New perspectives on probiotics in health and disease
Eric Bnn-Mwine Dliri,Byong H.Lee,b,c,∗
a School of Biotechnology,Jiangnan University,1800 Lihu Avenue,Wuxi 214122,China
b Department of Food Science and Biotechnology,Kangwon National University,Chuncheon 200-701,South Korea
c Department of Food Science / Agricultural Chemistry,McGill University,Ste-Anne-de-Bellevue,QC,Canada H9X 3V9
Abstract
Keywords:Probiotic;Heath benefits;Bile salt hydrolase;Dysbiosis;Microbiome
1.Introduction
Food fermentation and the consumption of fermented foods date far beyond human civilization.The transition from hunting and gathering to an agricultural lifestyle might have contributed to the further development of these food fermentations that are now practiced on industrial scales.However,human interactions with probiotics are more intimate and have a much longer history than the historic food fermentations.All parts of the human body such as the skin,oral cavity,gastrointestinal tract,and vaginal cavity are inhabited by trillions of microbes[1,2].At birth,the human gut is sterile but colonized immediately after birth[3].Factors such as the type of delivery(vaginal birthversuscesarean section)and the type of diet(breast feedingversusformula feeding)affect the colonization patterns[4].The pioneer microbes that‘infest’the gut make permanent adaptations and determine the physiological,immune,metabolic and behavioral development and also influence future disease susceptibility[132].Age and life style are some causes of many disease conditions since they contribute to alterations in the microbial flora in the body[5].Recent studies have demonstrated