Carvacrol attenuates N-nitrosodiethylamine induced liver injury in experimental Wistar rats
Balan Rajan,Rajendran Ravikumar,Thandavamoorthy Premkumar,Thiruvengadam Devaki
Department of Biochemistry,Guindy Campus,University of Madras,Chennai 600025,TN,India
Abstract
Keywords:Carvacrol;DEN;Antioxidants;Hepatotoxicity;Liver damage;Marker enzyme
1.Introduction
Liver disease remains a critical health problem globally,induced by viruses,alcohol,lipid peroxidative products and various drugs[1].Hepatic injury is a widespread common pathology sketched by fibrosis that can end up in chronic hepatitis,cirrhosis or cancer[2,3].It has been well seasoned that oxidative stress plays a causative role in the initiation,promotion and progression of hepatic diseases[4,5]and the liver is the main target for several toxic agents that can reduce free radical-mediated apoptosis[6].
N-nitroso alkyl compounds,in particular DEN are an eloquent hepatotoxin,carcinogen and mutagen[7,8].N-nitroso compounds are considered to be a lethal,and they crop up in tobacco products,cheddar cheese,cured and browned meals,occupational surroundings,cosmetics,agricultural toxicants,and pharmaceutical agents[9,10].DEN has been widely used as a precursor in initiating carcinogenesis in experimental animal models[2,7].Activation of DEN,which occurs chiefly in liver microsomes,has been demonstrated to stimulate Kupfer cells,leading to high levels of ROS,capable of damaging liver cells and inducing hepatocarcinogenesis[11].The cellular damage caused by ROS is measured in terms of lipid peroxidation[12].
Essential oils and their constituents have been favorably revered for their pharmaceutical actions.Plant-derived substances have in recent past turns out to be of great interest owing to their multifaceted pharmacological activities[13].Carvacrol(2-methyl-5-(1-methylethyl)-phenol)(Fig.1)is one such monoterpene,a natural isopropyl hydroxytoluene known to predominantly occurs in essential oil of aromatic plants includingOriganumVulgare(Oregano)andThymusvulgari(Thyme),which has been assessed for substantial pharmacological properties[14].Monoterpenoids explicit divergent exceptional biological effects like anti-oxidative,anti-inflammatory,and have preventive and therapeutic effects against many diseases[15].Drugs and/or essential oils containing Carvacrol have been extensively used in traditional medicine,and a huge number of feed additives based on this molecule are at present commercially obtainable[16].In addition,recent experimental data indicated that Carvacrol induces anxiolytic-like effect and antidepressant-like properties in tasks for anxiety and depression behavior in mice[17].It is prominent that essential oils,which are prolific in Carvacrol,bears rugged antioxidant character identical to those of ascorbic acid,butyl hydroxytoluene(BHT),and vitamin E[18]and can,truncate the risk of several degenerative disorders[19].Carvacrol also has a dynamic anti-microbial,anti-fungal,and anti-inflammatory effect[20].
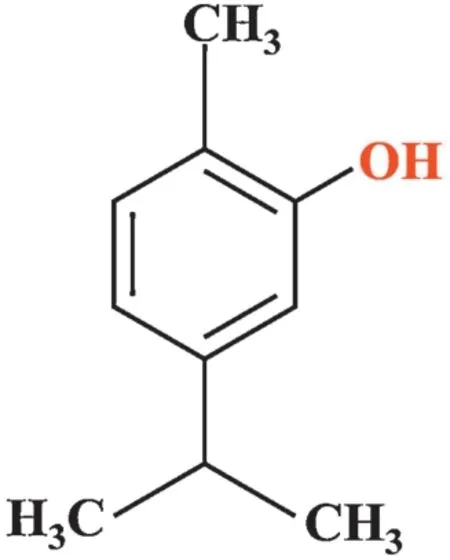
Fig.1.Structure of Carvacrol.
The present study was to embark upon the protective effect of Carvacrol against DEN-induced alterations on LPO and antioxidant enzyme activities in rats during liver damage.Further serum marker enzymes were also analyzed.Here,we report that Carvacrol partly attenuates DEN-induced liver injury in rats via induction of antioxidant defense system.
2.Materials and methods
2.1.Source of chemicals
DEN and Carvacrol were purchased from Sigma Chemicals Co.(St.Louis,MO,USA).All other chemicals used were of analytical grade obtained from SRL/HIMEDIA,India.
2.2.Animals
Male,Wistar strain albino rats weighing about 170–180 g were obtained.The animals were housed in cages under proper environmental conditions(26±2℃)and were fed with commercial pelleted diet(M/S Hindustan Foods Ltd,Bangalore,India).The animals had free access to water.All the experiments were designed and conducted according to the ethical norms approved by Institutional Animal Ethics Committee guidelines(IAEC No.04/02/2012).
