyses were carried out under gradient conditions using C18 column(4.6 mm×150 mm)packed with 5m diameter particles;the mobile phase was water containing 2% acetic acid(A)and methanol(B),and the composition gradient was:5%(B)for 2 min;25%(B)until 10 min;40,50%,60%,70% and 80%(B)every 10 min;following the method described by Freitas et al.[17]with slight modifications.The phenolic extracts were filtered through 0.45m membrane filter(Millipore)and then degassed by ultrasonic bath prior to use.The flow rate was 0.7 mL/min,injection volume 50L and the wavelength was 254 nm for gallic acid and resveratrol,280 nm for catechin and epicatechin,327 nm for caffeic and ellagic acids,and 366 nm for quercetin,rutin and kaempferol.The samples and mobile phase were filtered through 0.45m membrane filter(Millipore)and then degassed by ultrasonic bath prior to use.Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.010–0.100 mg/mL for resveratrol,catechin,epicatechin,quercetin,rutin and kaempferol;and 0.050–0.200 mg/mL for gallic,ellagic and caffeic acids.The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by DAD spectra(200–600 nm).Calibration curve for gallic acid was:Y=13,057x+1254.9(r=0.9993);catechin:Y=12,724x+1258.0(r=0.9997);epicatechin:Y=11,973x+1317.8(r=0.9999);resveratrol:Y=11,983x+1306.5(r=0.9993);caffeic acid:Y=11,872x+1570.3(r=0.9996);ellagic acid:Y=12,728x+1367.4(r=0.9998);quercetin:Y=13,149x+1267.8(r=0.9999);rutin:Y=12,657x+1340.5(r=0.9996)and kaempferol:Y=15,983x+1321.5(r=0.9992).All chromatography operations were carried out at ambient temperature and in triplicate.
3.Results and discussion
The dose-dependent inhibition of HMG-CoA reductase activity by the extracts as presented in Fig.1 and the IC50values in Table1 showed that there was no significant(P>0.05)difference in the inhibitory abilities of the two extracts.The control of elevated cholesterol levels has become imperative in recent times as hypercholesterolemia has been established as a risk factor for atherosclerosis and other cardiovascular complications.In most cases,the reduction of cholesterol synthesis is achieved by inhibition of HMG-CoA reductase,which is the rate limiting enzyme in cholesterol synthesis.Therefore,the inhibition of HMG-CoA reductase by the phenolic extracts from grapefruit peels would be beneficial in the management of cardiovascular complications.The HMG-CoA reductase inhibition by the phenolic extracts could be the possible mechanism for the cholesterol lowering effect of the peels in experimental animals as observed by previous studies[18].Although,as shown in this study the extracts favourably compared with pravastatin in inhibiting the HMGCoA reductase especially at higher concentrations,it should be noted that phenolic compounds,especially flavonoids are not structurally similar to statins.
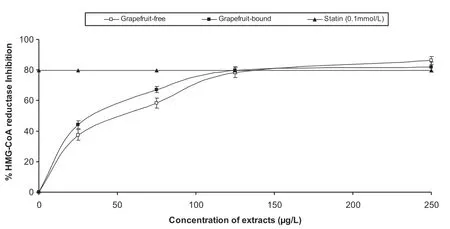
Fig.1.Inhibition of 3-hydroxy-methyl-3-glutaryl coenzyme A reductase(HMG-CoA reductase)activity by grapefruit peels’phenolic extracts.
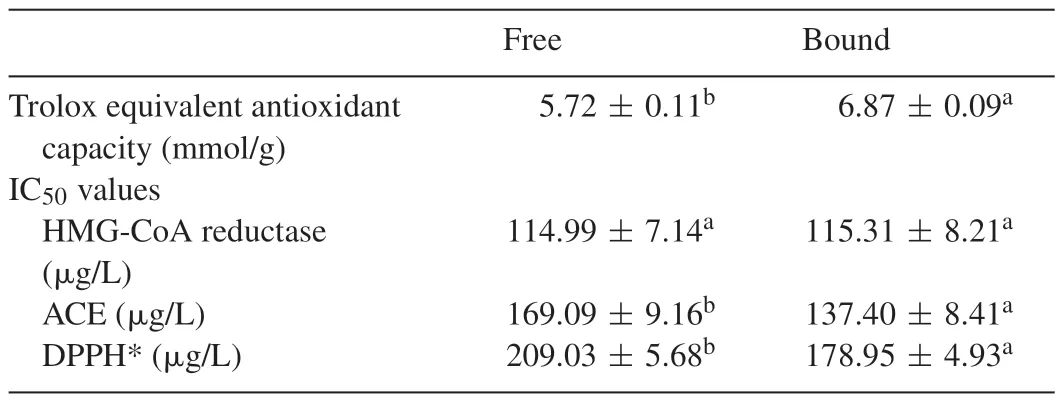
Table 1Antioxidant capacity and IC50 values.