that bacterial community composition is considerably altered in diseases such as obesity and periodontal disease,with healthy subjects usually exhibiting distinct,diverse and temporally stable bacterial populations at these sites when compared with patients displaying disease symptoms[6].As consumers become aware of the impact of what they eat on their health,they tend to search for functional foods.Attention has been paid to prevention of diseases than cure and hence,probiotic containing foods are abundant on the market.
2.Diseases and disorders caused by alterations in the human gut microbiota
It is evident that prenatal maternal exposure influences postnatal microbial colonization[3]and this plays pivotal roles in gut-associated lymphoid tissue(GALT)development[7],specific aspects of immune system development[8,9]and the integrity of the mucosal barrier[10].Therefore the development of the gut microbiota in the early stages of life may be linked to future disease susceptibility.Many studies have associated diseases such as Inflammatory bowel disease[3],obesity[6]colon cancer[11]and some allergies[9]to alterations in the gut microbiome(Table 1).In many instances,there is an imbalance in the population densities of gut microbiota(dysbiosis)and this results in an overgrowth of pathogenic microbes.In obesity,the altered microbial population is associated with a shift in function of the cells,resulting in increased energy harvest from ingested food;unexpended excess energy is deposited as adipose tissue[12].
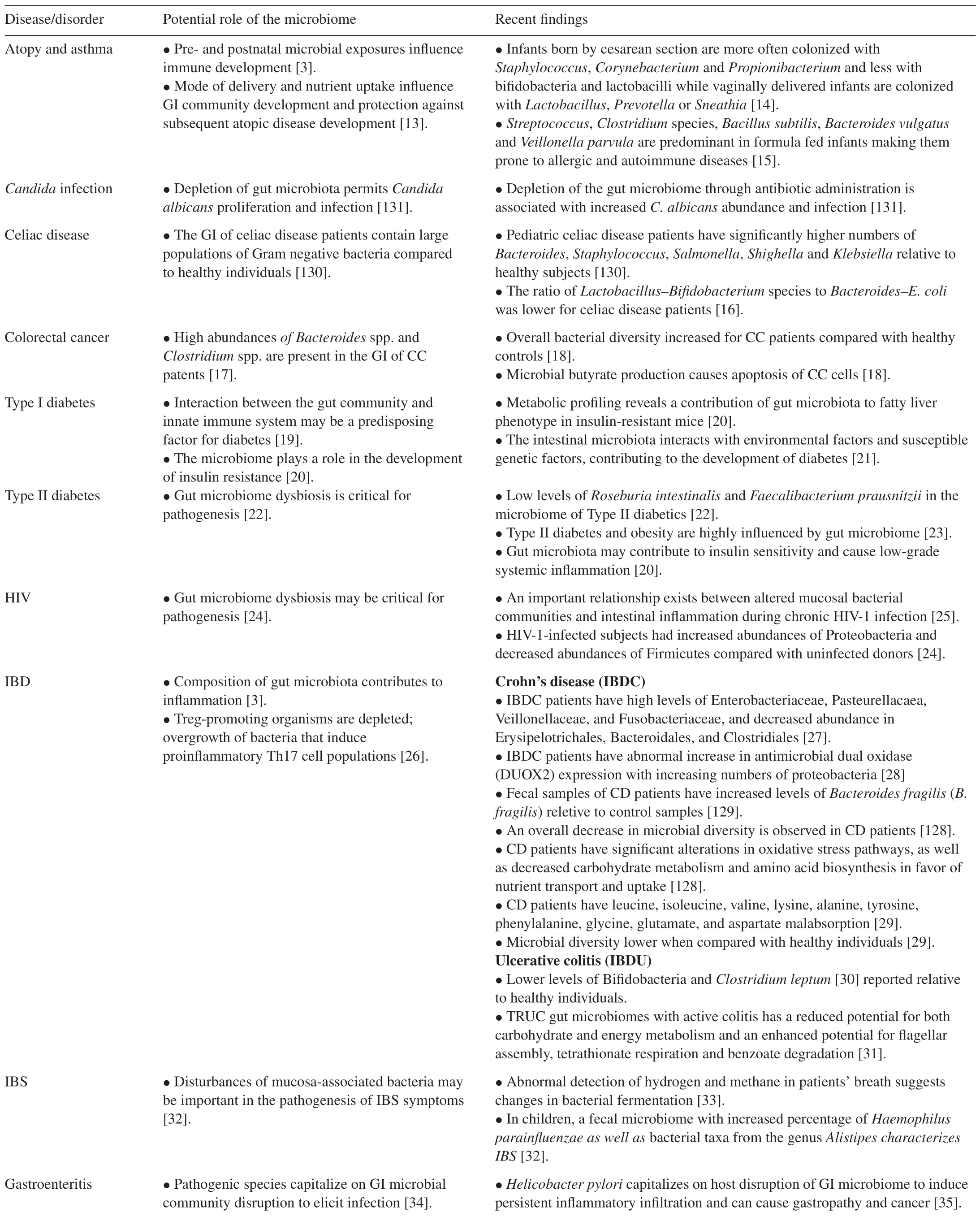
Table 1Diseases and disorders caused by alterations in the gut microbiome.
