老年2型糖尿病患者下肢周围动脉病变与心率变异率相关性分析
于 玲,杨 磊,宋彬彬,王永慧,李连霞,高 珊*
老年2型糖尿病患者下肢周围动脉病变与心率变异率相关性分析
于 玲1,杨 磊2,宋彬彬3,王永慧1,李连霞1,高 珊1*
(首都医科大学附属北京朝阳医院西区:1内分泌科,3心电图室,北京 100045;2首都医科大学附属北京朝阳医院神经内科,北京 100020)
探讨老年2型糖尿病患者(≥60岁)下肢周围动脉病变(PAD)与心率变异率(HRV)的关系。选择2012年6月至2014年7月在首都医科大学附属北京朝阳医院西区内分泌科住院的128例老年2型糖尿病患者,根据有无PAD(ABI<0.9定义为PAD)分为两组。测定体质量指数(BMI)、血压、血脂、周围神经病变、尿微量白蛋白(UAER)、双下肢踝肱指数(ABI)和动态心电图,由仪器自动分析计算出HRV各项时域及频域指标。对两组间各项指标进行统计学分析。共128例患者,其中90例无PAD、38例合并PAD。2型糖尿病合并PAD组年龄、糖尿病病程、糖化血红蛋白(HbA1c)、甘油三酯(TG)、UAER及高血压发生率均高于无PAD组(<0.05)。PAD组患者HRV指标下降,包括大部分时域指标SDNN、SDNN-index、SDANN及频域指标低频功率、高频功率。校正年龄、糖尿病病程、HbA1c、TG、UAER、高血压后,HRV指标与PAD程度呈负相关。老年2型糖尿病合并PAD者具有更低的HRV,表明心脏自主神经系统调节能力下降。
糖尿病,2型;老年人;下肢周围动脉病变;心率变异率
下肢周围动脉病变(peripheral artery disease,PAD)是糖尿病的常见慢性并发症之一,既是全身血管病变的一个局部反映,又是造成糖尿病足溃疡、坏疽乃至截肢的主要原因[1],其病情重、致残致死率高,严重影响着患者生活和生存质量。踝肱指数(ankle brachial index,ABI)指踝动脉压与肱动脉压的比值,是筛查和诊断PAD的一种简便、有效、无创的方法,ABI值的异常提示患者存在下肢闭塞性动脉粥样硬化性疾病。心脏自主神经病变(cardiac autonomic neuropathy,CAN)也是糖尿病重要的慢性并发症之一,当病变累及交感神经而表现为体位性低血压时,临床预后不良,可发生猝死[2]。糖尿病患者常存在多种代谢紊乱,高血糖、脂代谢紊乱、氧化应激反应增加等均为神经和血管并发症发生的共同基础。目前,关于老年2型糖尿病(type 2 diabetes mellitus,T2DM)患者PAD与心率变异率(heart rate variability,HRV)的研究较少,为研究二者之间关系,我们通过分析合并或不合并PAD的T2DM患者的HRV指标,以及不同程度的HRV指标间PAD的发生率,探讨两者间的关系。
1 对象与方法
1.1 研究对象
选择2012年6月至2014年7月在首都医科大学附属北京朝阳医院西区内分泌科住院的老年T2DM患者128例,均符合1999年WHO糖尿病诊断标准,既往无冠心病史,伴或不伴有四肢肢端麻木、便秘等神经病变的临床表现,排除严重的肝肾疾病、静脉曲张、急性感染性疾病、酮症等。其中,男69例,女59例,年龄63~83岁,病程6~348个月。
1.2 检测指标
检测并记录年龄、性别、糖尿病病程、身高、体质量、吸烟、血压、糖尿病性周围神经病变(diabetic peripheral neuropathy,DPN)、糖化血红蛋白(glycosylated hemoglobin A1c,HbA1c)、血肌酐(serum creatinine,SCr)、血尿酸(serum uric acid,SUA)、总胆固醇(total cholesterol,TC)、甘油三酯(triglycerides,TG)、高密度脂蛋白胆固醇(high-density lipoprotein cholesterol,HDL-C)、低密度脂蛋白胆固醇(low-density lipoprotein cholesterol,LDL-C)、尿白蛋白排泄率(urinary albumin excretion rate,UAER)、空腹血糖(fasting blood glucose,FBG)、空腹胰岛素(fasting insulin,FINS)。
DPN由MEDELEC肌电图诱发电位系统(英国牛津)测定,诊断标准参照2010年《中国T2DM防治指南》中的规定:明确的糖尿病病史;在诊断糖尿病时或之后出现的神经病变;临床症状和体征与DPN的表现相符;以下4项检查中如果任1项异常则诊断为DPN:(1)踝反射异常(或踝反射正常、膝反射异常);(2)针刺痛觉异常;(3)振动觉异常;(4)压力觉异常。本研究中各患者均行双下肢神经传导速度检测协助诊断[3]。
1.3 HRV测定
