Super solid-base supported Cu catalysts for hydrogenolysis of glycerol to 1,2-propanediol
ZHENG Liping, YUAN Zhenle, XIA Shuixin, CHEN Ping, HOU Zhaoyin
(Institute of Catalysis,Department of Chemistry,Zhejiang University,Hangzhou310028,Zhejiang,China)
Introduction
Solid-base catalysts have a number of advantages over homogeneous base [NaOH, Ca(OH)2] as they are noncorrosive, environmentally benign, present fewer disposal problems and they are easily separated from the liquid products. Solid-base catalysts are widely used in varieties of industry reactions such as the removal of hydrogen sulfide from light oil[1], the conversion of vegetable oil into biodiesel[2], industrial condensation reactions[3], and Michael addition of methyl crotonate[4]and so on. Nowadays, many typesof solid-base catalysts have been developed, and according to the strength of surface base sites which can be measured by Hammett indicators and expressed in terms of the acidity function (H0), they can be divided to mild solid-base catalysts (10
Glycerol, as a byproduct, is produced in larger amounts in the production of biodieselviatransesterification of triglycerides with methanol in the presence of basic or acidic catalysts. More than 3.5×105t glycerol per year was produced in the United States and it has tripled within the past 10 years in Europe. Recently disposal of this surplus glycerol is by incineration to produce energy. How to do with the large quantities of superfluous glycerol is a serious concerning facing all over the world. Until now, many efforts have been put toward the conversion of glycerol to more valuable chemicals such as propanediols[12-15], glyceric acid[16-20]and glycols.Among of which, converting glycerol to 1,2-propanediol (1,2-PDO) by an alternative route involving selective hydrogenolysis of glycerol (Scheme 1) has attracted much attention in recent years[21-23]. And this process is a clean and economically competitive for the utilization of renewable glycerol instead of nonrenewable petroleum.