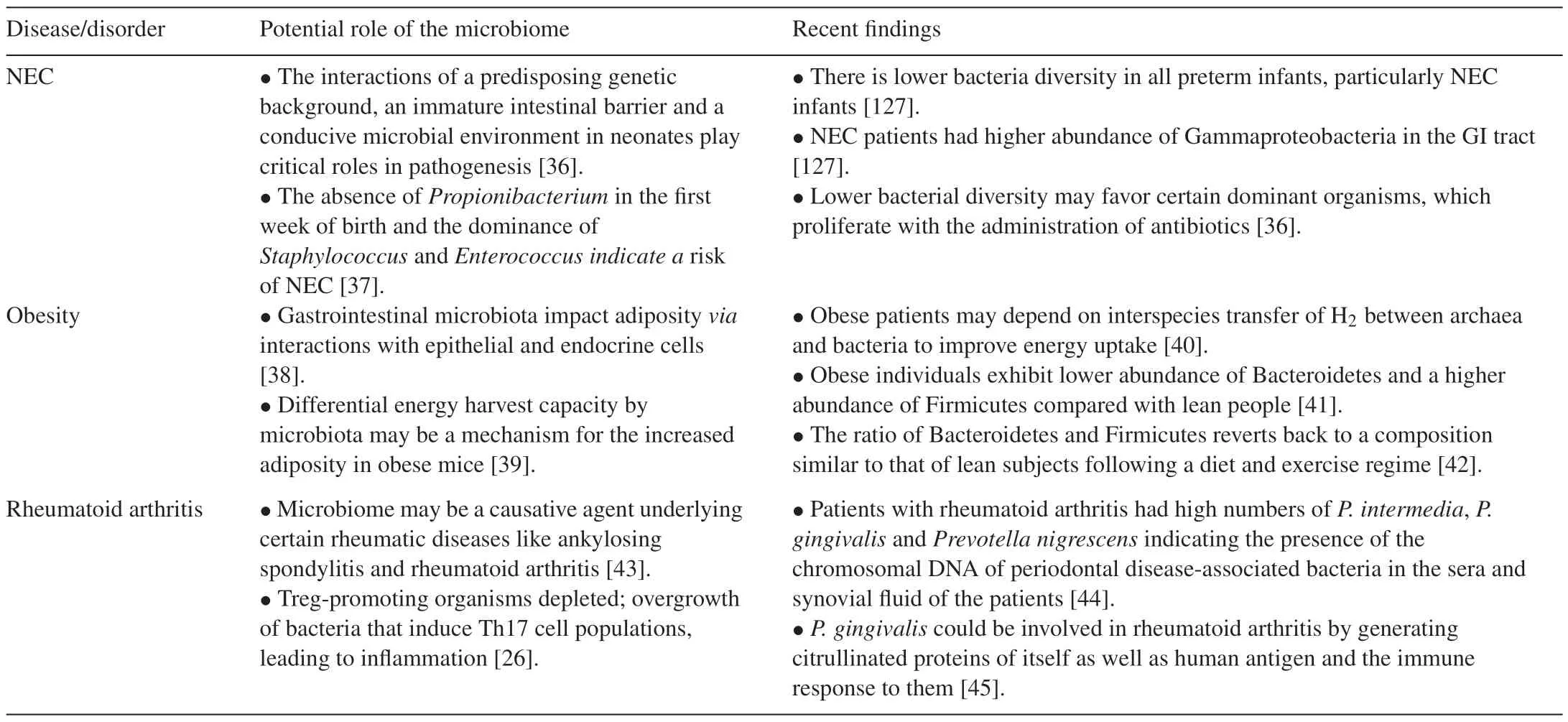
Table 1(Continued)
3.Probiotics and probiotic selection criteria
Probiotics are live microorganisms which,when administered in adequate amounts,confer health effects on the host and prebiotics are non-digestible food ingredients that stimulate the growth and or activity of probiotics[125].Though some non-living cells may have probiotic properties[46,47]living cells tend to function better.The stomach is highly acidic due to the presence of HCl.Therefore,one of the first barriers probiotics must endure is the gastric acidity of the stomach as well as the bile in the upper digestive tract before they get to the small intestines[35].Many types of bacteria have probiotic properties,however,the most documented groups comprise of lactic acid bacteria(LAB)and bifidobacteria.WhileL.caseiandLactobacillusacidophilussurvive in the acidic conditions of artificial gastric juice at pH 3.0 at 37℃,Lactobacillusdelbruekiissp.bulgaricusdoes not.Strains ofBifidobacteriumvary in their ability to survive transit through the stomach.The initial screening and selection of probiotics also include testing of the phenotype and genotypestability,including plasmid stability;intestinal epithelial adhesion properties;protein and carbohydrate utilization patterns;production of antimicrobial substances;antibiotic resistance patterns;ability to inhibit known pathogens,spoilage organisms,or both;and immunogenicity[124].Table 2 shows the properties and benefits of good probiotic strains.It is necessary that probiotic strains survive,proliferate and colonize their specific locations.They must neither be pathogenic nor trigger allergic response in the host.However,they may serve as adjuvants to stimulate the immune system against pathogens.Practically and for commercialization purposes,probiotics must be easily culturable on a large scale and must resist technological manipulations such as heating and low oxygen conditions in packages.
