模拟失重对雄性大鼠背根神经节尼氏小体形态和胶质细胞源性神经营养因子的影响
解放军总医院,北京 00853;第四军医大学西京医院 骨科,陕西西安 7003
模拟失重对雄性大鼠背根神经节尼氏小体形态和胶质细胞源性神经营养因子的影响
任宁涛1,张 恒1,李 洁1,雷 伟2,刘 宁2,毕 龙2,吴子祥2,张 然1,张永刚1,崔 赓1
1解放军总医院,北京 100853;2第四军医大学西京医院 骨科,陕西西安 710032
目的探讨模拟失重对背根神经节(dorsal root ganglia,DRG)及胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factor,GDNF)的影响。方法健康雄性SD大鼠80只,随机分为尾部悬吊(HU)组(n=40)和正常对照(NC)组(n=40),4周后处死各组大鼠,取腰5背根神经节,甲苯氨兰染色观察背根神经节内尼氏小体变化,免疫组化观察GDNF的变化,Western-blot方法检测GDNF的蛋白表达,实时PCR检测GDNF mRNA表达情况。结果与NC组相比,HU组尼氏体染色浅,尼氏体变小,弥散分布。免疫组化结果显示,与NC组比较,HU组GDNF量及积分光密度(integral optical density,IOD)值减少(P<0.05)。Western-blot结果显示,HU组较NC组GDNF蛋白表达减少(P<0.05)。实时PCR结果显示,与NC组相比,HU组GDNF mRNA表达降低(P<0.05)。结论4周模拟失重可导致DRG内尼氏小体形态发生变化,GDNF数量减少,GDNF蛋白表达及mRNA表达降低,推测失重状态下可引起大鼠背根神经节发生损伤。
模拟失重;背根神经节;动物模型
地球上一切生命都在重力环境中,当各种生命体离开地球重力环境进入太空后,他们的生理功能将受到不同程度的影响[1-5],如骨质疏松[6-8]、心血管功能紊乱[9-12]、肌肉萎缩[13]、脊髓方面相关改变[14-16]等。但由于空间搭载实验的机会较少,且成本较高,Morey-Holton和Globus[17]提出了通过尾部悬吊来模拟失重状态,有学者通过此模型发现模拟失重下可引起大鼠L5背根神经节(dorsal root ganglia,DRG)动作电位传导速度下降,髓鞘呈退变性改变[18],但未深入研究。胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factor,GDNF)作为多巴胺能神经营养因子,对神经元有强大的神经营养作用,能够抑制其发生退变[19],而DRG作为功能性脊柱单位的“大脑”,与腰背痛和根性痛有着密切的关系,因此本研究旨在通过建立大鼠尾部悬吊模型,从背根神经节内尼氏小体的形态及神经营因子GDNF的改变来探讨失重状态下是否可引起大鼠背根神经节损伤,进一步完善失重下引起背根神经节损伤的原因。
材料和方法
1实验动物及模拟失重模型制备 选用鼠龄为12 ~ 14周(体质量约300 g)健康雄性SD大鼠80只(由解放军总医院实验动物中心提供),随机分为尾部悬吊(HU)组(n=40)和正常对照(NC)组(n=40),各组大鼠按照标准条件单笼饲养。参照Morey-Holton和Globus[17]方法制备模拟失重模型。
2背根神经节采集及制备 尾部悬吊4周后,各组大鼠腹腔内注射戊巴比妥钠(45 mg/kg)进行麻醉。将其俯卧于平板上固定,去除背部皮肤,消毒皮肤,后正中切口,显露椎板,在手术显微镜下,用蚊钳咬去椎板,显露背根神经节,NC组和HU组各取右侧L5背根神经节40个,NC组和HU组各取20个背根神经节放入含4%多聚甲醛的磷酸盐缓冲液(phosphate buffer,PB)中后固定4 ~ 6 h (4℃),再移入30%蔗糖的PB中4℃过夜,沉底,OCT胶包埋,垂直DRG长轴行横断切片,片厚4 ~ 5 μ m,其余40个(NC组20个,HU组20个) DRG用于Western-blot及实时PCR检测。
3甲苯氨兰染色观察DRG内尼氏小体形态变化取DRG冷冻切片20个,NC组和HU组各10个,室温放置1 h,PBS洗涤3次,染色,75%乙醇分色,丙酮脱水,封片,镜下观察结果。