Statins inhibit HMG-CoA reductase activity by competing with HMG-CoA for the enzyme active site[19].The competitive inhibition of pravastatin is made possible by its structural similarity to HMG-CoA by the possession of a double cyclic ring structure[20].However,in the case of phenolic compounds,flavonoids such as genistein and diadzein have been shown to inhibit HMG-CoA reductase activity both competitively with HMG-CoA and non-competitively with NADPH[21].While statins are widely used in the management of cardiovascular complications[22],recent studies have supported the use of HMG-CoA reductase inhibitors from natural sources due to the side effects of statins[23].
Furthermore,the extracts inhibited ACE activity in a dosedependent manner(Fig.2)with the bound phenolics having a stronger inhibitory ability than the soluble free phenolics.Another mechanism for the management of cardiovascular complications such as hypertension is the use of ACE inhibitors.ACE inhibition prevents the enzyme from catalysing the conversion of angiotensin I to the powerful vasoconstrictor,angiotensin II[24].Phenolic-rich extracts from natural products have been shown from previous studies to inhibit ACE activity[11,25,26].The HPLC analysis of the extracts revealed the presence of kaempferol,rutin and quercetin which have been shown from previous studies to be ACE inhibitors[27].Angiotensin II does not only stimulate vasoconstriction,but also oxidative stress.Angiotensin II-induced oxidative stress has been shown to play a major role in the development of hypertension[28,29].
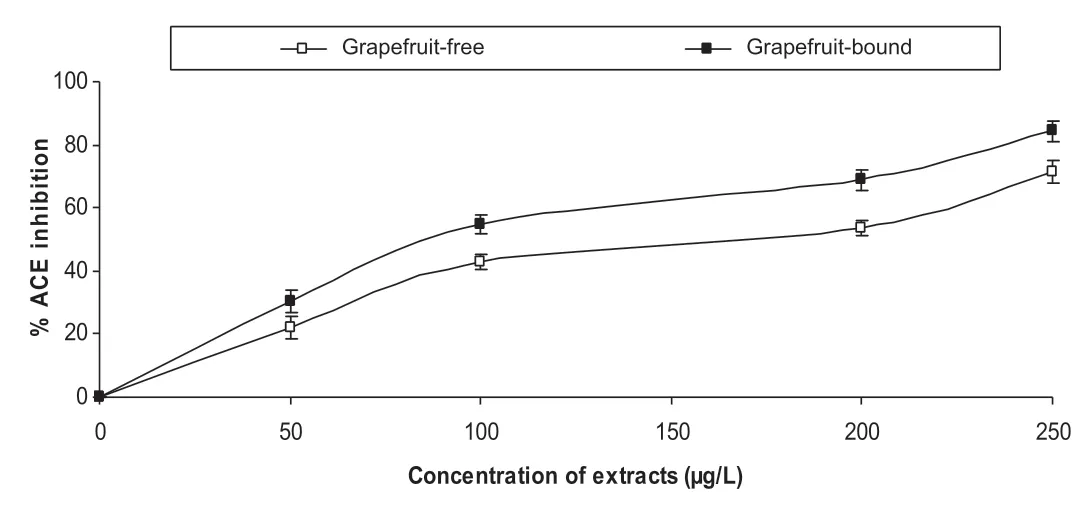
Fig.2.Inhibition of angiotensin-I converting enzyme(ACE)activity by grapefruit peels’phenolic extracts.
More so,the role of angiotensin II-induced reactive oxygen species(ROS)formation in atherogenesis has been well established[30].It is also noteworthy that the anti-oxidative effects of ACE inhibitors contribute to their therapeutic effects in patients suffering from cardiovascular complications[31].Also,hypercholesterolemia has been shown to induce oxidative stress in experimental animals[5].Generally,oxidative stress has been implicated in the development of major cardiovascular conditions such as atherosclerosis,ischaemic heart disease and hypertension[2].Therefore,the antioxidative effects of phenolic compounds might also contribute to the medicinal effects of grapefruit peels in the management of cardiovascular complications.