2.3.Experimental design (Fig.2)
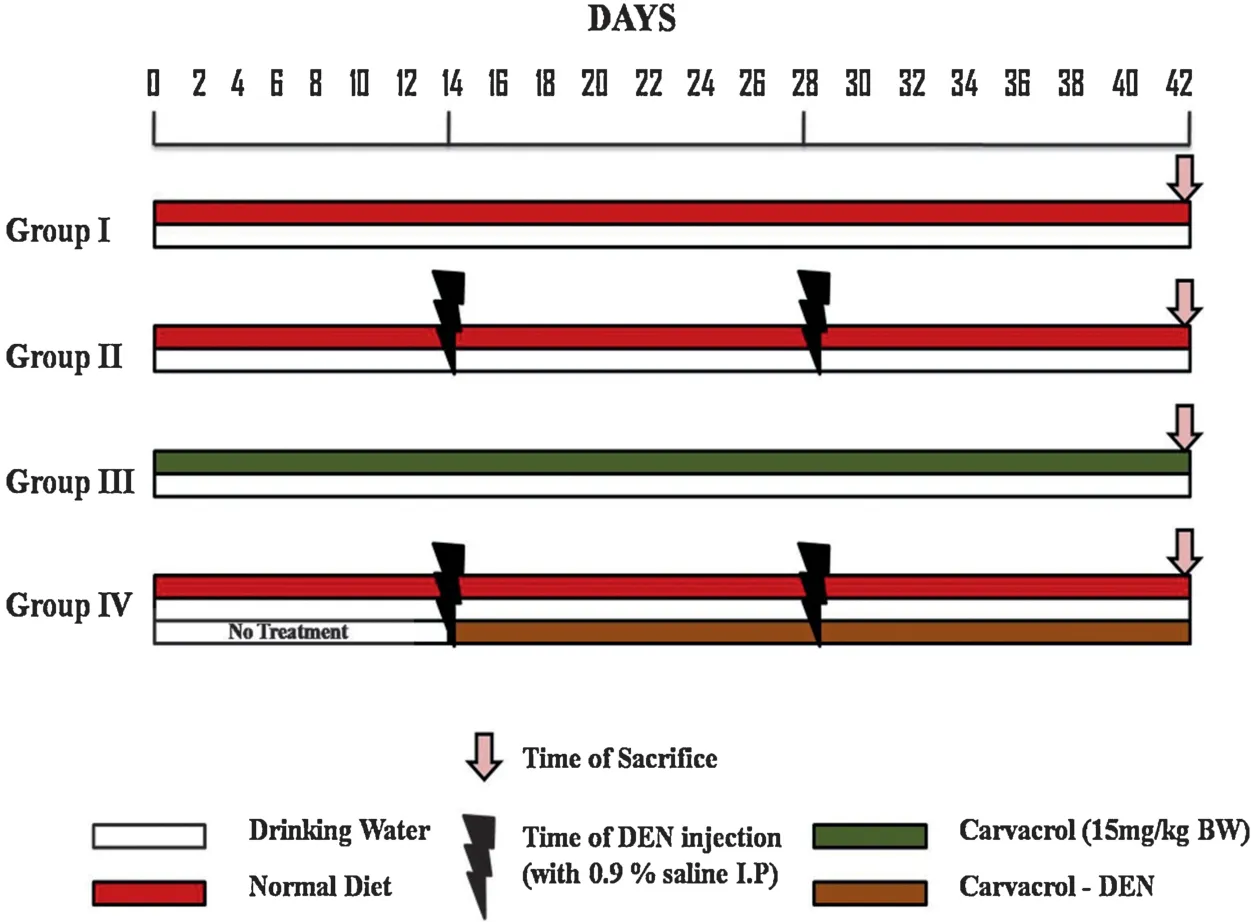
Fig.2.Schematic representation of the experimental design used for the study.
Group INormal control rats,provided with standard diet and drinking water.
Group IIRats received drinking water for the first 13 days,and on day 14 and 28 a single dose of DEN(200 mg/kg body weight,intraperitoneally using saline)was given[21]and maintained for 42 days.
Group IIIRats were treated with Carvacrol alone by orally,alternative days in a week at a dose of 15 mg/kg body weight(based on effective dose fixation studies)for 4 weeks,serve as drug control[22].
Group IVRats were treated with Carvacrol(15 mg/kg body weight)on alternative days to the DEN induced group of rats from day 14 till end of the experimental period.
At the end of the experimental period,the animals were fasted overnight and anesthetized with Xylazine(10 mg/kg)and Ketamine(90 mg/kg)and sacrificed by cervical decapitation.The blood was collected and allowed to coagulate at ambient temperature for 30 min.Serum was separated by centrifugation at 3000 r/min for 15 min at 4℃.The liver was immediately separated and washed in ice-cold saline.The tissues were sliced and homogenized in 0.1 mol/L Tris–HCl buffer(pH 7.4).The homogenates were centrifuged at 1000 r/min for 10 min at 4℃ in a cooling centrifuge.The supernatants were separated and used for the determination of various parameters.
2.4.Biochemical parameters
2.4.1.Fixationofoptimumdosageschedule
The suitable optimum dosage of Carvacrol was found by treating various doses of Carvacrol(10 mg/kg,15 mg/kg,30 mg/kg and 45 mg/kg body weight of rat)orally to the DEN induced group of rats.Carvacrol dose for a maximum efficacy in the minimum dose was determined as elicited by the levels of serum marker enzymes for tissue damage.At the end of experimental period,the animals were sacrificed and the serum separated was used to assess the biochemical changes.The dosage that gives the maximum protective efficacy(as elicited by the activities of AST,ALT,LDH and GGT in the serum)was fixed up as the optimum dosage for the drug.It was found that 15 mg/kg body weight of Carvacrol had the maximum protective efficacy in the minimum dosage.This dose of Carvacrol was followed for all the experimental studies(Fig.3).