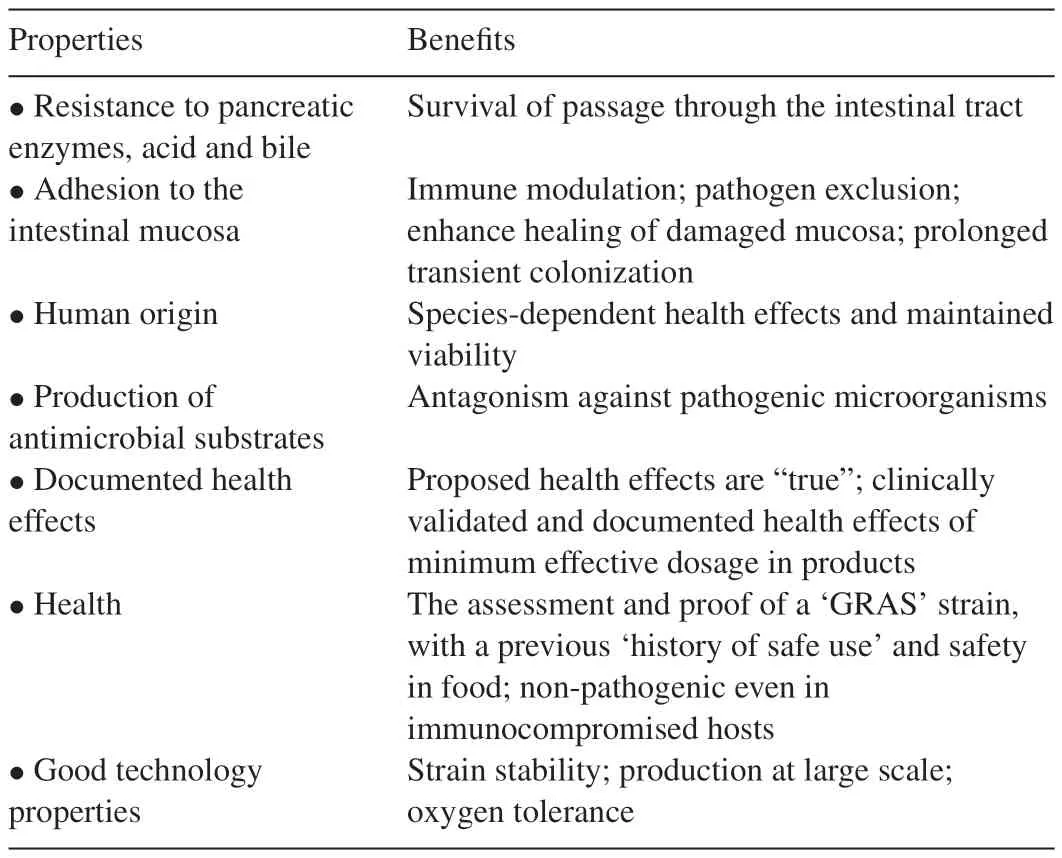
Table 2Properties and benefits of good probiotic strains.
