过表达肝细胞核因子4α脐带间充质干细胞促进小鼠大部肝切后肝再生
吴 宁,马 永,翟秀宇,张玉玲,张 勇,张祺祺,杭化莲,夏 强,卞建民*
过表达肝细胞核因子4α脐带间充质干细胞促进小鼠大部肝切后肝再生
吴 宁1,马 永1,翟秀宇2,张玉玲3,张 勇1,张祺祺4,杭化莲4,夏 强4,卞建民1*
(1南京医科大学附属南京医院普外科,南京 210006;2吉林大学第一医院泌尿外二科,吉林 130021;3遵义医学院附属医院贵州省细胞工程重点实验室,遵义 563003;4上海交通大学医学院附属仁济医院肝脏外科,上海 200127)
探讨人源性脐带间充质干细胞(UC-MSC)以及过表达肝细胞核因子4α(HNF4α)的UC-MSC能否促进小鼠大部肝切后肝再生。体外分离、培养、鉴定人UC-MSC,慢病毒转染过表达HNF4α。体外收集细胞培养上清液,将L02与上清液共培养,通过细胞增殖实验试剂盒(CCK8)检测细胞增殖活性。体内实验建立肝大部切除模型(约70%),分别经尾静脉向肝切除小鼠移植0.9%生理盐水(NS)、MSC、MSC-HNF4α。48h后比较3组肝切后肝再生的变化,通过免疫组化来观察肝标本Ki67的表达。成功分离鉴定UC-MSC,并且成功建立稳定过表达HNF4α的MSC。体外实验MSC组和MSC-HNF4α组中L02的增殖能力都明显高于NS组(<0.01),MSC组高于MSC-HNF4α组(<0.05)。同样体内实验相对于NS组,经MSC或MSC-HNF4α细胞处理的肝脏,其Ki67的表达明显高于NS组(<0.01),同样MSC组高于MSC-HNF4α组(<0.05)。UC-MSC和过表达HNF4α的MSC都分泌一系列因子促进肝再生。
小鼠;肝再生;间充质干细胞;肝细胞核因子4α
肝脏作为机体重要的代谢器官,在受到各种因素的损伤后表现出极强的自我修复能力。虽然肝细胞属于终末分化细胞,生理条件下主要处于静息状态,但一旦肝受到损伤后便会通过各种途径对受损肝进行代偿修复,在短期恢复原来大小,以满足机体需求[1]。然而很多严重肝损伤及肝衰竭患者,肝再生能力无法满足机体需求,既不耐受肝切除手术,也因为不能获得及时进行原位肝移植的机会从而错失治疗时机。
间充质干细胞(mesenchymal stem cells,MSC)作为一种多能干细胞,具备强大的增殖能力、多向分化能力以及免疫调节能力,已获广泛认可。目前,很多学者将目光投向采用MSC治疗各种类型的肝脏疾病,结果证明MSC能够改善多种肝脏疾病[2−4]。MSC移植治疗丙肝性肝硬变等在临床试验中也均取得了较好的疗效[5]。还有研究发现将MSC在体外通过人工诱导成为“肝样细胞”后移植于患者,同样具有改善肝功能不全的效应,且这种肝样细胞聚集到肝的能力强于MSC[6−8]。
肝细胞核因子4α(hepatocyte nuclear factor 4α,HNF4α),作为细胞核受体超家族成员,是肝细胞分化和功能维持最为关键的转录因子[9]。我们前期研究发现,在同样诱导条件下将HNF4α过表达于MSC后发现其诱导成为肝样细胞的效果明显强于对照组[10]。
本次试验主要探讨MSC是否能够促进大部肝切后肝再生,以及过表达HNF4α的MSC对肝再生的效果。
1 材料与方法