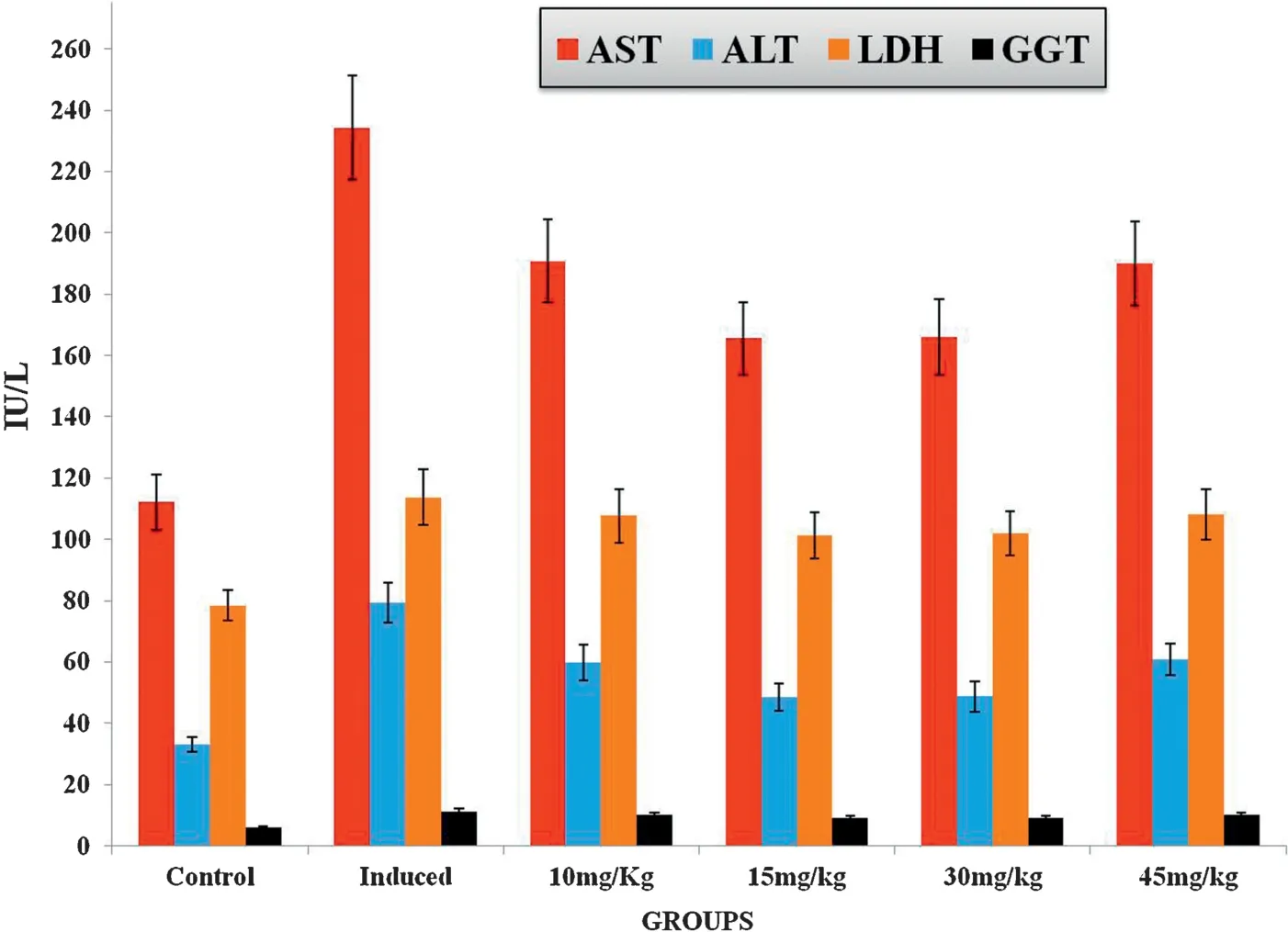
Fig.3.Effect of different dose of Carvacrol on control and DEN induced experimental groups of rats.Results are expressed as mean±S.D.for six rats in each group.
2.4.2.Estimationofmarkerenzymes
Activities of aspartate transaminases(AST)[23],alanine transaminases(ALT)[23],lactate dehydrogenase(LDH)[24],alkaline phosphatase(ALP)[25],5-Nucleotidase(5NT)[26]and-glutamyl transpeptidase(GGT)[27]were estimated in serum samples.
2.4.3.Estimationofmacromoleculardamages
2.4.3.1.Assayoflipidperoxidation(LPO).Malondialdehyde(MDA)level,the main product of lipid peroxidation was determined as described by Ohkawa et al.,1979[28].MDA is defined as nmol per mg of protein.
2.4.3.2.Estimationofenzymicandnon-enzymicantioxidants.Enzymatic antioxidant such as superoxide dismutase(SOD)[29],catalase(CAT)[30],glutathione peroxidase(Gpx)[31],glutathione reductase(GR)and Non-enzymic antioxidant such as glucose-6-phosphate dehydrogenase(G6PD)[32],vitamin C(VIT C)[33],vitamin E(VIT E)[34],vitamin A(VIT A)[35]and glutathione(GSH)[36]content were assayed in tissue homogenate.
2.4.3.3.EstimationofPhaseIandPhaseIIdrugmetabolizingenzymes.Phase I drug metabolizing enzymes such as Cytochrome P450(CYTP 450)[37],Cytochrome b5(CYTB5)[37],Cytochrome P450 reductase(CYTP 450 RED)[37],Cytochrome b5reductase(CYTB5 RED)[38]and Phase II drug metabolizing enzymes like glutathione S-Transferase(GST)[39]and UDP Glucuronyl Transferase(UDP-GT)[40]were also analyzed in tissue homogenate.
2.5.Histological evaluation
Histological evaluation was performed on a portion of the liver tissue after fixation with 10% formalin,embedded in paraffin wax,sectioned were stained with hematoxylin and eosin to assess the pathological changes.
2.6.Statistical analysis
The results were computed statistically(SPSS/10 Software Package;SPSS Inc.,Chicago,IL,USA)using one-way ANOVA.Post hoc testing was performed for inter comparisons using the LSD.In all tests,the level of statistical significance was set atp<0.05.