Scheme 1 Selective hydrogenolysis of glycerol to propanediols
Many catalysts have been extensively investigated for glycerol hydrogenolysis to 1,2-PDO, but most of them are noble metals, such as well-known supported noble metals Ru, Rh, Pt, and their alloys[24-26]. And these catalysts always catalyzed the cleavage of C—C bond, resulting in a low selectivity to 1,2-PDO.Copper-based catalysts, as a less expensive alternative,have a superior performance in this reaction due to their high efficiency for C—O bond hydro-dehydrogenation and lower activity for C—C bond cleavage[27-29]. Cu/ZnO catalyst prepared by coprecipitation was also reported active for this reaction[30], whereas the catalytic activity and selectivity were lower, moreover, these catalysts were not stable as the active species are easily to sinter during the reaction.
According to the reaction mechanism of glycerol hydrogenolysis to 1,2-PDO[31-32], a solid bi-functional catalyst consisting of metal and acidic/basic sites should be capable for this reaction. Choosing solid base and/or solid acid as a component and support of metal catalyst would be an alternative of the hybrid catalyst. Previous works[5-11]found that solid base supported Cu catalysts are more suitable for hydrogenolysis of glycerol, and the activity of these catalysts increased with their base strength.
In present work, a simple and convenient impregnation-combustion method was used to prepare highly dispersed copper particles supported on combined alkaline-earth metal oxides. And these catalysts were tested in hydrogenolysis of glycerol to 1,2-PDO. The relation between the performance for hydrogenolysis of glycerol to 1,2-PDO and the physico-chemical properties of the catalyst were discussed.
1 Experimental
1.1 Catalyst preparation
First, calculated amount of MgO (AR, Sinopharm Chemical Reagent Co., Ltd., China) pretreated at 823 K for 4 h was dispersed into water under stirring. Then calculated amount of Cu(NO3)2·3H2O (AR,Sinopharm Chemical Reagent Co., Ltd., China) was added into the suspended solution with stirring for 20 min. At last, quantitative NaOH solution (0.5 mol·L-1)was dripped within 1 h to deposit Cu2+onto the surface of MgO. After that, the precipitate was filtrated and washed with distilled water. This precipitate was dried in ambient air at 383 K overnightand calcined at 723 K for 4 h. Catalyst prepared according to this method with copper loading amount of 10%(mass) was identified as Cu/MgO.
The above prepared Cu/MgO catalyst was impregnated into an aqueous solution of Ca(NO3)2·4H2O or Sr(NO3)2mixed with C6H8O7·H2O(citric acid), the mole ratio between C6H8O7·H2O and Ca(NO3)2·4H2O or Sr(NO3)2was controlled to 8:1.Then the impregnated catalyst was dried at 383 K for 12 h followed by calcination at 723 K for 4 h.Catalysts prepared according to this method were identified as Cu/MgO-CaO and Cu/MgO-SrO. In this experiment, the amount of CaO or SrO was controlled to 10%(mol) of MgO.
1.2 Catalytic reactions
All fresh Cu-solid base catalysts were firstly reduced in H2at 623 K for 1 h before they were used for hydrogenolysis of glycerol. The hydrogenolysis of glycerol reaction was carried out in a custom-designed 50 ml stainless steel autoclave equipped with inner Teflon coating and a thermoelectric couple. Aqueous solution of glycerol [75%(mass), 8.0 ml] and 1.0 g reduced Cu/solid-base catalyst were firstly added to the reactor. The autoclave was purged with H2for 5 times, the initial hydrogen pressure was controlled at 3.0 MPa (without further addition during the reaction),then put in an oil bath preheated to the required temperature and maintained at that temperature for a given period time under vigorously stirring with a magnetic stirrer (MAG-NEO, RV-06M, Japan). After reaction, the reactor was cooled to room temperature;vapor phase was collected by a gas-bag and analyzed using a gas chromatograph (Shimadzu, 8A) equipped with an active carbon column and a thermal conductivity detector (TCD). Liquid phase was centrifuged to remove the solid catalyst powder and analyzed using a gas chromatograph (Shimadzu, 14 B)equipped with a 30 m capillary column (DB-WAX 52 CB, USA) and a flame ionization detector (FID).
1.3 Characterizations
The actual loading amount of copper in prepared catalysts was checked and confirmed by inductively coupled plasma-atomic emission spectroscopy (ICP,Plasma-Spec-Ⅱ spectrometer). BET surface areas were measured by N2physisorption at its normal boiling point (77 K) using an ASAP 2010 analyzer(Micromeritics). The catalysts were first degassed at 523 K for 4 h (<2.66 Pa) before adsorption. XRD patterns were collected on a Rigaku D/MAX-2500 diffractometer in a 2θrange of 5°—70° using Cu Kαradiation (λ=0.15406 nm). The crystallite size (D) of CuO and Cu particles were calculated by Scherrer equation,D= 0.90λ/βcosθ, whereθis the diffraction angle andβis the full width at half-maximum(FWHM).
TEM images of reduced catalysts were recorded using an accelerating voltage of 200 kV (JEOL-2010 F). Before TEM analysis, catalysts were reduced in a stream of H2at 623 K for 1 h, the reduced powders were poured into alcohol under the protection of H2and suspended with an ultrasonic dispersion for 10 min, and then the resulted solution was dropped on carbon film of nickel grid.
TPR experiments were conducted in a quartz reactor. Samples were loaded and pretreated firstly at 623 K for 1 h under N2flow (30 ml·min-1). After sample was cooled to room temperature, reduction agent (10% H2/N2mixture, 30 ml·min-1) was shifted;sample was heated to 723 K at a rate of 10 K·min-1.Effluent gas was dried by powder KOH and consumption of hydrogen was analyzed by thermal conductivity detector (TCD). The amounts of hydrogen consumption were calibrated using known amounts of CuO.
The number of surface metallic copper sites was determined by dissociative N2O adsorption at 323 K using the procedure described in reference[7]. Catalysts were firstly reduced in the procedure described in above TPR experiment in 10% H2/N2mixture (30 ml·min-1) until 723 K. The amount of hydrogen consumption in first TPR was denoted asX. And then,the catalyst bed was purged with He and cooled to 323 K. Surface copper atoms were oxidized in a 20%N2O/N2(30 ml·min-1) at 323 K for 0.5 h. Finally,samples were flushed with He to remove the oxidant and cooled to room temperature to start another TPR run. Hydrogen consumption in the second TPR was denoted asY. The dispersion of Cu and exposed Cusurface area are calculated in the following equations:All copper atoms were reduced in the first TPR:

hydrogen consumption =X
Surface copper atoms that were oxidized to Cu2O by N2O at 323 K were reduced in the second TPR:

hydrogen consumption =Y
And the dispersion of Cu was calculated as:

The alkalinity of the catalysts was carried outviaCO2-TPD. Sample was first pretreated at 823 K(except for Cu/MgO-SrO at 1330 K) in purified Ar flow of 50 ml·min-1for 1 h, and then cooled to 323 K,exposed to 20% CO2/Ar for 30 min, purged by Ar for 5 h at 323 K in order to eliminate the physical adsorbed CO2. Temperature-programmed desorption was conducted by ramping to 823 K (except for Cu/MgO-/SrO at 1330 K) at 10 K·min-1and CO2(m/z =44) in effluent was detected and recorded as a function of temperature by a quadrupole mass spectrometer (OmniStarTM, GSD301, Switzerland).
2 Results and Discussion
2.1 XRD
The XRD patterns of calcined catalysts are shown in Fig.1. After calcination at 723 K, copper species in all samples were converted into CuO. The diffraction peaks of CuO (at 2θ= 35.6° and 39.0°) in Cu/MgO catalyst are broader and less intense,suggesting that CuO/MgO prepared in this work has smaller CuO crystalline size (2.6 nm) and higher Cu dispersion. The CuO crystalline size in Cu/MgO-CaO and Cu/MgO-SrO was bigger than that of Cu/MgO,which is 4.3 and 5.1 nm respectively. This may be attributed to the combustion reaction of Sr(NO3)2or Ca(NO3)2with C6H8O7·H2O proceed on the surface of Cu/MgO can cause the sintering of CuO particles.Neither CaO nor SrO phase was detected in these combined solid-base supported Cu catalysts, whereas CaCO3and SrCO3formed on catalysts Cu/MgO-CaO and Cu/MgO-SrO, which suggested that the formed CaO or SrO during the combustion reaction easily reacted with the carbon dioxide in air or the existed CaO or SrO particles are too small to be detected.

Fig.1 XRD patterns of calcined Cu-solid base catalysts
Fig.2 shows the XRD patterns of the reduced catalysts. It can be found that the peaks of CuO in all catalysts disappeared after reduction.