The anti-oxidative effects of the phenolic extracts from the grapefruit peels was tested in endothelial cells EA.Hy 926 and also in cell free system using DPPH and ABTS assays.The dose-dependent DPPH*scavenging ability(Fig.3)revealed that the bound phenolics had significantly higher scavenging ability than the free phenolics.Table 1 showed that the bound phenolics had significantly higher ABTS*scavenging ability than the free soluble phenolic extracts.Fig.4 showed the time courses for peroxyl radical-induced oxidation of DCFH to DCF in human endothelial cells(EA.Hy 926)cells and the inhibition of oxidation by grapefruit peel phenolic extracts.Fig.5 showed that the CAA units for the extracts at the concentrations tested(0.1–100g/L)were 6.5–31.42% and 8.88–46.42% for the free and bound phenolics respectively.
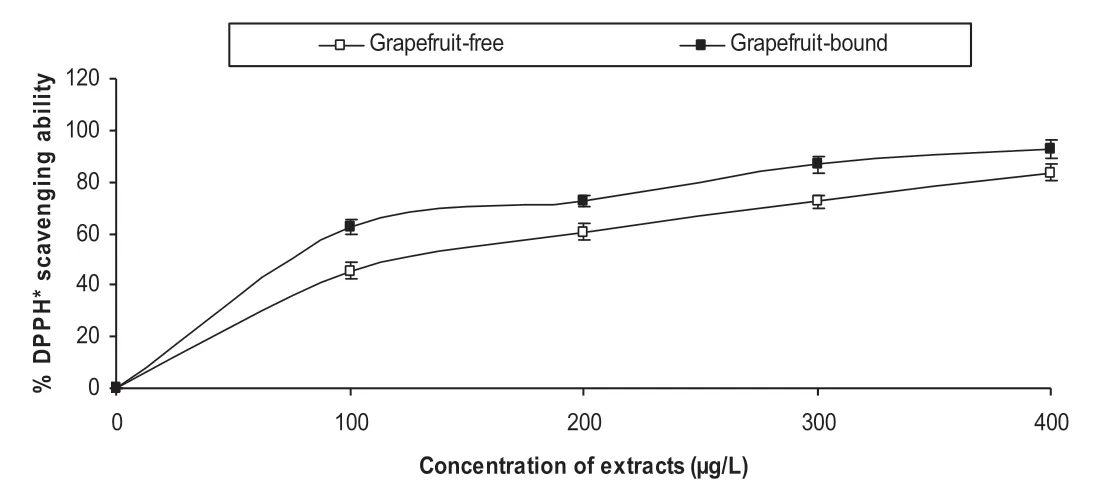
Fig.3.1,1-Diphenyl-2 picrylhydrazyl(DPPH)radical scavenging ability of grapefruit peels’phenolic extracts.
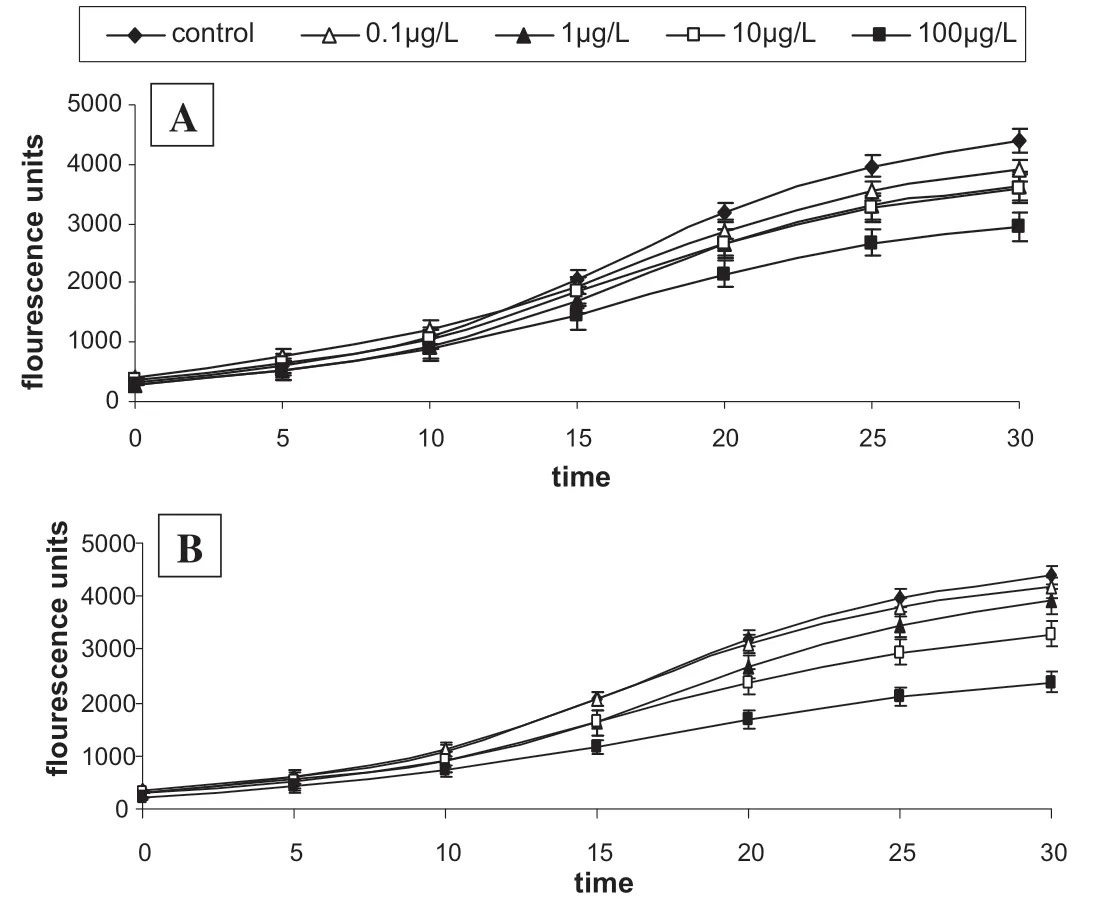
Fig.4.Time course for peroxyl radical-induced oxidation of DCFH to DCF in EA.Hy 926 cells and the inhibition of oxidation by grapefruit peels’[A]free and[B]bound phenolic extracts.
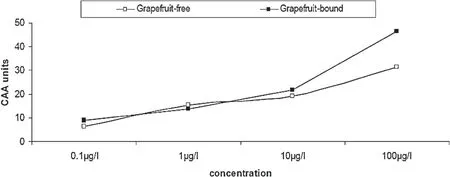
Fig.5.Dose–response curves for inhibition/increase of peroxyl radical-induced DCFH oxidation grapefruit peels phenolic extracts.
These results showed that the phenolic extracts scavenged the radicals and prevented the oxidation of the membrane lipids and DCFH,thereby reducing the formation of DCF.DCF is formed from the reaction between DCFH trapped in the cells and the ABAP-induced peroxyl radicals.The cellular antioxidant activity(CAA)of the phenolic extracts in EA.Hy 926 cells showed that the phenolic extracts were able to exhibit anti-oxidative properties in physiological