3.Results
3.1.Effect of Carvacrol on food consumption,body weight,liver weight and relative liver weight
The hepatoprotective effect of Carvacrol against DENinduced liver injury was elucidated in male Wistar albino rats.Fig.4 shows the food consumption of normal and induced rats,there is no notable decline in the food intake in induced rats when compared to normal rats.Figs.5 and 6 show Initial body weight,Final body weight,Liver weight and relative liver weight(g/100 g body weight)of control and experimental group of animals.In DEN-induced group 2 animals,there is a significant decrease in the final body weight and significant increase in liver weight when compared with group 1 control animals.The Carvacrol treated group 4 showed a significant increase in the final body weight when compared with group 2 animals.
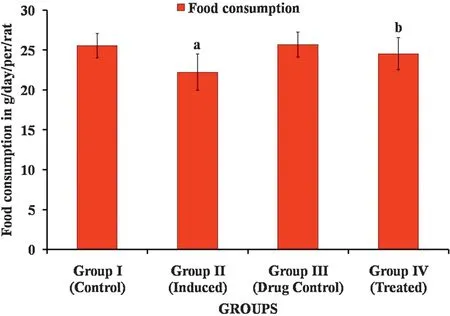
Fig.4.Effect of Carvacrol on food consumption of control and experimental groups of rats.Results are expressed as mean±S.D.for six rats in each group.Statistical significance at p <0.05 compared with a Compared with group 1 and b Compared with group 2.
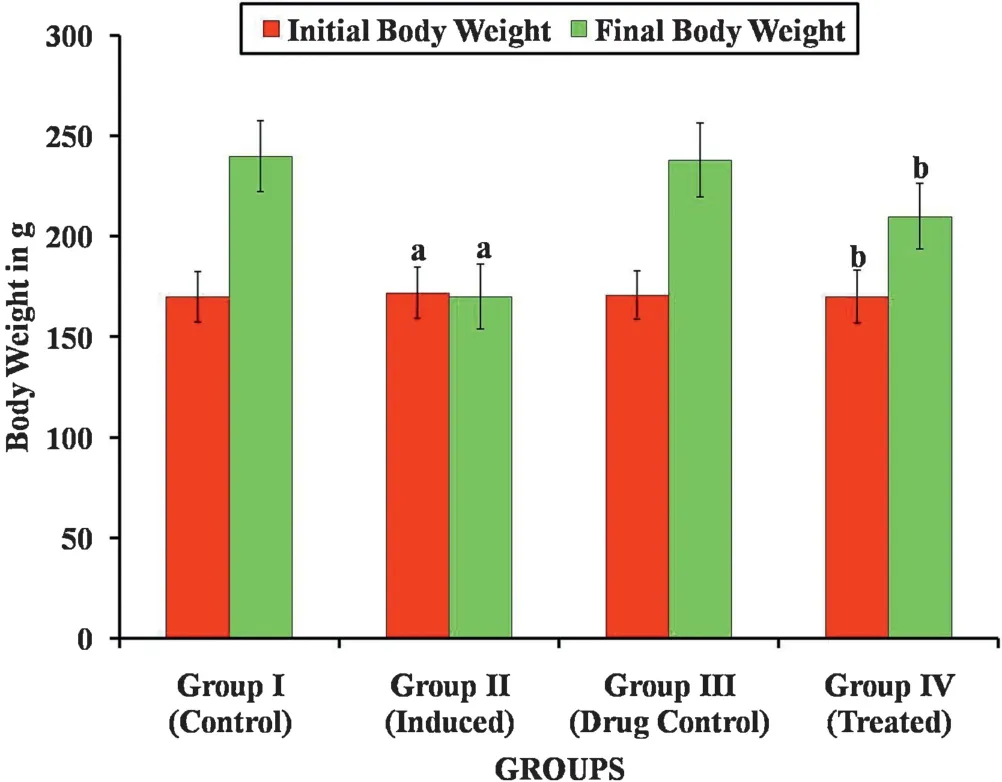
Fig.5.Effect of Carvacrol on Initial body weight and final body weight of control and experimental groups of rats.Results are expressed as mean±S.D.for six rats in each group.Statistical significance at p <0.05 compared with a Compared with group 1 and b Compared with group 2.
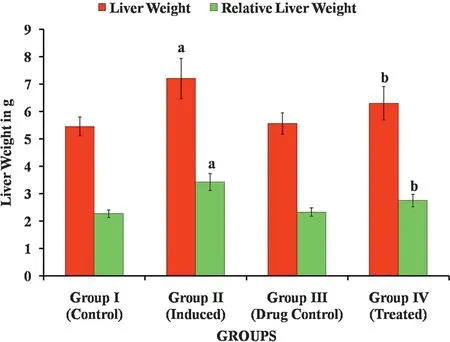
Fig.6.Effect of Carvacrol on Liver weight and Relative weight of control and experimental groups of rats.Results are expressed as mean±S.D.for six rats in each group.Statistical significance at p <0.05 compared with a Compared with group 1 and b Compared with group 2.
During the course of the experiment,all rats showed the greater tolerance to treatment with Carvacrol.In group 2 animals,the relative liver weight significantly increased when compared with group 1 animals and there is a significant decrease in the liver weight in Carvacrol treated groups 4 animals when compared with group 2 animals.No obvious changes were observed between the control and Carvacrol alone treated group which is an indicative of nontoxic nature of Carvacrol.
3.2.Carvacrol attenuates lipid peroxidation
Fig.7 shows the level of LPO in the serum and liver of control and experimental groups of animals which was analyzed for oxidative stress.In DEN-induced group 2 animals,there is a significant increase in the levels of lipid peroxides when compared with group 1 normal control animals.Whereas in Carvacrol treated groups 4 animals,there is a significant decrease in the levels of lipid peroxides when compared with group 2 induced animals.However,animals treated with Carvacrol alone(group 3)did not show any significant changes when compared with group 1 control animals.