1.1 材料
足月剖宫产脐带(上海交通大学医学院附属仁济医院妇产科);改良型RPMI1640培养基、磷酸盐缓冲液(phosphate buffered saline,PBS)(HyClone,美国);DMEM/F12培养基、胎牛血清(fetal bovine serum,FBS),脂肪诱导液、成骨诱导液、软骨诱导液(Gibco,美国);碱性成纤维细胞生长因子(basic fibroblast growth factor,bFGF)(Invitrogen,美国);HNF4α过表达慢病毒(吉凯基因,中国);流式抗体、免疫组化抗体(BD,美国);细胞增殖实验试剂盒(cell counting kit-8,CCK-8)(同仁,日本);6周龄BALB/c小鼠(上海斯莱克,中国)。仪器包括高速台式冷冻离心机(Eppendorf,德国),酶标仪(Biotek,美国),FACS Calibur流式细胞仪(BD,美国),免疫共聚焦(莱卡,德国)。
1.2 实验方法
1.2.1 脐带间充质干细胞(umbilical cord mesenchymal stem cells,UC-MSC)的分离培养 在征得家属同意签署知情同意书并且经医院伦理委员会同意后取健康足月剖宫产胎儿脐带,冲洗脐带中残留血液,剥离脐带外层羊膜以及内部2根动脉1根静脉,将脐带剪碎成肉糜状(1mm×1mm×1mm的组织块),培养皿中贴壁1h后加入适量含10%FBS、1μg/L bFGF的DMEM/F12完全培养基,5%CO2、37℃培养箱中培养,7d后半量换液,待MSC贴壁后移除组织块,观察细胞生长情况,至细胞融合度约达80%,0.25%胰酶消化后按1∶3比例传代培养。
1.2.2 UC-MSC流式鉴定和分化鉴定 取第3代融合度80%UC-MSC,消化离心后PBS洗涤2次,计数每流式管中加入细胞2×105/ml,200μl PBS重悬细胞,按抗体使用说明书中的量加入流式直标抗体各10μl CD90、CD105、CD29、CD31、CD34、HLA-DR,4℃暗室孵化30min,PBS离心洗涤2次,流式固定液固定后上机检测,数据采用Flowjo7.6分析。取第3代UC-MSC接种于预置盖玻片的6孔板中,分别添加不含血清脂肪诱导液、成骨诱导液和软骨诱导液诱导培养,1周换液2次,持续3周。分别将诱导后的细胞通过油红染色、茜红染色和检测Ⅱ型胶原表达的方式检测诱导效果。
1.2.3 构建人HNF4α慢病毒载体 设计HNF4α全长引物,上游5′-GAGGATCCCCGGGTACCGGT- CGCCACCATGCGACTCTCCAAAACCCTC-3',下游5′-TCCTTGTAGTCCATACCGATAACTTCCTGCTT-GGTGATG-3′。提取原代肝细胞总RNA进行RT-PCR,反应条件为94℃预变性2min,94℃解链30s,55℃退火1min,72℃延伸1min,循环33次,72℃总延伸10min,4℃终止反应。扩增产物在琼脂糖凝胶上电泳,采用琼脂糖凝胶DNA回收试剂盒回收并纯化目的基因片段。将纯化的目的基因片段交由公司测序验证,构建慢病毒载体。所构建载体包括含HNF4α过表达载体,以及不含HNF4α的对照载体,上述慢病毒均自带绿色免疫荧光蛋白(green fluorescent protein,GFP)基因。