conditions.The CAA of the extracts in endothelial cells is also therapeutically important as reactive oxygen species are produced at all layers of the vascular wall,especially at the endothelium[31].Therefore,the prevention of oxidative stress-induced endothelial dysfunction has become a major therapeutic target in the management of cardiovascular complications[32,33].
Clinical trials have shown that the use of HMG-CoA reductase inhibitors in patients at coronary risk improved endothelial function through reduction of oxidative stress and/or up regulation of NO activity[34].Furthermore,some studies have shown the synergistic effect of the use of HMG-CoA reductase inhibitors and ACE inhibitors in reducing inflammatory markers in atherosclerotic rabbits[35].Therefore,the anti-oxidative properties of the phenolic extracts from the peels,combined with their inhibition of HMG-CoA reductase and ACE could be useful in the management/prevention of oxidative stress-induced endothelial dysfunction mediated cardiovascular complications.
HPLC fingerprinting of grapefruit peels(free and bound)revealed the presence of gallic acid(tR=11.87 min;peak 1),catechin(tR=15.69 min;peak 2),resveratrol(tR=20.03 min;peak 3),caffeic acid(tR=23.91;peak 4),ellagic acid(tR=28.17 min;peak 5),epicatechin(tR=34.29 min;peak 6),rutin(tR=42.67 min;peak 7),quercetin(tR=47.21 min;peak 8)and kaempferol(tR=51.26 min;peak 9)(Fig.6 and Table 2).Although the bound phenolic extracts had lower concentrations of most of the phenolic compounds identified,it had higher bioactivity than the free phenolic extracts in the assays used in this study.It could be speculated that the method of extraction for the bound phenolics makes a wider variety of phenolic compounds available which might not have been identified by the finger-printing[36].