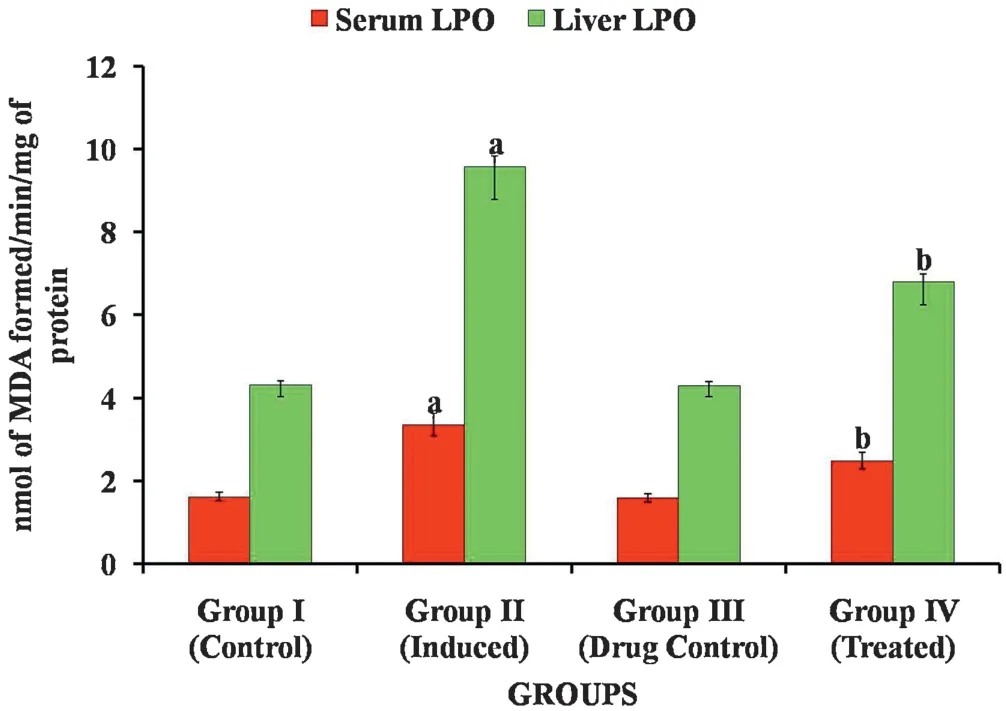
Fig.7.Effect of Carvacrol on Lipid Peroxidation in control and experimental groups of rats.Results are expressed as mean±S.D.for six rats in each group.Statistical significance at p <0.05 compared with a Compared with group 1 and b Compared with group 2.
3.3.Effect of Carvacrol on the activities of marker enzymes
Tables 1 and 2 portray the effect of Carvacrol on the activities of marker enzymes AST,ALT,ALP,LDH,GGT and 5NT in the serum and liver of control and experimental group of rats.These marker enzymes are significantly(p<0.05)increased in DENinduced group 2 animals when compared with group 1 normal control animals.Carvacrol-treated groups 4 showed a significant decrease in the activities of these enzymes when compared with group 2 DEN-induced animals.This may prove that Carvacrol has better restoration potential of liver tissue.
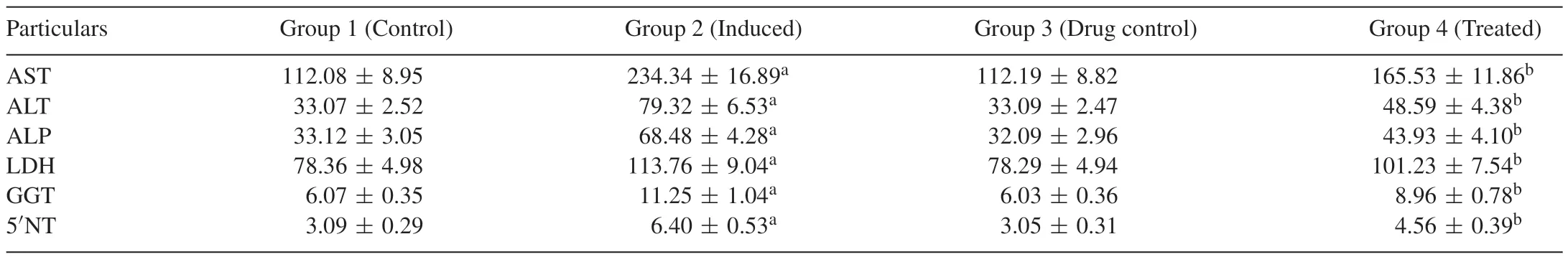
Table 1Effect of Carvacrol on the activities of marker enzymes in the serum of control and experimental groups of rats.
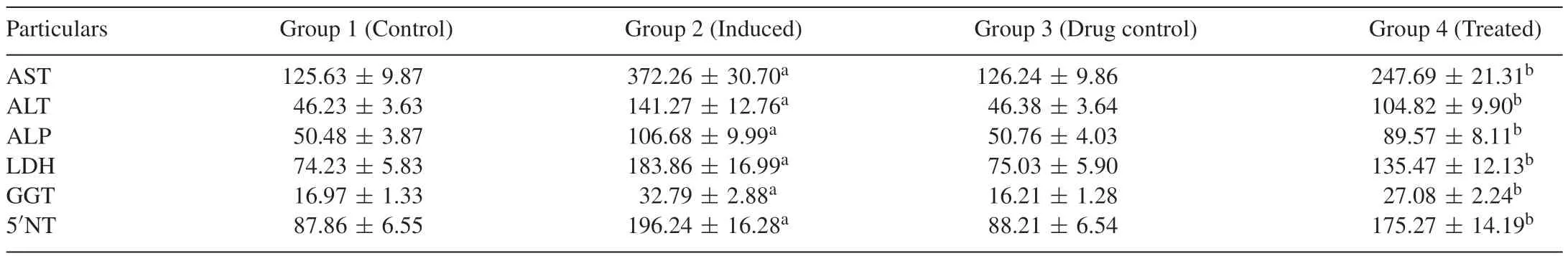
Table 2Effect of Carvacrol on the activities of marker enzymes in the liver of control and experimental groups of rats.