4GDNF免疫组化染色 取DRG冷冻切片20个,NC组和HU组各10个,室温放置30 min后,入4℃丙酮固定10 min,PBS洗,用3%过氧化氢溶液孵育5 ~ 10 min,PBS洗,5% ~ 10%正常山羊血清(PBS稀释)封闭,室温孵育10 min。滴加一抗:多克隆的兔抗鼠GDNF抗体,37℃孵育2 h。PBS冲洗,滴加二抗:Cy-3;驴抗兔抗体;(1∶200;Jackson Immuno Research,PA,USA and DyLight488;),37℃孵育30 min,PBS冲洗,滴加辣根酶标记链霉卵白素,37℃孵育30 min,PBS冲洗,封片,观测,测定比较各组积分光密度(integral optical density,IOD)值。
5Western-blot检测GDNF蛋白表达 取右侧L5背根神经节样本20个,NC组和HU组各10个,进行低温匀浆,在4℃ 800 g条件下离心15 min,取上清液,4℃ 105 g条件下离心1 h。离心沉淀后,加入匀浆液,待沉淀充分溶解后,重新在4℃ 105 g条件下离心1 h,上清即为胞膜蛋白。测定样本蛋白浓度后,加入1/4体积的5倍样本缓冲液,于95℃水浴中变性5 min。取等量的40μ g蛋白样本放置在12% SDS-聚丙烯酰胺凝胶上恒压电泳,然后湿转法转移至硝酸纤维素膜。分别加入一抗(1∶200兔抗鼠GDNF,Santa Cruz公司),室温孵育4 h,后滤膜漂洗3次,每次10 min。再加入小牛抗兔二抗(1∶500,SantaCruz公司),于37℃条件下平缓摇动温育1 h。增强化学发光法检测信号,拍摄并扫描照片。反应条带做半定量分析。
6实时PCR检测GDNF mRNA表达 用总RNA抽提试剂盒(Invitrogen life technologies,美国)提取腰5 DRG总RNA。逆转录合成cDNA:RNA 2 μ g,加无RNA酶的H2O至总体积10 μ l;加10 μ l的RT反应液到10 μ l退火混合物中,37℃水浴60 min,95℃变性5 min,得到的RT终溶液即为cDNA溶液。实时定量PCR扩增:25 μ l反应体系,在Rotor-Gene 3 000 Realtime PCR仪中扩增。引物用Primer 5.0软件设计,GDNF上游引物5-'CAGAG GGAAAGGTCGCAGAG-3',下游引物5-'TCGTAGC CCAAACCCAAGTC-3',PCR产物长度96 bp。
7统计学处理 采用SPSS17.0软件处理所得数据,计量资料以表示,组间比较采用t检验,P<0.05为差异有统计学意义,
结 果
1各组大鼠背根神经节切片甲苯氨兰染色分析与NC组相比,HU组尼氏体染色浅,尼氏体变小,弥散分布。见图1。
2各组大鼠背根神经节内GDNF免疫组化分析免疫组化结果显示,与NC组相比,HU组背根神经节内GDNF数量减少(图2),IOD值降低(P<0.05)。
3各组大鼠背根神经节GDNF蛋白表达 HU组背根神经节内GDNF蛋白表达较NC组减少(P<0.05)。见图3。
4各组大鼠背根神经节GDNF mRNA表达 HU组GDNF mRNA表达量明显低于NC组(P<0.05)。见图4。

图 1 与NC组相比,HU组尼氏体染色浅,尼氏体变小,弥散分布(甲苯氨兰染色×100)Fig. 1 Nissl bodies were stained light, smaller and scattering distribution in HU group (×100)
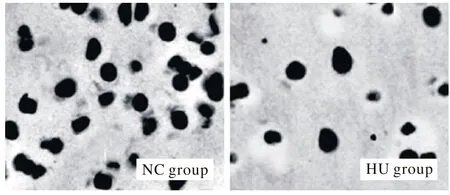
图 2 HU组背根神经节内GDNF数量较NC组减少(免疫组化×200)Fig. 2 Number of GDNF in DRG detected by immunohistochemistry (×200). In HU group, the number of GDNF and IOD were signifi cantly lower than that of NC group

图 3 各组大鼠脊髓背根神经节内GDNF蛋白表达 (aP<0.05)Fig. 3 Protein expression of GDNF in DRG of rats in different treatment group (aP<0.05)