1.2.4 转染HNF4α 将第3代MSC接种于6孔板,确保次日贴壁后融合度约为30%。次日换液,将1×108TU慢病毒(MOI=50)加于2ml含5mg/L聚凝胺(Polybrene)的感染增强液(enhanced infection solution)中,混匀用于培养细胞。8h后弃上清转染液,PBS清洗3遍,加10%FBS培养液2ml,常规培养,2d换液,5d后共聚焦观察荧光以检测病毒转染效率。
1.2.5 收集上清检测分泌蛋白 将MSC和MSC-HNF4α分别接种于25cm2培养瓶,待其融合度约达70%,PBS清洗3遍,换不含血清DMEM/F12培养基6ml,常规培养48h后收集上清。两组分别3个不同批次各取上清2ml,送至上海康成生物工程有限公司采用RayBio™人L系列抗体芯片Ⅰ(507)检测上清液中各种因子表达情况,以及比较两组间分泌蛋白表达差异。其余上清-80℃长期保存,待用。
1.2.6 CCK-8检测细胞增殖活性 本次实验体外采用L02肝细胞系作为普通肝脏细胞,消化离心对数生长状态L02细胞,含10% FBS的RPMI1640完全培养基重悬计数使其密度为1×105/ml,100μl接种至96孔板,过夜待其完全贴壁,吸去培养基。设3组(=5):对照组,MSC组和MSC-HNF4α组分别添加DMEM/F12,MSC和MSC-HNF4α上清各75μl,最终加RPMI1640完全培养液使每孔补足100μl,5%CO2、37℃培养箱中培养24h。每孔加10μl CCK-8溶液,孵箱内继续孵化1.5h后用450nm波长酶标仪分别检测OD值。
1.2.7 小鼠肝切模型建立和检测 8周龄,体质量约20g雄性BALB/c小鼠18只。术前2h,随机分3组(=6),分别注射生理盐水(normal saline, NS)、5×106/ml MSC、5×106/ml MSC-HNF4α 200μl。小鼠肝切术参照姚菊芳小鼠大部肝切术的改进,60mg/kg戊巴比妥麻醉,仰卧位固定,于剑突下腹中线剪开约2cm长切口,打开腹腔。显微镜下,分别结扎左外叶及中叶(约70%肝体积)血管,并切除,观察创面无渗血后缝合关闭腹腔。48h后统一活杀小鼠,下腔静脉采血1ml做丙氨酸氨基转移酶(alanine transaminase,ALT)、天门冬氨酸氨基转移酶(aspartate transaminase,AST)和白蛋白(albumin,ALB)检测,取剩余部分肝4%多聚甲醛固定,其余肝组织立即投入液氮长期保存。石蜡包埋固定24h肝组织,切片,常规HE染色观察肝组织病理变化。按照免疫组化说明书检测肝组织中增殖细胞核抗原Ki67表达,判定标准:以细胞核呈界限清楚的棕色反应为阳性,每张切片随机观察5个高倍视野(×400倍),对其中的表达阳性细胞进行计数。
1.3 统计学处理
采用SPSS19.0统计软件进行统计分析。数据均用均数±标准差表示,指标比较采用单因素方差分析,两组间比较采用检验。<0.05表示差异有统计学意义。
2 结 果
2.1 MSC的形态与鉴定