全部患者行24h动态心电图检查,记录其24h心电变化,由仪器自动分析计算出HRV各项时域及频域指标,时域指标包括:正常R-R间期标准差(standard deviation of the R-R intervals,SDNN),每5min R-R间期均值标准差(standard deviation of averages of R-R intervals calculated in 5-min segments,SDANN),每5min正常R-R间期均值标准差(mean of the standard deviation of R-R intervals calculated in 5-min segments,SDNN-index),相邻正常R-R间期差值均方根值(root mean square of successive differences of adjacent R-R intervals,RMSSD),相邻正常R-R间期>50ms百分比(percentage of differences between adjacent R-R intervals>50ms,PNN50)。频域指标包括:低频功率(0.04~0.15Hz,low frequency,LF)、高频功率(0.15~0.40Hz,high frequency,HF)、低频/高频(LF/HF)。将各指标分别取<33%为1st组,33%~67%为2nd组,>67%为3rd组,观察各组中PAD的发生率。
1.4 ABI的测定
用ES-1000 SPM多普勒血流探测仪测定。根据心血管和介入放射学协会(Society of Cardiovascular and Interventional Radiology,SCVIR)标准[4],检查前,患者休息10~15min,室温下,仰卧位,分别置袖带于双上臂,用多普勒探头于肘部肱动脉处获取信号,测得双侧肱动脉收缩压(brachial systolic blood pressure,BSBP),取两者中的高值,置相同的袖带于踝部,用多普勒探头于胫后动脉、足背动脉处获取信号,测得踝动脉收缩压(ankle systolic blood pressure,ASBP),取其高值,ASBP高值/BSBP高值即为ABI,相同方法测对侧肢体。按2011年美国心脏病学会基金会(American College of Cardiology Foundation,ACCF)及美国心脏联合会(American Heart Association,AHA)的标准[5]测定ABI,双侧胫后或足背动脉ABI有1项<0.9,为PAD组,取4个ABI数值中最小的一个纳入研究,双侧ABI中有1项<0.9,即选入PAD组。ABI均≥0.9者选入非PAD组。
1.5 统计学处理
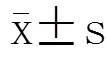
2 结 果
2.1 PAD组与非PAD组患者一般资料的比较
128例老年T2DM患者中ABI<0.9者占38例,非PAD组90例。与非PAD组比较,PAD组年龄更大,病程更长,TG、高血压发生率、HbA1c及UAER更高,上述指标差异均具有统计学意义(<0.05;表1)。
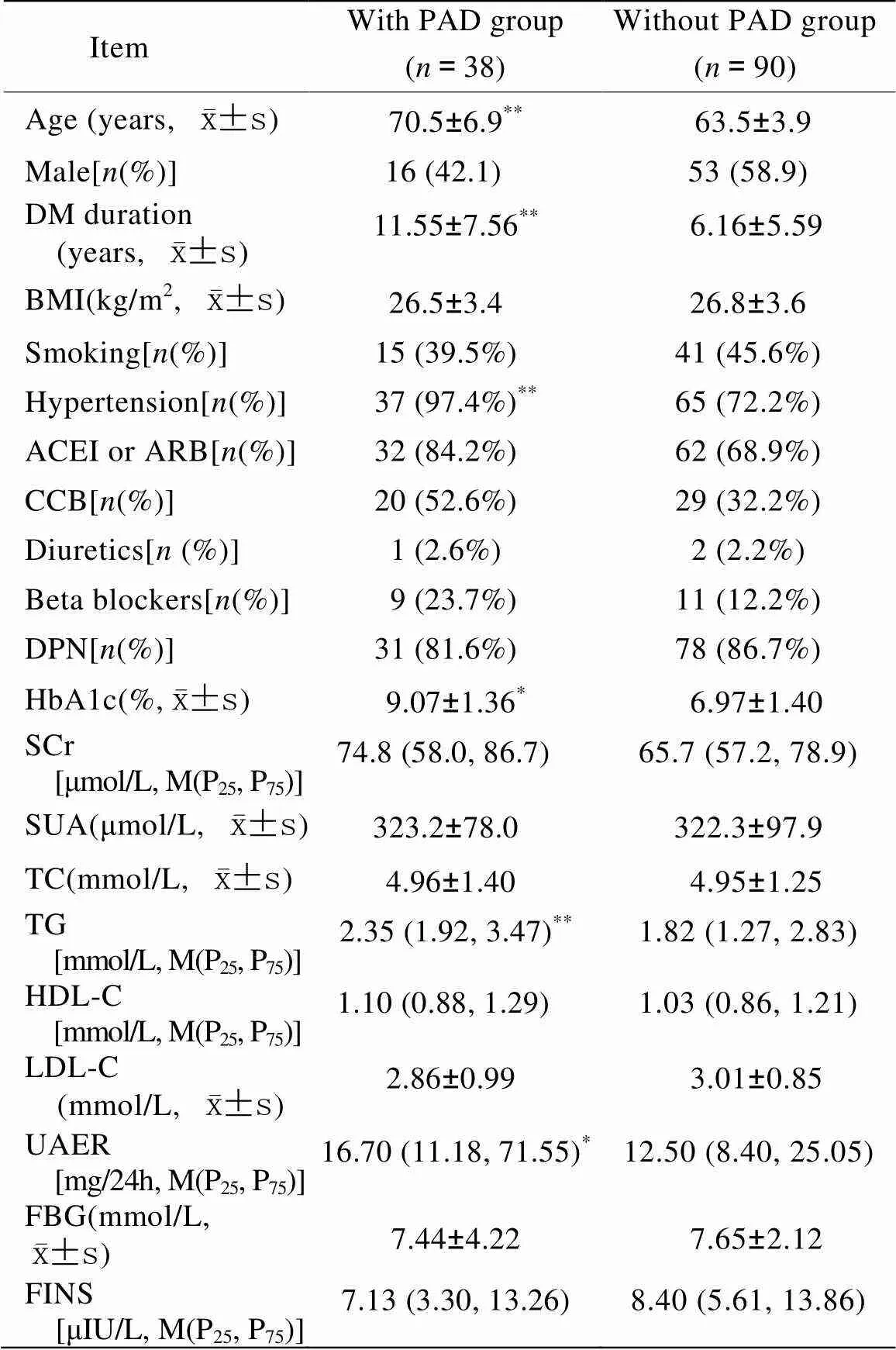
表1 两组T2DM患者临床资料比较
T2DM: type 2 diabetes mellitus; PAD: peripheral artery disease; DM: diabetes mellitus; BMI: body mass index; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCB: calcium channel blocker; DPN: diabetic peripheral neuropathy; HbA1c: glycosylated hemoglobin A1c; SCr: serum creatinine; SUA: serum uric acid; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; UAER: urinary albumin excretion rate; FBG: fasting blood glucose; FINS: fasting insulin. Compared with without PAD group,*<0.05,**<0.01
2.2 两组患者HRV各项指标比较
两组患者HRV时域指标:SDNN、SDANN、SDNN-index差异均具有统计学意义(<0.01);PNN50、RMSSD差异无统计学意义(>0.05);两组患者HRV频域指标:LF、HF差异均具有统计学意义(<0.05),LF/HF差异无统计学意义(>0.05;表2)。
图1结果表明SDNN、SDANN、SDNN-index、LF中各自1st组PAD发生率>3rd组,差异有统计学意义(<0.05),即在HRV越低的组PAD的发生率相对更高。