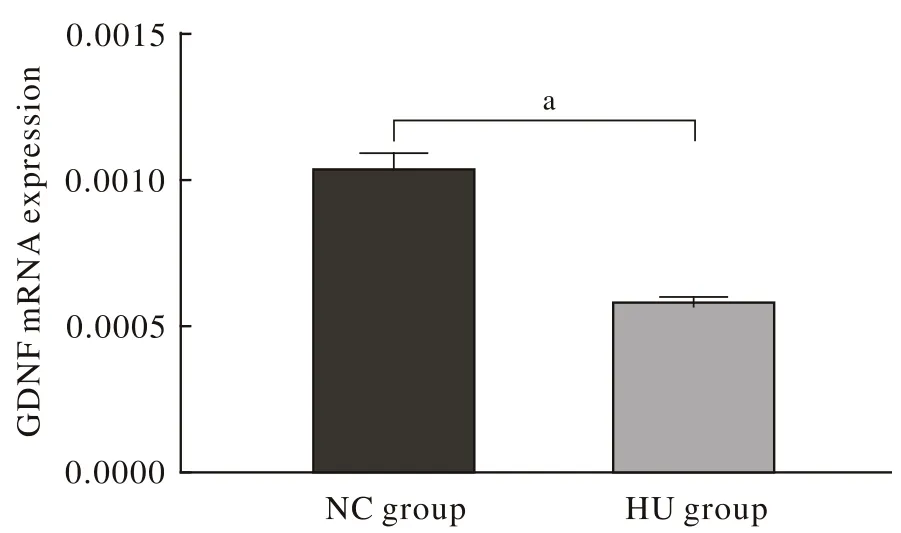
图 4 HU组GDNF mRNA表达量较NC组降低 (aP<0.05)Fig. 4 HU group displayed lower expression of mRNA of GDNF than that of NC group (aP<0.05)
讨 论
尼氏小体可合成神经元所需的蛋白,尼氏小体的形态可反应神经元的功能活性。本实验结果示,经4周的模拟失重, HU组尼氏体较NC组染色浅,尼氏体变小,弥散分布。模拟失重后DRG内尼氏小体形态的改变验证了模拟失重状态下可引起大鼠背根神经节发生损伤,与其他学者研究结果类似。
GDNF由Lin等[20]从大鼠神经胶质细胞系B49的培养液中首先纯化并命名,GDNF对神经元有强大的神经营养作用,抑制其发生退变[19],当神经受损后,GDNF含量或蛋白表达会出现异常。本实验免疫组化、Wester-blot及实时PCR结果示,与NC组相比,HU组大鼠腰5背根神经节中GDNF数量、蛋白表达减少,GDNF mRNA表达量降低。GDNF作为神经营养因子,其含量的减少可导致神经失营养,进而引起神经的损伤。
此实验结果与前期其他学者研究结果相一致,但在失重后神经营养方面的研究却较少,本实验从神经营养方面分析了背根神经节损伤的原因,结果显示,失重可引起神经营养因子GDNF减少,背根神经节失去营养导致损伤,为后期预防和治疗提供了一定的理论基础。
综上所述,4周的模拟失重可以引起大鼠DRG发生损伤性变化,但模拟失重后引起DRG损伤是否具有性别差异、失重条件去除后损伤是否可逆、损伤机制及防治方法还有待于后期进一步研究。
1 Blaber E, Marçal H, Burns BP. Bioastronautics: the influence of microgravity on astronaut health[J]. Astrobiology, 2010, 10(5):463-473.
2 Buckey JC Jr. Preparing for Mars: the physiologic and medical challenges[J]. Eur J Med Res, 1999, 4(9):353-356.
3 Williams DR. Bioastronautics: optimizing human performance through research and medical innovations[J]. Nutrition, 2002,18(10):794-796.
4 Clement G, Slenzka K. Fundamentals of Space Biology: Research on Cells, Animals, and Plants in Space[M]. New York: Springer,2006:51-80.
5 Kalb R, Solomon D. Space exploration, Mars, and the nervous system[J]. Arch Neurol, 2007, 64(4):485-490.
6 LeBlanc A, Schneider V, Shackelford L, et al. Bone mineral and lean tissue loss after long duration space flight[J]. J Musculoskelet Neuronal Interact, 2000, 1(2):157-160.
7 刘宁,崔赓,雷伟,等.尾部悬吊大鼠骨质疏松模型骨的微观结构及力学性能变化的研究[J].中国骨与关节杂志,2012,1(2):169-173.
8 刘宁,雷伟,崔赓,等. 尾部悬吊模拟失重大鼠模型骨密度测量与骨折风险预测[D]. 西安:第四军医大学,2012.
9 Zhang R, Ran HH, Cai LL, et al. Simulated microgravity-induced mitochondrial dysfunction in rat cerebral arteries[J]. FASEB J,2014, 28(6):2715-2724.