组织块贴壁培养2周后可见从组织块边缘游离出部分MSC,移除组织块后继续培养1周使细胞融合度约达80%传代,传代后细胞生长速度加快,排列规则,形态大多呈梭形、多边形及不规则形(图1A)。在脂肪、成骨、软骨诱导液中诱导3周后的MSC形态明显发生改变,多趋向扁平状,油红染色、茜红染色和Ⅱ型胶原表达阳性(图1B,1C,1D)。细胞流式鉴定发现细胞高表达间充质细胞相关抗原CD29、CD90、CD105,而低表达造血细胞相关抗原CD31、CD34和HLA-DR(图2)。
2.2 免疫共聚焦检测HNF4α转染效率
慢病毒转染5d后,免疫共聚焦观察MSC中GFP表达情况以鉴定其转染效率,结果显示转染效率>90%(图3),细胞形态未发生明显改变。
2.3 细胞上清液促进L02增殖活性
将各组上清培养L02细胞24h后,通过CCK-8测得OD值,结果发现MSC-HNF4α组L02增殖活性明显高于NS组[(1.278±0.071)(1.031±0.047),<0.01],同样MSC组也高于NS组[ (1.964±0.074)(1.031±0.047),<0.01;图4]。提示:在体外条件下,MSC和MSC-HNF4α培养上清液中的物质都具备促进L02细胞增殖的分泌物质,且在没有免疫调节的环境下MSC分泌物对肝细胞的增殖作用强于过表达HNF4α的MSC细胞(<0.05;图4)。
2.4 移植细胞促进大部肝切肝再生
尾静脉注射细胞(或NS)2h后,开腹行70%肝切除,48h后18只小鼠均存活,并且活力饮食基本恢复正常。统一活杀后,各组小鼠肝大小大致相似。同一部位HE染色未见明显差别,均可见少量炎性细胞浸润,胆小管增生,肝细胞增生(图5)。免疫组化测得肝再生指标Ki67表现一致,均为MSC组明显高于NS组[(45.000±5.263)(12.333±2.516),=0.001]和MSC-HNF4α组[(45.000±5.263)(23.000±4.000),=0.007],同时MSC-HNF4α组上述指标阳性率高于NS组[(23.000±4.000)(12.333±2.516),=0.017;图6]。提示在体内存在免疫调节环境下,MSC和MSC-HNF4α同样具有促进肝再生的能力,并且过表达HNF4α后对肝再生的促进能力弱于正常MSC。
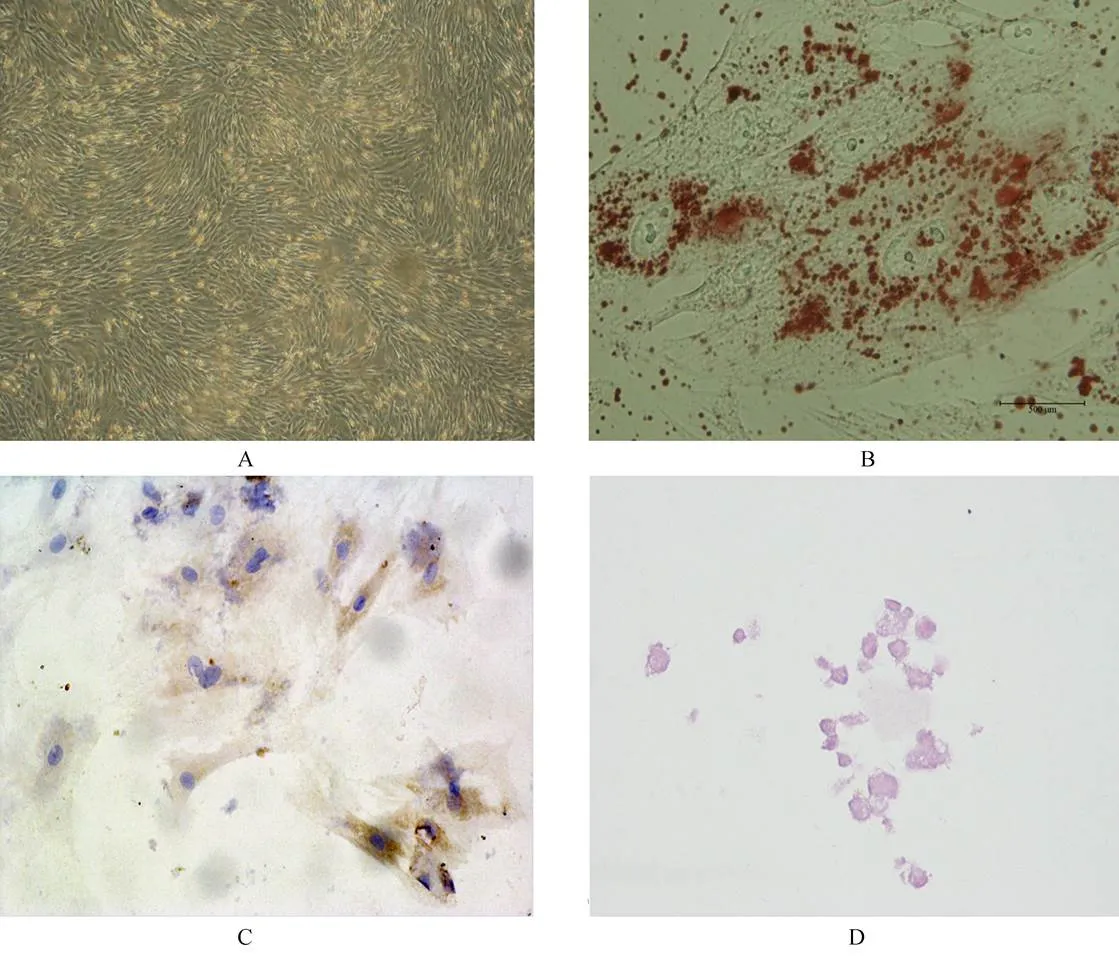
图1 MSC形态以及分化鉴定
Figure 1 Identification of MSC morphology and differentiation
MSC: mesenchymal stem cells; A: the third-passage UC-MSC (×100); B: after adipogenic induction, many small lipid vacuoles are observed by Oil Red O staining (×400); C: after chondrogenic induction, MSC differentiate into chondrogenic-like cells and are positive for typeⅡcollagen (×100); D: after osteogenic-specific induction, MSC are positive for Alizarin red staining (×100)