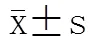
表2 两组糖尿病患者HRV各指标比较
PAD: peripheral artery disease; HRV: heart rate variability; SDNN: standard deviation of the R-R intervals; SDNN-index: mean of the standard deviation of R-R intervals calculated in 5-min segments; SDANN: standard deviation of averages of R-R intervals calculated in 5-min segments; RMSSD: root mean square of successive differences of adjacent R-R intervals; PNN50: percentage of differences between adjacent R-R intervals>50ms; LF: low frequency; HF: high frequency. Compared with without PAD group,*<0.05,**<0.01

图1 SDNN、SDANN、SDNN-index、LF、HF各取三分位后PAD的发生率
Figure 1 The incidence of PAD categorized by tertiles of SDNN, SDANN, SDNN-index, LF, and HF
PAD: peripheral artery disease; SDNN: standard deviation of the R-R intervals; SDANN: standard deviation of the averages of R-R intervalscalculated in 5-min segments; SDNN-index: mean of the standard deviation of the R-R intervals calculated in 5-min segments; LF: low frequency; HF: high frequency. The 1st group:<33%; the 2nd group: 33−67%; the 3rd group:>67%. Compared with the 3rd group,*<0.05
2.3 PAD与否与各指标进行多因素相关及回归分析
Spearman相关分析显示,校正年龄、性别、糖尿病病程、HbA1c、TG、UAER、高血压后,所有患者大部分HRV各指标与ABI呈负相关(<0.05)。结果如下:SDNN(=-0.347,<0.01)、SDNN-index(=-0.287,<0.01)、SDANN(=-0.370,<0.01)、LF(=-0.192,<0.05),HF(=-0.180,<0.05);上述各心率变异率指标取三分位后分为3组行logistic回归,除HF1st与3rd组比较差异无统计学意义外,各指标1st组与3rd组比较差异均有统计学意义(<0.05),即SDNN、SDNN-index、SDANN、LF中各自1st组PAD发生率均>3rd组,OR值分别为5.149、3.100、6.727、2.643(表3)。

表3 多元logistic回归:HRV取三分位后作为自变量,PAD与否为因变量
PAD: peripheral artery disease; HRV: heart rate variability; SDNN: standard deviation of the R-R intervals; SDNN-index: mean of the standard deviation of R-R intervals calculated in 5-min segments; SDANN: standard deviation of averages of R-R intervals calculated in 5-min segments; LF: low frequency; HF: high frequency. The odds ratio was adjusted for age, diabetic duration, hypertension, HbA1c, TG, and UAER in each model; upper index tertile (3rd) for each index was considered as the reference (OR=1)
3 讨 论