4.Functional genomics of LAB
Recently,the number of sequenced LAB genomes has increased exponentially and the genomic data from several LAB species and strains are available to give a better understanding of their gene content,their properties and their roles in human health and food fermentation[48].The most important LAB used as starters in dairy fermentations areLactococcuslactis,Streptococcusthermophilus,L.delbruekiisubsp.bulgaricus,while in some cases also someLeuconostocor otherLactobacillusspp.are used[49].Because LAB do not contain a functional respiratory system,they obtain energy by substrate level phosphorylation.They use either the homofermentative pathway to virtually produce only lactate or the heterofermentative pathway to produce large amounts of CO2,and ethanol in addition to lactate.LAB compete with other bacteria based on their rapid growth and their lactic acid production in their habitats.Luesink et al.[50]have reported that the main factor controlling sugar degradation in LAB is the catabolite control protein CcpA which acts as a transcriptional activator of the lactic acid synthesis(las)operon with the orderpfk-pyk-ldh.In many LAB,theccpAgene is colocated with the prolidase-encoding pepQ gene but divergently transcribed from each other,indicating a link between carbon and nitrogen metabolism[2].Of all the nitrogen control systems present in LAB,GlnR and CodY are the most studied.All LAB genomes posses GlnR but CodY is only present inLactocccus,StreptococcusandEnterococcusspp.GlnR is involved in controlling the import of nitrogenous compounds and the synthesis of intracellular ammonia under high nitrogen concentrations[51],while CodY controls the proteolytic system ofL.lactisand particularly the cell-wall proteinase(PrtP),the key enzyme in milk degradation[52].
5.Mechanism of action of probiotics
The actual mechanism of action of probiotics has not been clearly understood,however,documented results are those obtained from animal models andinvitroexperiments.One mode of action of probiotics may be an improvement of the barrier functions of the gut mucosa(Fig.1).Several strains ofLactobacillusandBifidobacteriumas well as structural components,and microbial-produced metabolites are able to stimulate epithelial cell signaling pathways[53].The Nuclear Factor Kappa-Light-Chain-Enhancer of activated Bcells(NF-kB)pathway is modulated by probiotics at many different levels with effects seen on I Kappa B protein(IKB)degradation and ubiquitination[123],proteosome function[122]and nuclearcytoplasmic movement of RelA through a PPAR-gamma dependent pathway.Some probiotics such asS.thermophilusandL.acidophilusalter the expression of tight junction proteins and/or their localization in bothinvivoandinvitromodels[54].LactobacillusplantarumMB452 has been shown to alter expression levels of genes coding for occludin,tubulin,proteasome and certain cytoskeleton anchoring proteins[55].Other probiotics boost gut barrier function through increased production of cytoprotective molecules such as heat-shock proteins.In addition,probiotics are able to prevent cytokine and oxidant-induced epithelial damage thereby promoting cell survival[121].
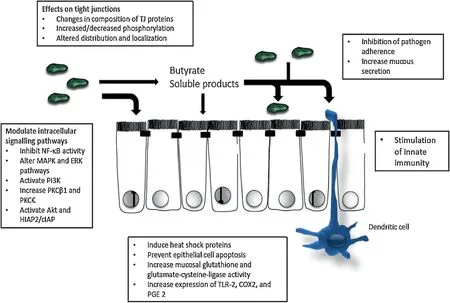
Fig.1.An overview of mechanisms involved in probiotic-induced enhancement of epithelial barrier function.These include direct modulation of epithelial cell signaling pathways and tight junctions,as well as effects on microbial ecology,innate and adaptive immune function[116].
Probiotics may also modulate the immune system functions.For instance,L.acidophilushas been found to modulate toll-like receptors and the proteoglycan recognition proteins of enterocytes,leading to activation of dendric cells and lymphocytes T-helper 1 responds.The resulting stimulation of lymphocytes T-helper 1 cytokines can suppress lymphocyte T-helper 2 responses which provoke the atopic issues[120].By this mechanism,the probiotics such asL.acidophilus,andRhamnococcusGG decrease skin sensitivity in children and can reduce disorders like eczema[56,57].Another possible mechanism of action of probiotics may be their ability to suppress the growth of pathogenic bacteria by producing broad spectrum bacteriocins[58].ProbioticssuchasB.infantisY1,L.acidophilusMB443,L.plantarumMB 452,L.paracaseiMB 451,L.bulgaricusMB453 inhibit pathogens from binding to gut cell walls and also produce short chain fatty acids(SCFA)which decrease the pH of the gut to selectively favor the growth of desirable microbes[118,119].Some strains of lactobacilli express human mucus-binding pili,which would enhance their ability for colonization[117].