Fig.2 XRD patterns of reduced Cu-solid base catalysts
2.2 H2-TPR
The H2-TPR profiles of the calcined samples are illustrated in Fig.3. Mainly one reduction peak from 483 to 573 K was detected in Cu/MgO catalyst, which could be ascribed to the reduction of highly dispersed copper oxides that has a weak interaction with MgO support[6-11]. When Cu/MgO was modified with CaO,the reduction temperature shifted to higher temperature than that of Cu/MgO, and another reduction peak from 573 to 623 K was detected. And the ratio of the second peak increased obviously in Cu/MgO-SrO, which means that CaO and SrO promotes the interaction between the copper species and the surface of the MgO support[33-35]. This strong interaction could protect the reduced Cu species from sintering.
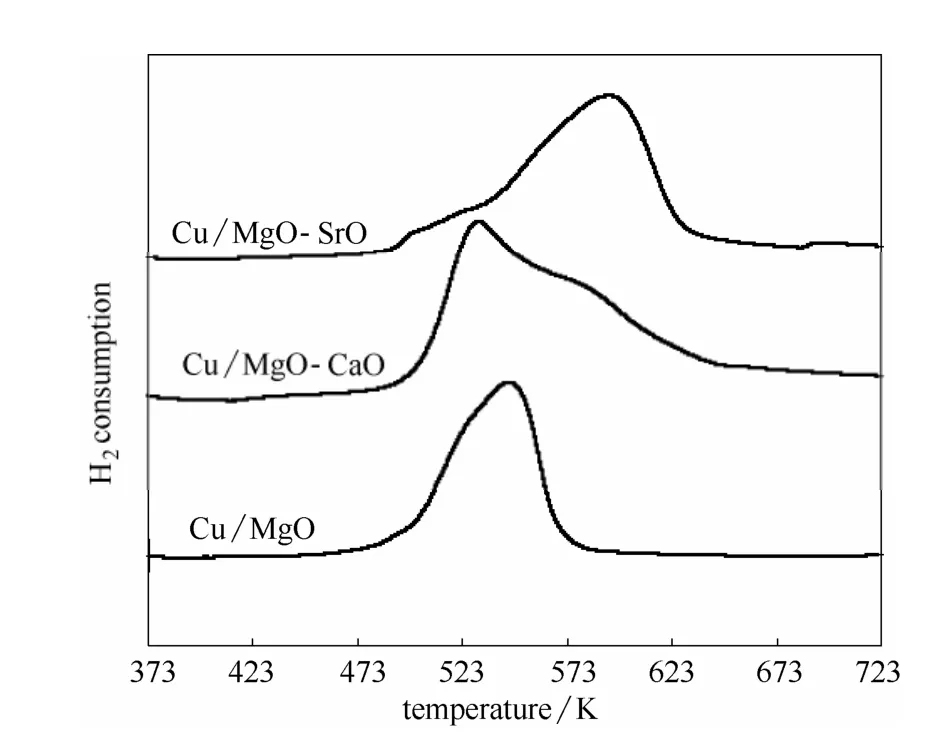
Fig.3 H2-TPR profiles of calcined Cu-solid base catalysts
2.3 TEM analysis
The morphologies of the calcined samples are shown in Fig.4. The outline of a Cu/MgO catalyst particle is sized in 350—600 nm, on which copper species sized 2.0—7.1 nm disperse uniformly. The copper particles exhibit a narrow size distribution with an average diameter of 4.1 nm. While it is interesting to find that outline of a separated Cu/MgO-CaO and Cu/MgO-SrO particle decreased obviously to 50—100 and 50—85 nm, respectively. And copper species sized 2.5—9.0 nm dispersed uniformly on the surface of the combined solid-base particles. These results further indicated that the combustion reaction of Ca(NO3)2or Sr(NO3)2with citric acid on Cu/MgO decreased the particle size of catalyst and didn’t make the copper particles sintered obviously.
2.4 Surface area of catalysts and Cu dispersion
The specific surface area of Cu/solid-base, Cu dispersion and particle size are summarized in Table 1.It was found that the surface area of Cu/MgO-CaO and Cu/MgO-SrO increased from 30.6 m2·g-1(of Cu/MgO) to 51.3 and 57.5 m2·g-1, respectively. This phenomenon can be attributed to that the smaller catalysts particles formed in Cu/MgO-CaO and Cu/MgO-SrO (see Fig.4) after the combustion reaction.
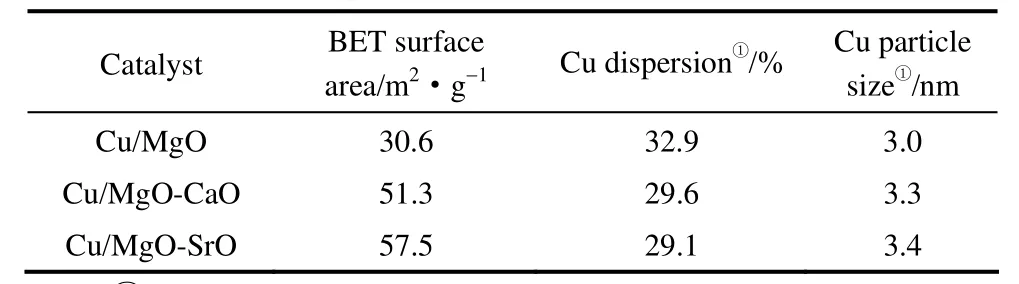
Table 1 Text properties of Cu/solid-base catalysts