近年来,糖尿病发病率明显增高,成为影响人类健康的重要慢性疾病。糖尿病合并PAD是糖尿病患者下肢截肢致残的主要原因。国内有报道显示,糖尿病患者下肢截肢率比正常人高5~15倍[6]。早期诊断和治疗PAD可预防糖尿病足坏疽乃至截肢的发生[7]。应用较广的ABI测定,其操作方法简单、费用低、无创伤。随着研究的深入,我们进一步发现,ABI除了能作为较为准确的下肢动脉硬化闭塞症的筛选性检查外,同时也是动脉粥样硬化所造成心血管事件率的新的危险预测因子。以ABI<0.9诊断为周围动脉疾病,其敏感度和特异度均为95%[8]。
糖尿病下肢动脉硬化的发病原因涉及许多方面,发病机制比较复杂,是多种因素长期综合性作用引起的。在本文所观察的病例中,合并PAD的老年糖尿病患者具有高龄、病程长、血糖控制差、TG高、高血压发生率高、UAER高等特点。大量研究证据支持餐后血糖持续升高与血管病变密切相关[9,10]。血脂异常、高血压是公认的动脉硬化的危险因素。UAER的升高也是导致动脉硬化的危险因素,在T2DM患者伴微量白蛋白尿的阶段,已存在广泛的内皮细胞功能紊乱,血浆蛋白可通过受损的内皮细胞渗透至血管内膜下,促进动脉硬化的发生[11]。
糖尿病自主神经病变是糖尿病最常见的并发症之一,因诊断标准不尽相同,文献报道其发生率为2.5%~40.0%[12]。糖尿病患者发生CAN,使恶性心律失常、心绞痛、无痛性心肌梗死、心力衰竭、心源性休克、卒中、运动耐力下降等发生率上升,猝死发生率明显增加[12−16]。HRV有时域分析和频域分析两种指标,临床应用较多的为时域指标。一般认为SDNN代表总体的心率变异程度,SDANN和SDNN-index代表心率缓慢变化的成分,反映了交感神经的功能,RMSSD和PNN50代表心率速度变化的成分,反映了迷走神经功能。频域分析中高频功率反映迷走神经调节功能,低频功率与压力反射调节有关,它反映交感和副交感神经系统对窦房结的复合调节作用[17,18]。通常认为糖尿病患者发生CAN时早期表现为副交感神经的损害,患者表现为静息时心率增快,而体位性低血压是晚期交感神经病变的表现。
本研究中合并与未合并PAD的患者相比,HRV除RMSSD、PNN50外各指标均低于无PAD者,差异均有统计学意义(<0.05)。在校正年龄、性别、病程、HbA1c、TG、UREA后,HRV各指标仍与PAD呈负相关。HRV取三分位后,除HF外,各1st组(即心率变异率越低组)PAD的发生率高于3rd组,差异有统计学意义(<0.05)。文献报道长期血糖升高者血管活性因子产生减少,血液的高凝状态以及糖、蛋白质、脂肪代谢紊乱均造成动脉粥样硬化和微血管病变,神经缺血及营养障碍导致自主神经功能损害[19]。国外文献也指出,高血压、胰岛素抵抗、肥胖、高TG、吸烟、中心性肥胖等均与糖尿病患者HRV下降有关[20−22]。以PAD为因变量,各因素进入logistic回归方程后,SDNN、SDNN-index、SDANN、LF与其独立相关,OR>1。说明HRV与PAD的发生独立相关。目前国内外有关下肢动脉硬化与心率变异之间的相关性研究较少。在1型糖尿病患者中发现下肢动脉硬化与自主神经病变独立相关[23]。Canani等[24]发现在T2DM患者中下肢动脉硬化与自主神经病变独立相关,与本文观察到的结果一致。
临床上我们对老年糖尿病患者动脉硬化的筛查更为关注,而自主神经及心率变异检测方面相对不足。在老年T2DM患者中,若出现下肢动脉硬化者,应加强对其HRV的监测,及早干预,以防止心脏等不良事件的发生,减少糖尿病患者的死亡率。由于本研究样本量较少,在今后的研究中,还需大样本资料对二者的关系进行进一步研究。
[1] Cacoub P, Cambou JP, Kownator S,. Prevalence of peripheral arterial disease in high-risk patients using ankle-brachial index in general practice: a cross-sectional study[J]. Int J Clin Pract, 2009, 63(1): 63−70.
[2] Schroeder EB, Chambless LE, Liao D,. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study[J]. Diabetes Care, 2005, 28(3): 668−674.
[3] Chinese Society of Diabetes, Chinese Medical Association. China Guideline for Type 2 Diabetes (2010)[J]. Chin J Diabetes, 2012, 20(1): 1−36. [中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2010年版)[J]. 中国糖尿病杂志, 2010, 20(1): 1−36.]