6.Health effects of probiotics
Though many human and animal studies have proved the health effects of probiotic consumption[59–61,103],health authorities have only approved claims on(a)lactose intolerance and lactose digestion and(b)cholesterol reduction mostly because of biomarker deficiency.Probiotics research is still in the early stages,and far more studies need to be conducted to determine the health benefits and safety of probiotics.
6.1.Cholesterol reducing ability
Cholesterol plays a vital role in many functions of the body,such as in the synthesis of steroidal hormones,but excessive cholesterol in the blood causes arterial clogging and increases the risk of heart disease and/or stroke.The risk of heart attack is three times higher in those with hypercholesterolemia,compared to those who have normal blood lipid profiles[115].The cholesterol reducing ability of probiotics has been extensively reviewed by Ishimwe et al.[62].In a randomized,double-blind,placebo-controlled,and parallel-designed study,L.acidophilusCHO-220 and inulin was administered to thirty-two hypercholesterolemic men and women.After 12 weeks,their plasma total cholesterol and low-density lipoprotein(LDL)-cholesterol reduced by 7.84 and 9.27%,respectively[63].Many hypotheses have been proposed for the mechanism by which probiotics lower cholesterol levels.The hypothesis include deconjugation of bileviabile salt hydrolase[111],binding of cholesterol to probiotic cellular surface and incorporation of cholesterol molecules into the probiotic cellular membrane,production of short-chain fatty acids from oligosaccharides,co-precipitation of cholesterol with deconjugated bile[64]and cholesterol conversion to coprostanolin[114].Among all the hypothesis,the bile salt hydroxylase theory is most popular.Liver hepatocytes produce bile salts which enhance dietary cholesterol and fat transport across the intestinal epithelium.The primary bile acids conjugate with either glycine to form glycocholic acid(cholylglycine)or taurine to form taurocholic acid.Since the conjugated bile salts are very soluble,most of them enter into enterohepatic circulation after absorption[113]resulting in an accumulation in the blood.Studies have shown that manyBifidobacteriumandLactobacillusspecies produce bile salt hydrolase(BSH)which cleaves amide bonds between bile acids and their conjugates[65,112].Probiotics BSH may therefore hydrolyze conjugated bile acids to liberate free primary bile acids which are less efficiently reabsorbed from the intestinal lumen and excreted in feces[111,112].Such probiotics containing active BSH increase the production of bile salts from cholesterol in their colonized area,thus reducing cholesterol associated problems.SomeLactobacillusspecies such asL.acidophiluspossess protease-sensitive receptors on their cell surface with which they bind tightly to exogenous cholesterol or phosphatidylcholine vesicles and incorporate them into their cell membranes[64,111].Bacteria such asSterolibacterium denitrificansproduce cholesterol dehydrogenase/isomerase which catalyzes the conversion of cholesterol to cholest-4-en-3-one which is converted into coprostanol and excreted in feces[63].Since probiotic cholesterol lowering ability is strain specific[62],strains that exhibit excellent properties still need to be identified.Secondary bile acids have been reported to disrupt DNA repair pathway and cause oxidative stress in epithelial cells[66].This thus calls for more research on how BSH producing probiotics may prevent risks such as sepsis or colon cancer due to the secondary bile salts[67].