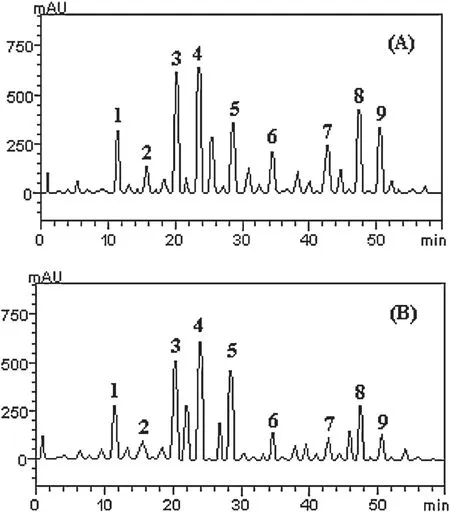
Fig.6.Representative high performance liquid chromatography profile of[A]grapefruit free phenolic extracts and[B]bound phenolic extracts.Detection UV was at 327 nm.Gallic acid(peak 1),catechin(peak 2),resveratrol(peak 3),caffeic acid(peak 4),ellagic acid(peak 5),epicatechin(peak 6),rutin(peak 7),quercetin(peak 8)and kaempferol(peak 9).
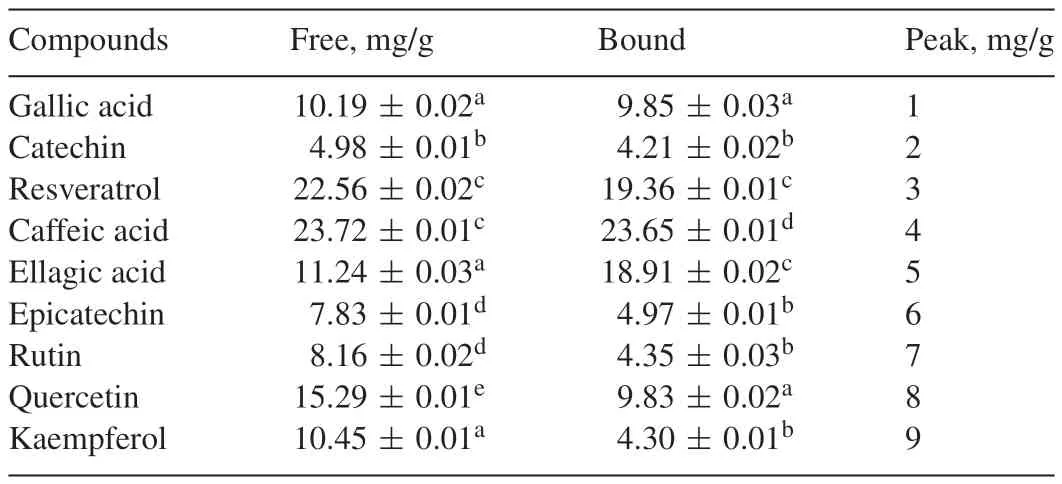
Table 2Phenolic composition of grapefruit(free and bound)extract.
4.Conclusion
The inhibition of HMG-CoA reductase and ACE activities,as well as the anti-oxidative abilities of the phenolic extracts from grapefruit peels in endothelial cells could be part of the mechanism by which the peels manage and/or prevent cardiovascular diseases.However,further in vivo experiments and clinical trials are recommended.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Ayokunle Ademosun’s stay in Italy was funded by Education Trust Fund(ETF)of the Federal Government of Nigeria/Federal University of Technology,Akure and Training and Research in Italian Laboratory(TRIL)Programme of The Abdus Salam(REF.421)International Centre for Theoretical Physics,Trieste,Italy.
- 食品科学与人类健康(英文)的其它文章
- Detection of Salmonella and several common Salmonella serotypes in food by loop-mediated isothermal amplification method
- Carvacrol attenuates N-nitrosodiethylamine induced liver injury in experimental Wistar rats
- New perspectives on probiotics in health and disease
- A review:Health promoting lactic acid bacteria in traditional Indonesian fermented foods
- GUIDE FOR AUTHORS