[4] Sacks D, Bakal CW, Beatty PT,. Position statement on the use of the ankle brachial index in the evaluation of patients with peripheral arterial disease: a consensus statement developed by the Standards Division of the Society of Cardiovascular & Interventional Radiology[J]. J Vasc Interv Radiol, 2002, 13(4): 353.
[5] 2011 Writing Group Members; 2005 Writing Committee Members; ACCF/AHA Task Force Members. 2011ACCF/AHA Focused Update of the Guideline for the Management of Association Task Force on Practice Guidelines Report of the American College of Cardiology Foundation/American Heart Patients with Peripheral Artery Disease (Updating the 2005 Guideline): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines[J]. Circulation, 2011, 124(18): 2020−2045.
[6] Pan CY, Gao Y, Yuan SY,. The prevalence of vascular lesions in the lower extremities and their risk factors in type 2 diabetes mellitus[J]. Chin J Diabetes, 2001, 9(6): 323−326. [潘长玉, 高 妍, 袁申元, 等. 2型糖尿病下肢血管病变发生率及相关因素调查[J]. 中国糖尿病杂志, 2001, 9(6): 323−326.]
[7] Walker R. Diabetes and peripheral neuropathy: keeping people on their own two feet[J]. Br J Community Nurs, 2005, 10(1): 33−36.
[8] Hiatt WR. Medical treatment of peripheral arterial disease and claudication[J]. N Engl J Med, 2001, 344(21): 1608−1621.
[9] Aronow WS, Ahn C, Weiss MB,. Relation of increased hemoglobin A1c levels to severity of peripheral arterial disease in patients with diabetes mellitus[J]. Am J Cardiol, 2007, 99(10): 1468−1469.
[10] Zheng Q, Zhou N. Clinical significance of ankle brachial index in diagnosis of peripheral artery disease in patients with diabetes mellitus[J]. Hainan Med J, 2008, 19(7): 89−90. [郑 倩, 周 宁. 踝肱指数用于诊断糖尿病下肢动脉病变的意义[J]. 海南医学, 2008, 19(7): 89−90.]
[11] Deckert T. Nephropathy and coronary death: the fatal twins in diabetes mellitus[J]. Nephrol Dial Transplant, 1994, 9(8): 1069−1071.
[12] Tesfaye S, Boulton AJ, Dyck PJ,. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments[J]. Diabetes Care, 2010, 33(10): 2285−2293.
[13] Gerritsen J, Dekker JM, Ten Voorde BJ,. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study [J]. Diabetes Care, 2001, 24(10): 1793−1798.