6.2.Urogenital and vaginal health
The dominant microflora in a healthy human vagina is a variety ofLactobacillusspecies which play essential roles in protecting women from genital infections.An alteration in the population of lactobacilli can result in microbial imbalance in the vagina,causing a quantitative and qualitative shift from normally occurring lactobacilli to a mixed microflora dominated by anaerobic bacteria such asGardnerellavaginalis,Bacteroides,Prevotella,andMobiluncusspecies[68].Such a condition is termed bacterial vaginosis.Infections that involve urogenital microbial flora imbalance such as yeast vaginitis,candidiasis,bacterial vaginosis,and urinary tract infection can be recurrent[35].Current available antimicrobial treatments can often lead to diarrhea,super infections,depression and even renal failure.Moreover,antimicrobial resistance tends to decrease the effectiveness of this therapy over time.Lactobacilli have been shown to produce biosurfactants and collagen-binding proteins that inhibit pathogen adhesion to cells.This may account for why the vaginal mucosa is dominated by lactobacilli making it less receptive to pathogens[69].Cell to cell communication could also be a mechanism by which probiotics stimulate mucus production which serves as a barrier to pathogens and also signaling the anti-inflammatory cytokine production[70].Falagas et al.[71]observed that lactobacilli can inhibit the growth ofCandidaalbicansand its adherence on the vaginal epithelium in small sample sizes.Presently,the only strains clinically shown to have an effect areLactobacillusrhamnosusGR-1 andLactobacillusreuteri[72],when administered intra-vaginally once weekly or twice daily orally,reduced recurrences of UTI and restored a normal lactobacilli dominated vaginal flora in patients[73].A recent study on the role of probiotics in a woman’s urogenital health confirms that supplemental probiotics containingL.rhamnosusGR-1 andL.reuteripromote the colonization of beneficial microbiota and may help to support overall vaginal health[110].Daily oral intake ofL.rhamnosusandLactobacillusfermentumhave also been shown to modify the vaginal flora[74].Though many properties required by probiotics to confer urogenital protection have been identified,yet evidence of their expressioninvivois scanty.To make this approach successful,proper selection of strains,proof of concept,and efficacy must accompany products used in patients.
6.3.Oral health
The human mouth harbors diverse microbiomes in the human body such as viruses,fungi,protozoa,archaea and bacteria.The bacteria cause two common diseases namely dental caries(tooth decay)and the periodontal(gum)diseases[75].The balance of all these microorganisms can easily be disturbed and a prevalence of pathogenic organisms can lead to different oral health problems such as dental caries,periodontitis,and halitosis[76].Although the evidence for periodontitis is less than dental caries,the use of probiotics to manage the oral microflora appears to be an effective method to control oral conditions[109].
Probiotics marketed for oral health include species ofLactobacillusandBifidobacterium[77].Many studies have shown that they can reduce oral levels of the cariogenic speciesStrepto coccusmutans[78,79,108].StreptococcussalivariusK12 has also been identified to produces bacteriocins against pathogens such asStreptococcuspyogenesandStreptococcuspneumonia,and prevents recurrent pharyngitis,otitis media,and tonsillitis[80,81].In a double-blind,placebo-controlled trial administration ofS.salivariusK12 reduced the occurrence of plaque and reduced levels ofS.mutansin subjects[82].Species such asStreptococcusuberisandStreptococcusoralisalso suppress periodontal pathogens[83].Probiotics was effective on halitosis and prevented the production of volatile sulfur compounds.In addition,Vivekananda et al.[107]observed a reduction in gingivitis and gum bleeding afterL.reuteriadministration.The mechanism by which these probiotics colonize and affect the oral cavity is needed to better understand how they improve oral health.
6.4.Lactose intolerance
Lactose is an important nutrient in all mammalian neonates that almost all have the ability to digest lactose to glucose and galactose for a variable time after birth.In most human populations,lactase activity decreases during mid-childhood(about five years of age),resulting in low levels from that age onwards.However,some people retain high levels of activity throughout adult life.In humans,inheritance of lactase persistent(LP:adults retain ability to digest lactose)is dominant and lactase-non persistent(LNP:adults lose ability to digest lactose)is recessive[104].When milk is ingested and the small intestine fails to produce enough lactase,Lactose intolerance(LI)or lactose malabsorption occurs.Colonic bacteria then metabolize unabsorbed lactose producing hydrogen,methane and short chain fatty acids[106].Lactose intolerance is determined by blood glucose concentrations,and breath hydrogen test following ingestion of a lactose load.Also,a genetic detection of C/T polymorphism at−13,910 upstream of LPH(lactase-phlorizin hydrolase)gene can also be used.Lactose maldigestion may be classified as primary type(hypolactasia)or secondary type.Primary maldigestion is due to an autosomal recessive condition which results in reduced lactase activities in the intestine[104].On the other hand,secondary-type lactose maldigestion is thought to be due to a loss of small intestinal mucosa.Symptoms of lactose intolerance include abdominal pain,bloating,flatulence and diarrhea,but the cause may be multifactorial.In humans,a decline in intestinal lactase cannot be reversed by consuming lactose regular.Lactose ingestion and digestion have several effects on health.People with lactose intolerance may avoid milk consumption and other dairy products,take lactase tablets or take probiotic supplements to manage the condition.Another approach to manage lactose intolerance is to increase the lactose load steadily in one’s diet.This aids the colon to adapt slowly.Since lactase from intestinal brush border is not an inducible enzyme,the reduction in symptoms may be explained by colonic adaptation[102].