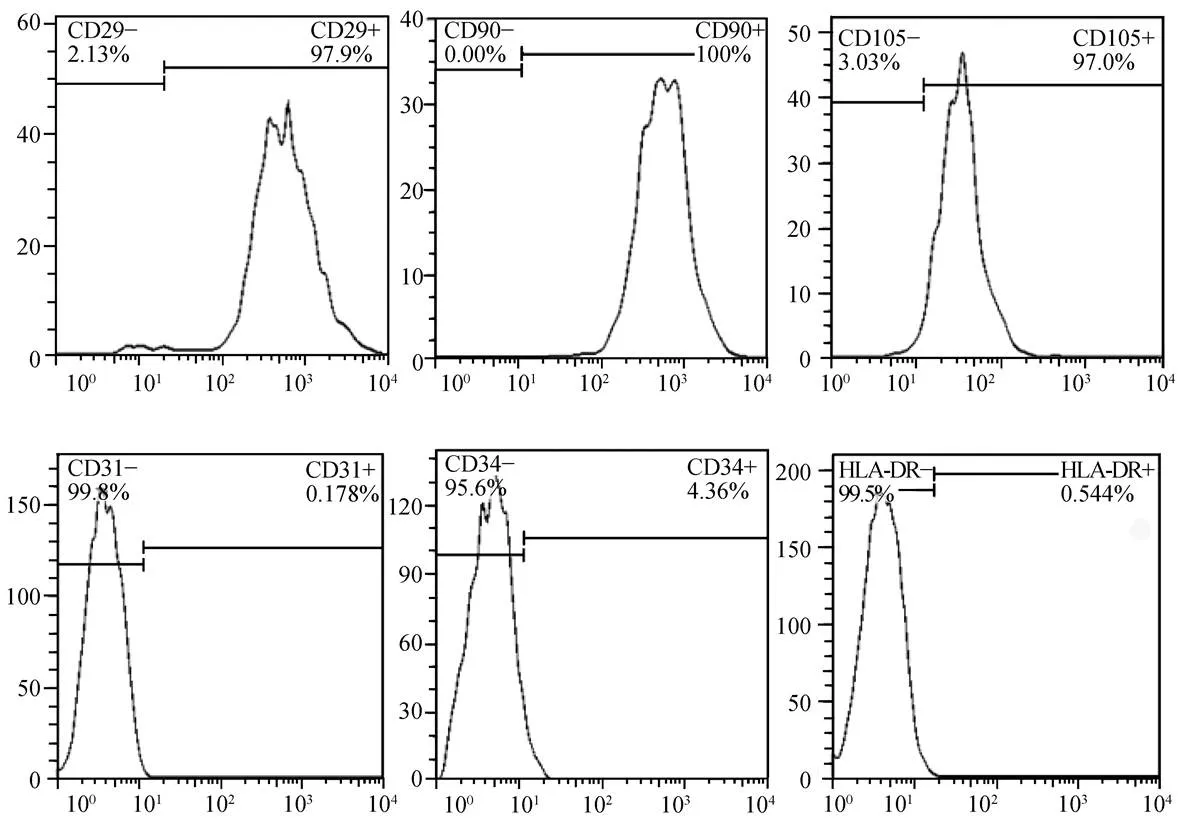
图2 第3代MSC流式细胞鉴定结果
Figure 2 Identification of the third-passage human UC-MSC by flow cytometry
UC-MSC: umbilical cord mesenchymal stem cells. Flow cytometric analysis shows MSC cells are positive for CD29, CD 90 and CD105, and negative for CD31, CD34 and HLA-DR
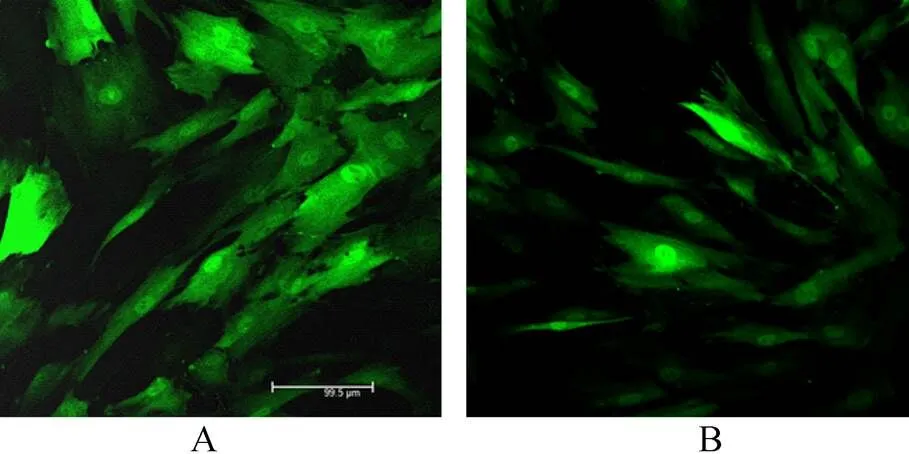
图3 MSC转染慢病毒
Figure 3 Transfection of HNF4α into MSC (×200)
MSC: mesenchymal stem cells; HNF4α: hepatocyte nuclear factor 4α; A: control group; B: overexpression HNF4α group
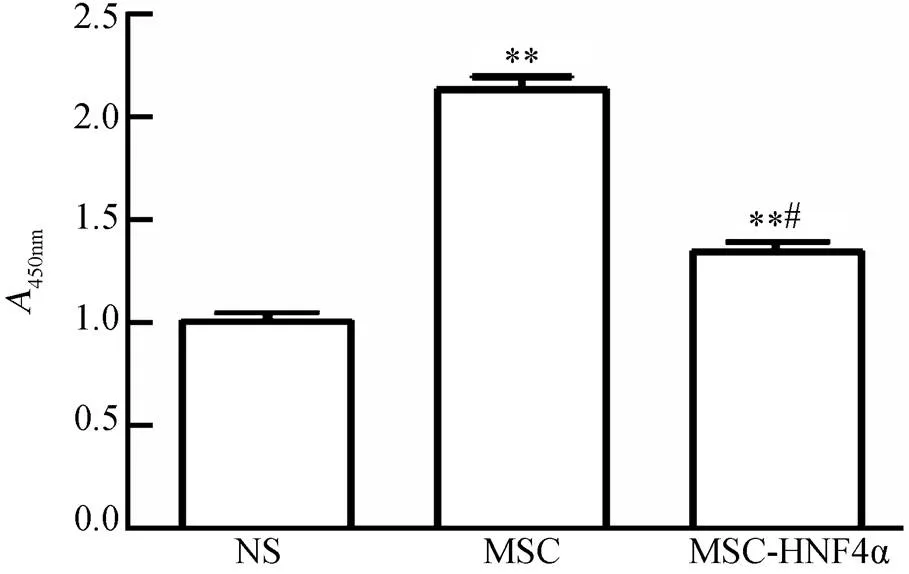
图4 体外实验MSC和MSC-HNF4α促进L02增殖
Figure 4 MSC and MSC-HNF4α promote L02 proliferation
NS: normal saline; MSC: mesenchymal stem cells; HNF4a: hepatocyte nuclear factor 4α. Compared with NS group,**<0.01; compared with MSC group,#<0.05
2.5 蛋白芯片两组细胞分泌物差异
收集两组细胞上清各3份,采用RayBio™人L系列抗体芯片Ⅰ(507)检测上清液中各种因子表达情况,发现两组上清液中分泌因子存在较大差异(图7)。而在这些因子中我们发现,MSC和MSC-HNF4α能分泌大量促进肝细胞再生因子:肝细胞生长因子(hepatocyte growth factor,HGF)、转化生长因子β、(transforming growth factor-beta,TGFβ)、成纤维生长因子(fibroblast growth factor,FGF)、白细胞介素6(interleukin-6,IL-6)、胰岛素等,而过表达HNF4α的MSC表达更多的HGF、血小板衍生生长因子(platelet-derived growth factor,PDGF)及分泌型卷曲蛋白1(secreted frizzled-related proteins 1,sFRP-1)等多种因子,差异具有统计学意义。
3 讨 论