[14] Maser RE, Mitchell BD, Vinik AI,. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis[J]. Diabetes Care, 2003, 26(6): 1895−1901.
[15] Jellis CL, Stanton T, Leano R,. Usefulness of at rest and exercise hemodynamics to detect subclinical myocardial disease in type 2 diabetes mellitus[J]. Am J Cardiol, 2011, 107(4): 615−621.
[16] Low PA, Benrud-Larson LM, Sletten DM,. Autonomic symptoms and diabetic neuropathy: a population-based study[J]. Diabetes Care, 2004, 27(12): 2942−2947.
[17] Risk M, Bril V, Broadbridge C,. Heart rate variability measurement in diabetic neuropathy: review of methods[J]. Diabetes Technol Ther, 2001, 3(1): 63−76.
[18] Moraes RS, Ferlin EL, Polanczyk CA,. Three-dimensional return map: a new tool for quantification of heart rate variability[J]. Auton Neurosci, 2000, 83(1−2): 90−99.
[19] Hu FL. Clinical study of diabetic autonomic neuropathy[J]. Chin J Pract Nerv Dis, 2008, 11(1): 14−16. [胡逢来. 糖尿病自主神经病变临床分析[J]. 中国实用神经疾病杂志, 2008, 11(1): 14−16.]
[20] Perciaccante A, Fiorentini A, Paris A,. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus[J]. BMC Cardiovasc Disord, 2006, 6: 19.
[21] Grassi G, Dell’Oro R, Facchini A,. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives[J]. J Hypertens, 2004, 22(12): 2363−2369.
[22] Laitinen T, Lindstrom J, Eriksson J,. Cardiovascular autonomic dysfunction is associated with central obesity in persons with impaired glucose tolerance[J]. Diabet Med, 2011, 28(6): 699−704.
[23] Costacou T, Huskey ND, Edmundowicz D,. Lower-extremity arterial calcification as a correlate of coronary artery calcification[J]. Metabolism, 2006, 55(12): 1689−1696.
[24] Canani LH, Copstein E, Pecis M,. Cardiovascular autonomic neuropathy in type 2 diabetes mellitus patients with peripheral artery disease[J]. Diabetol Metab Syndr, 2013, 5(1): 54.
(编辑: 周宇红)
Correlation of lower extremities peripheral arterial disease and heart rate variability in elderly patients with type 2 diabetes mellitus
YU Ling1, YANG Lei2, SONG Bin-Bin3, WANG Yong-Hui1, LI Lian-Xia1, GAO Shan1*
(1Department of Endocrinology,3Department of Electrocardiography, Jingxi Branch, Beijing Chaoyang Hospital, Capital University of Medical Sciences, Beijing 100045, China;2Department of Neurology, Beijing Chaoyang Hospital, Capital University of Medical Sciences, Beijing 100020, China)
To investigate the correlation of lower extremities peripheral arterial disease (PAD) and heart rate variability (HRV) in the elderly patients (over 60 years) with type 2 diabetes mellitus (T2DM).A total of 128 T2DM patients admitted to our hospital from June 2012 to July 2014 were included in this study. All subjects were divided into 2 groups according to having PAD or not [ankle brachial index (ABI)<0.9 defined as PAD]. Their body mass index (BMI), blood pressure, serum lipids, peripheral neuropathy, urinary albumin excretion rate (UAER), ABI and HRV were measured and analyzed.In the 128 T2DM patients, there were 90 patients having no PDA and 38 having. Those with PAD had older age, longer diabetes duration, higher UAER, higher incidence of hypertension, and higher levels of HbA1c and triglycerides (TG) than the patients without PAD (<0.05). And they had lower HRV indices, including those in time domain, such as, the standard deviation of the R-R intervals (SDNN), mean of the standard deviation of the R-R intervals calculated in 5-min segments (SDNN-index) and the standard deviation of the averages of R-R intervals (SDANN), and those infrequency domain, high-frequency activity and low-frequency activity, for example. After adjustment for age, diabetes duration, UAER, levels of HbA1c and TG, and incidence of hypertension, HRV indices were negatively correlated with the severity of PAD.In the cohort of the elderly T2DM patients, those with PAD have lower HRV indices than those without, suggesting a dysfunction of cardiovascular autonomic regulation.
diabetes mellitus, type 2; elderly; peripheral artery disease, lower extremities; heart rate variability
R592; R587.1
A
10.11915/j.issn.1671−5403.2015.01.013
2014−09−29;
2014−11−15
高 珊, E-mail: gaoshanmw@163.com