7.Future perspectives
From general gut health,to immune support,skin health,cholesterol control,maybe even sensorimotor behaviors,the research thus continues to build.Over the past decade,there has been extensive work in animal models on how probiotics and prebiotics modulate host metabolism.Studies with animal models have shown that the gut microbiota can regulate inflammation,adiposity,satiety,energy expenditure and glucose metabolism.As more knowledge on the mechanisms frominvivoexperiments is unraveling,there is a growing need to translate the results into humans.However,there are very few good,doubleblind,placebo-controlled clinical trials that can prove causality of pro-and prebiotics on modulating human metabolism[62].At present,high-quality human trials have demonstrated the potential for gut microbiota in manipulating and preventing or treating disease such as hypercholesterolemia and obesity[12,85,86].Cholesterol lowering effects of fermented dairy products and encapsulated bile salt hydrolase(BSH)were reported in animal trials,with a reduction of 58% serum cholesterol level in rats by oral feeding of encapsulated BSH[87].Though cholesterol reducing probioticL.reuteriNCIMB 30242(Micropharma,Canada)has been on the market as the first recognized biomaker of disease,in human trials,however,there are mixed outcomes[88,89].This therefore calls for more work to carry out to identify strains with excellent activities as well as their mechanism of action.Lebeer et al.[90]have reported an increase in antiinflammatory activity when lipoteichoic acid is removed from lactobacilli cell walls and this opens a new door to unraveling the mechanism by which probiotics work.Specific bacterial strains can therefore be genetically modified to study their mechanisms of action.Some animal studies have revealed that probiotics produce bioactive compounds which significantly contribute to functionality within the gastrointestinal tract[91].Bifidobacteriumbreve,B.bifidum,B.pseudolongumandLactobacillusconvert linoleic acid(LA)into conjugated linoleic acid,CLA[92,93]which suppresses multistage carcinogenesis at different sites[94].LactobacillushelveticusandBifidobacteriumlongumhave also been reported to produce and respond to mammalian serotonin[95]and affect behavior modulation[96,97].The ability of these bacteria to produce as well as respond to neurochemicals substantiates the potential of probiotics to influence psychological health and general behavior as observed by Hsiao et al.[98]and Tillisch et al.[99].It is therefore probable that modulating the gut microbiota with such biotherapeutics may target stress-related CNS disorders,including stress-induced cognitive deficits[100].However,elucidation of mechanisms and substantiation of animal studies in humans remain essential research goals.
Conflict of interest
The authors declare no conflict of interest.
- 食品科学与人类健康(英文)的其它文章
- Phenolics from grapefruit peels inhibit HMG-CoA reductase and angiotensin-I converting enzyme and show antioxidative properties in endothelial EA.Hy 926 cells
- Detection of Salmonella and several common Salmonella serotypes in food by loop-mediated isothermal amplification method
- Carvacrol attenuates N-nitrosodiethylamine induced liver injury in experimental Wistar rats
- A review:Health promoting lactic acid bacteria in traditional Indonesian fermented foods
- GUIDE FOR AUTHORS