3.4.Evaluation of antioxidant status in liver
Table 3 depicts the antioxidant status in the liver of control and experimental group of animals.DEN-induced group 2 animals exhibited a significant decrease in the activities of SOD and CAT when compared with group 1 normal control animals,Carvacrol-treated groups 4 showed a significant increase in the activities of SOD and CAT when compared with group 2 DEN-induced animals.The activities of GPx,GR,GSH,G6PD,VIT A,VIT C and VIT E also significantly decreased in DEN induced group 2 animals when compared with group 1 control animals.In Carvacrol-treated groups 4 animals,there is a significant increase in the activities of GPx,GR,GSH,G6PD,VIT A,VIT C and VIT E when compared with group 2 DEN-induced animals.No adverse effect was observed in group 3 animals.This clearly proves the antioxidant capacity of Carvacrol due to its free radical encountering activity.
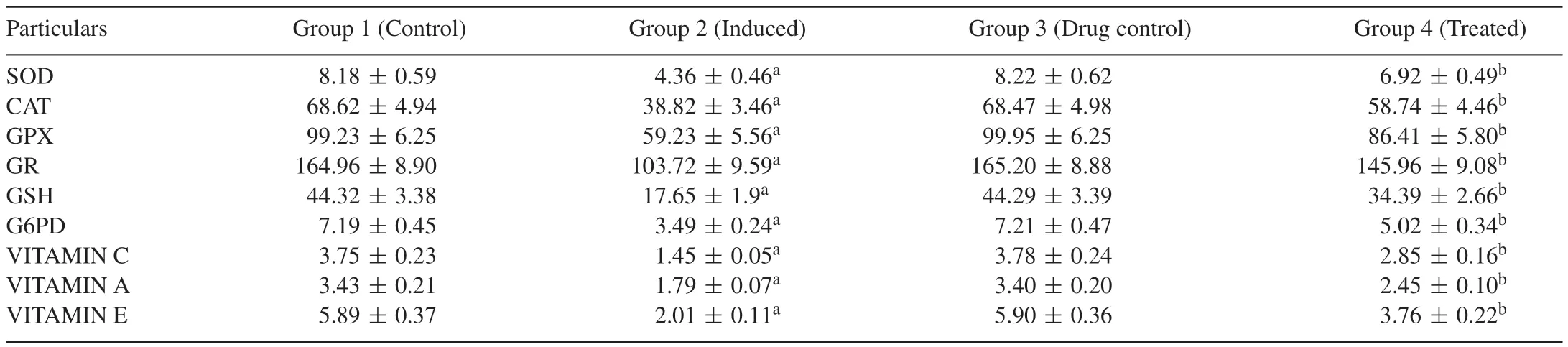
Table 3Effect of Carvacrol on the activities of antioxidant enzymes in the liver of control and experimental groups of rats.
3.5.Evaluation of Phase I and Phase II drug metabolizing enzymes
Table 4 portrays the effect of Carvacrol on the activities of Phase I and Phase II drug metabolizing enzymes in the liver of control and experimental group of rats.CYTP450,CYTB5,CYTP450 REDUCTASE,CYTB5 REDUCTASE enzymes are significantly(p<0.05)increased in DEN-induced group 2 animals when compared with group 1 normal control animals.Carvacrol-treated groups 4 showed a significant decrease in the activities of these enzymes when compared with group 2 DEN-induced animals.Whereas GST and UDP-GT enzymes are significantly(p<0.05)decreased in DEN-induced group 2 animals when compared with group 1 normal control animals.Upon Carvacrol treatment groups 4 showed a significant increase in the activities of these enzymes.This clearly reveals that Carvacrol has maximum renovation potential of liver tissue.
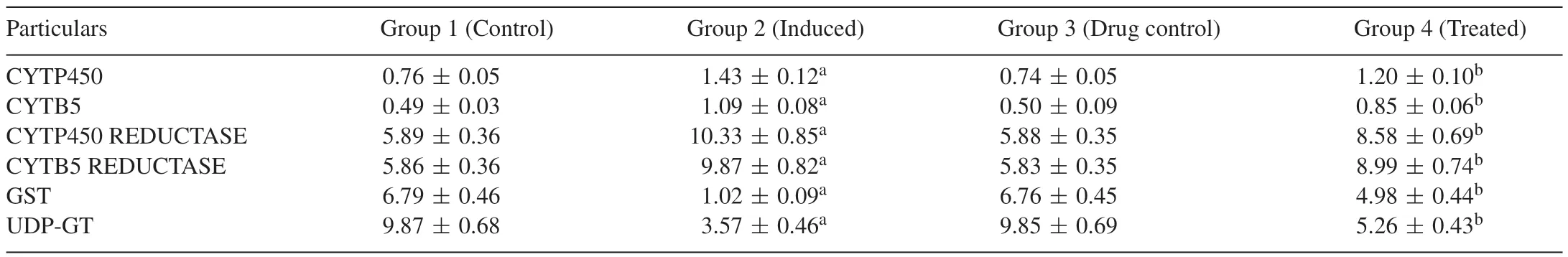
Table 4Effect of Carvacrol on the activities of Phase I and Phase II drug metabolizing enzymes in the liver of control and experimental groups of rats.