肝脏再生是一个极其复杂的过程,包括启动阶段、增殖阶段和终止阶段3个步骤。目前,普遍接受的学说认为,肝切后血液流体剪应力发生改变,从而促进肝间充质细胞分泌胰岛素、表皮生长因子(epidermal growth factor,EGF)、HGF、肿瘤坏死因子α(tumor necrosis factor alpha,TNFα)和IL-6等多种细胞因子[11],作用于肝实质细胞,并且激活wnt等多条相关信号通路[12],启动肝实质细胞通过有丝分裂代偿再生。一些研究发现,当大部分肝细胞死亡或肝毒性物质持续作用时,肝脏中的干细胞即卵原细胞通过快速增殖分化参与肝再生;肝受到更严重损伤后,骨髓MSC、胰腺干细胞等可经血流停留于肝参与肝再生[13,14]。有研究结果表明,体外诱导分化后的MSC具备治疗多种肝脏疾病的潜力[8,15,16]。本研究小组在前期研究中已证实,将HNF4α过表达于MSC能够诱导更趋成熟肝细胞的肝样细胞[10]。
本次实验,我们采用UC-MSC,是因为UC-MSC相对于传统骨髓或者脂肪来源的MSC具备更好的原始特性,并且具备更好的免疫调节能力,原料的获取和分离也较后者更加容易。我们将HNF4α通过慢病毒转染的方式过表达于MSC,并且收集MSC和MSC-HNF4α条件培养基。通过体外增殖实验,我们证实MSC和MSC-HNF4α的外分泌因子都能够促进L02的增殖效应,而且MSC组的促增殖作用较MSC-HNF4α组明显。经尾静脉注入MSC组动物残肝中Ki67的表达高于MSC-HNF4α组(但两组都明显高于对照组)。为了解释上述现象,我们采用抗体芯片技术检测两组细胞外分泌因子。通过比对两组芯片结果差异,我们发现MSC以及MSC-HNF4α能够分泌大量促进肝细胞再生的关键性因子,如HGF、TGFβ、FGF、IL-6、胰岛素样生长因子(insulin-like growth factor,IGF)等。不过,MSC-HNF4α不仅能够更多地分泌HGF(<0.05),而且还能大量分泌sFRP-1。sFRP-1作为wnt信号通路的上游竞争性抑制因子,能够有效抑制wnt信号通路的激活而导致其下游的关键性因子β−连环蛋白(catenin)抑制。肝切除后残肝再生启动过程中,β−连环蛋白的激活有至关重要的作用[17]。我们认为MSC-HNF4α组中抑制因子sFRP-1的高表达能够合理地解释MSC-HNF4α促再生能力弱于未经处理的MSC。
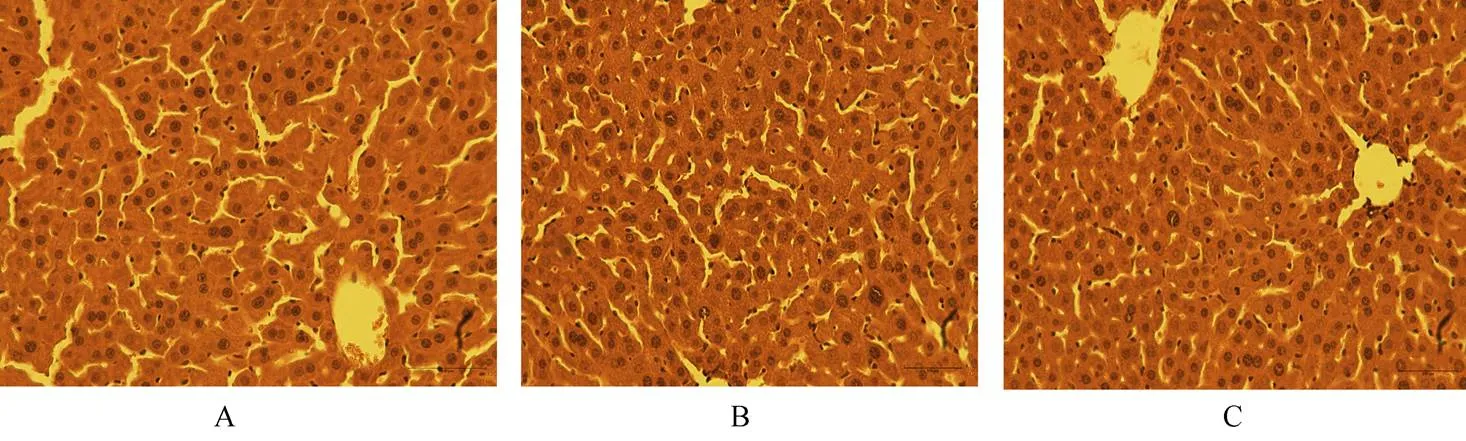
图5 各组小鼠肝脏HE染色未见明显差别
Figure 5 HE-staining of liver tissue shows no difference between different groups (×200)
MSC: mesenchymal stem cells; HNF4a: hepatocyte nuclear factor 4α; A: control group; B: MSC group; C: overexpression HNF4α group

图6 MSC和MSC-HNF4α促进肝再生(Ki67表达)
Figure 6 MSC and MSC-HNF4α promote liver proliferation(Ki67 experssion, ×400)