10 Zhang R, Ran HH, Peng L, et al. Mitochondrial regulation of NADPH oxidase in hindlimb unweighting rat cerebral arteries[J]. PLoS One, 2014, 9(4):e95916.
11 Zhang R, Jia G, Bao J, et al. Increased vascular cell adhesion molecule-1 was associated with impaired endothelium-dependent relaxation of cerebral and carotid arteries in simulated microgravity rats[J]. J Physiol Sci, 2008, 58(1):67-73.
12 Zhang R, Ran HH, Gao YL, et al. Differential vascular cell adhesion molecule-1 expression and superoxide production in simulated microgravity rat vasculature[J]. EXCLI J, 2010, 9:195-204.
13 Kawano F, Nomura T, Ishihara A, et al. Afferent input-associated reduction of muscle activity in microgravity environment[J]. Neuroscience, 2002, 114(4):1133-1138.
14 Sayson JV, Lotz J, Parazynski S, et al. Back pain in space and postflight spine injury: Mechanisms and countermeasure development[J]. Acta Astronautica, 2013, 86: 24-38.
15 Islamov RR, Rizvanov AA, Tyapkina OV, et al. Genomic study of gene expression in the mouse lumbar spinal cord under the conditions of simulated microgravity[J]. Dokl Biol Sci, 2011, 439:197-200.
16 Jiang B, Roy RR, Polyakov IV, et al. Ventral horn cell responses to spaceflight and hindlimb suspension[J]. J Appl Physiol, 1992, 73(S2):S107-S111.
17 Morey-Holton ER, Globus RK. Hindlimb unloading rodent model:technical aspects[J]. J Appl Physiol, 2002, 92(4): 1367-1377.
18 Ren JC, Fan XL, Song XA, et al. Prolonged hindlimb unloading leads to changes in electrophysiological properties of L5 dorsal root ganglion neurons in rats after 14 days[J]. Muscle Nerve, 2012,45(1): 65-69.
19 Höke A, Ho T, Crawford TO, et al. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers[J]. J Neurosci, 2003,23(2):561-567.
20 Lin LF, Doherty DH, Lile JD, et al. GDNF: a glial cell linederived neurotrophic factor for midbrain dopaminergic neurons[J]. Science, 1993, 260(5111):1130-1132.
Effects of simulated weightlessness on Nissl body morphology and GDNF in DRG of male rats
REN Ningtao1, ZHANG Heng1, LI Jie1, LEI Wei2, LIU Ning2, BI Long2, WU Zixiang2, ZHANG Ran1, ZHANG Yonggang1, CUI Geng1
1Chinese PLA General Hospital, Beijing 100853, China;2Department of Orthopedics, Xijing Hospital, The Fourth Military Medical University, Xi'an 710032, Shaanxi Province, China
CUI Geng. Email: cuigeng@aliyun.com
Objective To investigate the effect of simulated weightlessness on dorsal root ganglia (DRG) and GDNF in rat model. Methods Eighty male Sprague-Dawley rats were randomly divided into HU group (n=40) and normal control (NC) group (n=40). The experiment lasted for 4 weeks, then L5 DRG was excised, toluidine blue staining was used to detect the changes of nissl body morphology, and GDNF was detected using immunohistochemistry method. GDNF protein expression was measured by Westernblot and GDNF mRNA expression was measured by RT-PCR. Results Nissl body staining showed that, compared with NC group, nissl bodies were stained light, smaller and scattering distribution in HU group. Immunohistochemistry revealed that the number of GDNF and IOD value reduced significantly in HU group (P<0.05). Western-blot showed that GDNF protein expression in HU group were significantly lower than NC group (P<0.05). mRNA expression of GDNF decreased significantly in HU group (P<0.05). Conclusion Four weeks of stimulated weightlessness can cause damaged changes in DRG nissl body morphology and reduction of the number, protein expression and mRNA expression of GDNF, which suggests that simulated weightlessness can cause damage changes in rat dorsal root ganglion.
simulated weightlessness; dorsal root ganglia; animal model
R 856
A
2095-5227(2015)05-0502-04
10.3969/j.issn.2095-5227.2015.05.024
时间:2015-02-04 10:50
http://www.cnki.net/kcms/detail/11.3275.R.20150204.1050.003.html
2014-11-17
任宁涛,男,硕士,研究方向:脊柱外科。Email: ning taoren@163.com
崔赓,男,副主任医师,副教授。Email: cuigeng@aliyun. com