3.6.Effect of Carvacrol on histological features of liver
The histology of the liver tissue was examined under a light microscope.Group 1 control animals(Fig.8a)revealed the normal architecture of the liver cells with granulated cytoplasm and small uniform nuclei,and group 3 animals(drug control;Fig.8c)also showed the normal histological appearance of liver cells indicating the nontoxic nature of Carvacrol.The group 2 animals induced with DEN(Fig.8b)depicted the area of hepatocellular necrosis with the adjacent liver cells exhibiting dysplastic changes,and the animals treated with Carvacrol(Fig.8d)showed no necrosis with a decrease in hepatic damage.
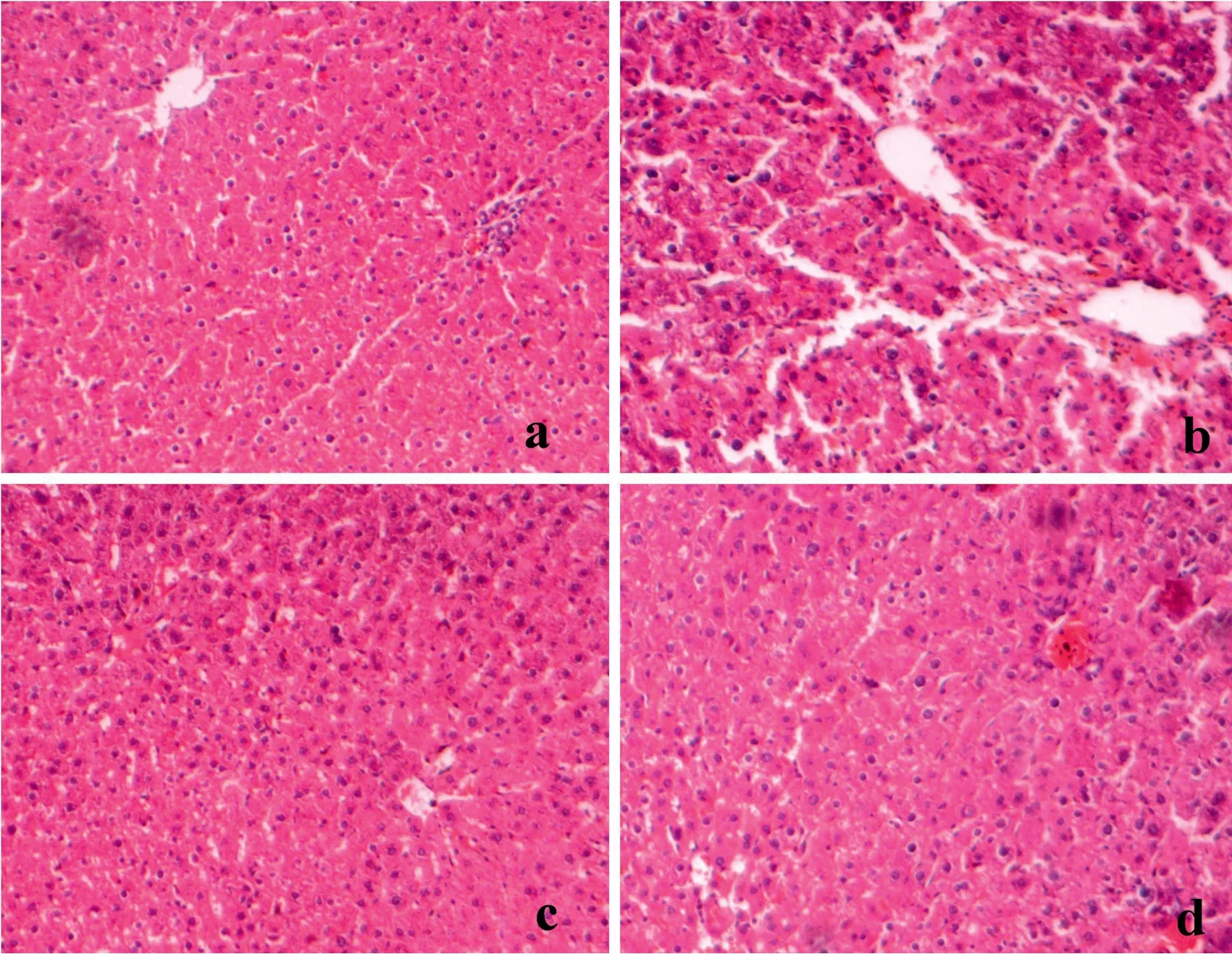
Fig.8.Photomicrographs obtained from(a)Control group,(b)DEN group,(c)Carvacrol alone group and(d)DEN+Carvacrol group.