MSC: mesenchymal stem cells; HNF4a: hepatocyte nuclear factor 4α; A: control group; B: MSC group; C: MSC-HNF4α group; D: the expression of Ki67 in each group. Compared with NS group,**<0.01; compared with MSC group,#<0.05

图7 抗体芯片检测MSC和MSC-HNF4α上清分泌蛋白
Figure 7 Detection of secreted proteins in supernatant of MSC and MSC-HNF 4α by antibody microarray
MSC: mesenchymal stem cells; HNF4a: hepatocyte nuclear factor 4α; A: MSC group; B: MSC-HNF4α group
在体外大量获取“肝样细胞”建立工程化肝细胞株用于细胞移植或组织工程肝,有望成为今后治疗肝衰竭的方法。我们认为,由于MSC-HNF4α能够分泌更多因子以及能够更好地分化成为肝样细胞,其可能会在肝衰竭尤其是慢性肝衰竭的治疗中发挥重要作用。
[1] Fausto N, Campbell JS, Riehle KJ. Liver regeneration[J]. J Hepatol, 2012, 57(3): 692−694.
[2] Kaibori M, Adachi Y, Shimo T,. Stimulation of liver regeneration after hepatectomy in mice by injection of bone marrow mesenchymal stem cellsthe portal vein[J]. Transplant Proc, 2012, 44(4): 1107−1109.
[3] Kuo TK, Hung SP, Chuang CH,. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells[J]. Gastroenterology, 2008, 134(7): 2111−2121.
[4] Seki T, Yokoyama Y, Nagasaki H,. Adipose tissue-derived mesenchymal stem cell transplantation promotes hepatic regeneration after hepatic ischemia-reperfusion and subsequent hepatectomy in rats[J]. J Surg Res, 2012, 178(1): 63−70.
[5] El-Ansary M, Abdel-AzizⅠ, Mogawer S,. PhaseⅡ trial: undifferentiateddifferentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis[J]. Stem Cell Rev, 2012, 8(3): 972−981.