4.Discussion
DEN has been reported to induce a relentless generation of free radicals in the liver,which in turn augment the demand of antioxidant enzymes.Subsequently,it ends in oxidative stress and initiation of carcinogenesis.Single dose of DEN injection on 14th and 28th day reduced body weight and noticeably increased the ratio of liver to overall body weight in comparison with normal rat.Depletion in food intake and therefore,the reduction of body weight gain noticed in DEN-treated animals,could be predominantly due to loss from skeletal muscle and adipose tissue as already shown,and it could be considered as an ancillary indication of the diminishing hepatic function following exposure to DEN.In addition,evaluation of the liver/body weights ratio was used to scrutinize potential changes in the liver size,but no changes were found.In the present investigation,the hoisted levels of marker enzyme in serum and liver are determinations of cellular damage and loss of functional integrity of the cell membrane due to DEN administration.Treatment with Carvacrol(15 mg/kg)brought back these enzymes to near routine level by possibly preserving the functional integrity of the hepatocytes,showing its defense action against DEN induced Hepatotoxicity.It was already reported that,the dosage of 25 mg/kg bodyweight of Carvacrol pretreatment resulted in the protection against thioacetamide-induced liver toxicity by attenuation of oxidative stress,inflammation,and apoptosis[41].Though,previous studies by Nafees et al.,showed 25 mg/kg body weight of Carvacrol as a maximum protective dose,our studies suggesting that even 30 mg/kg body weight of Carvacrol had similar protective effective as like in 15 mg/kg bodyweight of Carvacrol(based on dosage fixation study in figure).So we fixed up a dose of 15 mg/kg body weight of Carvacrol as a maximum efficacy with minimum dosage and we used this concentration as standard concentration for further experimental studies.GR plays a key role in cellular defense against oxidative stress by arresting accumulation of GSSG and thus maintaining the redox state.The decreased level of GR activity after DEN administration possibly reflects conformity to oxidative conditions.The reduction in the specific activities of antioxidant enzymes and drug metabolizing enzyme,after DEN administration may be associated to their inhibition by DEN through immediate interaction with the enzyme molecules or modification of the post-transcriptional and post-translational steps in the enzyme synthesis.DEN,an indirect acting carcinogen that requires metabolic activation to yield an ultimate carcinogen.It is widely accepted that metabolic activation of nitrosamines by Cytochrome P450 enzymes to reactive electrophiles is required for their cytotoxic,mutagenic and carcinogenic activity.It has been reported that DEN after its metabolic biotransformation promutagenic adducts that may initiate liver carcinogenesis.Carvacrol treated animals showed a significant decrease in the activities of these enzymes when compared with group 2 DEN-induced animals.Detoxification of activated procarcinogen and thereby protection against carcinogenic injury occurs in the presence of Phase II drug metabolizing enzymes.One of the important pathways is GSH conjugation catalyzed by GST that is involved in the removal of the proximate and ultimate carcinogens through formation of more water soluble and non electrophilic detoxification products.GST and UDP-GT enzymes are significantly decreased in liver injured animals when compared with normal control animals,this might be resulted because of enhanced covalent binding of DEN metabolites to cellular DNA and thereby carcinogenesis.Upon Carvacrol treatment liver injured rats showed a significant increase in the activities of these enzymes.A significant DEN-induced hepatotoxicity hoisted levels of Serum AST and ALT and a fall in their levels after 30 days.GGT is one of the important marker of liver injury,and their elevation disclose cholestasis and bile duct necrosis.As DEN causes liver injury,there was a significant increase in the activities of these enzymes in DEN-induced(group 2)animals when compared to control rats(Tables 1 and 2).On the other hand,the activities of these marker enzymes were significantly decreased in rats treated with Carvacrol(group 4)when compared to group 2 animals.Administration of DEN has been described to generate more LPO products like Malondialdehyde.Such discovery corroborate with our results as we had seen the elevated level of LPO in DEN-treated rats(Fig.7),which was significantly lowered on Carvacrol treatment.Natural antioxidants are capable of preventing ROS production,and thereby,it detracts the intracellular oxidative stress.GSH is an important naturally occurring cellular non-enzymatic antioxidant and is needed to maintain the normal reduced state of the cells and to counteract ROS thereby reducing the oxidative stress.GSH depletion ultimately stimulates oxidative stress,with a cascade of effects so that influencing functional and structural coherence of cell and organelle membrane.The Carvacrol treated rats administered with DEN showed significant raise in the levels of hepatic GSH when compared to DEN-treated animals(Table 3).This increase in GSH levels could have suppressed DEN-induced LPO.The decrease of GSH levels and GPx and catalase activities indicates the extremity of the oxidative stress-induced during the exposure to DEN.Interestingly,Carvacrol counteracted the hepatic oxidative damage by preventing the reduction of these parameters provoked by DEN.Enhancement of liver LDH activity,a notable marker of hepatocytes injury,indicates a non-detailed alteration in the plasma membrane integrity and permeability and/or may be due to its overproduction by tumor cells[10].From our observations,it can be concluded that Carvacrol may counteract the oxidative stress induced by DEN in rats by preventing LPO through scavenging of free radicals or by enhancing the activity of antioxidants,which could detoxify free radicals.Thus,the present study highlights the protective effect of Carvacrol on N-Nitrosodiethylamine induced oxidative stress,which may be involved in the free radical scavenging mechanism.
5.Conclusion
The present study from our findings clearly demonstrated that oral administration of Carvacrol exerts significant protective effects against DEN-induced oxidative stress and liver damage by augmenting host antioxidant defense mechanisms.This may be due to the prevention of chemical and drug-induced toxicity through enhancing the antioxidative and drug-metabolizing enzymes,as well as lowering the extent of lipid peroxidation.Hence,Carvacrol may very well be considered as an effective chemopreventive option for DEN induced Hepatotoxicity.
Acknowledgements
The authors,Rajan Balan and Premkumar Thandavamoorthy is thankful to the Indian Council of Medical Research(ICMR),Grant no:45/38/2013 and Grant no:3/2/2/221/13,New Delhi,Government of India for the financial support in the form of Senior Research Fellowship(SRF).
- 食品科学与人类健康(英文)的其它文章
- Phenolics from grapefruit peels inhibit HMG-CoA reductase and angiotensin-I converting enzyme and show antioxidative properties in endothelial EA.Hy 926 cells
- Detection of Salmonella and several common Salmonella serotypes in food by loop-mediated isothermal amplification method
- New perspectives on probiotics in health and disease
- A review:Health promoting lactic acid bacteria in traditional Indonesian fermented foods
- GUIDE FOR AUTHORS