[6] Li F, Liu P, Liu C,. Hepatoblast-like progenitor cells derived from embryonic stem cells can repopulate livers of mice[J]. Gastroenterology, 2010, 139(6): 2158−2169.
[7] Woo DH, Kim SK, Lim HJ,. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice[J]. Gastroenterology, 2012, 142(3): 602−611.
[8] Zhou R, Li Z, He C,. Human umbilical cord mesenchymal stem cells and derived hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model[J]. PLoS One, 2014, 9(8): e104392.
[9] Odom DT, Zizlsperger N, Gordon DB,. Control of pancreas and liver gene expression by HNF transcription factors[J]. Science, 2004, 303(5662): 1378−1381.
[10] Hang H, Yu Y, Wu N,. Induction of highly functional hepatocytes from human umbilical cord mesenchymal stem cells by HNF4alpha transduction[J]. PLoS One, 2014, 9(8): e104133.
[11] Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas[J]. Am J Pathol, 2010, 176(1): 2−13.
[12] Tan X, Behari J, Cieply B,. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration[J]. Gastroenterology, 2006, 131(5): 1561−1572.
[13] Bird TG, Lorenzini S, Forbes SJ. Activation of stem cells in hepatic diseases[J]. Cell Tissue Res, 2008, 331(1): 283−300.
[14] Cantz T, Manns MP, Ott M. Stem cells in liver regeneration and therapy[J]. Cell Tissue Res, 2008, 331(1): 271−282.
[15] Aurich H, Sgodda M, Kaltwasser P,. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissuepromotes hepatic integration[J]. Gut, 2009, 58(4): 570−581.
[16] Stock P, Bruckner S, Winkler S,. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury[J]. Int J Mol Sci, 2014, 15(4): 7004−7028.
[17] Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad[J]. Semin Cancer Biol, 2011, 21(1): 44−58.
(编辑: 周宇红)
Overexpression of hepatocyte nuclear factor 4α in umbilical cord mesenchymal stem cells promotes liver regeneration after subtotal hepatectomy in mice
WU Ning1, MA Yong1, ZHAI Xiu-Yu2, ZHANG Yu-Ling3, ZHANG Yong1, ZHANG Qi-Qi4, HANG Hua-Lian4, XIA Qiang4, BIAN Jian-Min1*
(1Department of General Surgery, Nanjing Hospital Affiliated to Nanjing Medical University, Nanjing 210006, China;2the Second Department of Urology, the First Hospital of Jilin University, Jilin 130021, China;3Guizhou Provincial Key Laboratory of Cell Engineering, Affiliated Hospital of Zunyi Medical College, Zunyi 563033, China;4Department of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China)
To investigate whether human umbilical cord mesenchymal stem cells (UC-MSC) and the cells with overexpression of hepatocyte nuclear factor 4α (HNF4α) can promote liver regeneration after subtotal hepatectomy in mice.After human UC-MSC were isolated, cultured and identified, the cells were transfected with lentiviral vector LV-HNF4α. Then the cell supernatant was collected and cultured with L02 cells, and the L02 cell viability was detected by cell counting kit-8 (CCK8) assay. In thestudy, a total of 18 mice were randomly divided into 3 groups (=6 for each group): normal saline (NS) group, MSC group and MSC-HNF4α group, and received an injection of NS, MSC and MSC-HNF4α (5×106/ml), respectively via tail vein in 2 h before the establishment of subtotal hepatectomy model (70%). In 48 h later, the expression of Ki67 in the liver tissues was detected by immunohistochemical assay, and the results were compared among 3 groups.UC-MSC was successfully isolated from human umbilical cord, and the UC-MSC with stable overexpression of HNF4α were established.study indicated that the L02 cells showed stronger proliferative ability when cocultured with MSC and MSC-HNF4α than cultured alone (<0.01), and those cocultured with MSC-HNF4α stronger than the cells with MSC (<0.05).study showed similar findings: the expression of Ki67 was stronger in the mice treated with MSC or MSC-HNF4α than those with NS (<0.01), and also overexpression of HNF4α resulted in more significant expression of Ki67 (<0.05).Both MSC and MSC-HNF4α secret some cytokines to promote liver regeneration.
mice; liver regeneration; mesenchymal stem cells; hepatocyte nuclear factor 4α
(201308014),(81100306)(134119a9501).
R333.4
A
10.11915/j.issn.1671−5403.2015.01.016
2014−09−17;
2014−11−06
南京市科学技术委员会基金(201308014);国家自然科学基金 (81100306);上海市科委医学引导类基金(134119a9501)
卞建民, E-mail: jmbian0324@gmail.com