Fig. 4 TEM images of reduced Cu-solid base catalysts
The dispersion of Cu detected by surface N2O oxidation experiment in reduced Cu/MgO catalyst reached 32.9%, and the calculated mean particle size of metallic Cu is 3.0 nm (Table 1). And when Cu/MgO was modified with CaO and SrO, Cu dispersiondecreased slightly and calculated copper particle size increased to 3.3 and 3.4 nm, respectively. These results are generally in agreement with that obtained from XRD and TEM.
2.5 CO2-TPD
CO2-TPD profiles of Cu/MgO, Cu/MgO-CaO and Cu/MgO-SrO are shown in Fig.5. Because the decomposition of SrCO3needs high temperature(> 1123 K), the thermoelectric couple in our group cannot endure that high temperature for twice times,so only Cu/MgO-SrO was detected in the temperature ranged from 323 to 1330 K (the figure insert in Fig.5).All catalysts have two desorption peaks. The desorption peaks detected in Cu/MgO are located at 413 and 489 K, respectively (Fig.5). After modified with CaO and SrO, both two peaks shifted to higher temperature, which indicated that the strength of the alkalinity increased (Fig.5). Both the number and strength of alkaline sites in Cu/MgO-SrO are higher than Cu/MgO-CaO. The figure insert in Fig.5 shows the CO2-TPD profile of Cu/MgO-SrO pretreated at 1330 K. A peak detected at 1219 K is ascribed to the decomposition of SrCO3.

Fig.5 CO2-TPD profiles of Cu-solid base catalysts
2.6 Glycerol hydrogenolysis
The activity of these catalysts for the hydrogenolysis of glycerol is summarized in Table 2.At 433 K, the best activity was achieved on Cu/MgO-SrO, with a 81.6% conversion of glycerol and a 91.0% selectivity of 1,2-PDO, which is about two times of that of Cu/MgO catalyst. These results indicated that the activity of catalysts for the hydrogenolysis of glycerol increased with the strength of its alkalinity.

Table 2 Glycerol hydrogenolysis over Cu/solid-base catalysts
Fig.6 illustrates the time course of hydrogenolysis of glycerol over these catalysts. It can be found that the conversion of glycerol increased quickly in the first 20 h for all catalysts, Cu/MgO-SrO exhibited the best performance in these experiments.The activities of these catalysts under different temperature are presented in Fig.7. Even at 413 K,which is significantly lower than those reported in the literatures (usually temperature higher than 473 K are needed to achieve high activity)[5-11], Cu/MgO-SrO is capable for the hydrogenolysis of glycerol and the detected conversion of glycerol reaches 30.1%, and it increased quickly to 97.0% with the reaction temperature (at 473 K). The selectivity of 1,2-PDO remains higher than 90% during these experiments.
3 Conclusions

Fig. 6 Time courses of glycerol hydrogenolysis on different Cu-solid base catalysts

Fig.7 Conversion of glycerol at different temperature
In this work, we found that the impregnatecombustion is a simple and efficient method to prepare a solid super base supported Cu catalyst with high dispersion and strong alkalinity, and these catalysts are capable for the hydrogenolysis of glycerol at low temperature. At 453 K, 3.0 MPa H2and 15 h, the conversion of glycerol on Cu/MgO-SrO reaches 91.2%, and the selectivity of 1,2-PDO remains higher than 90%. Characterizations indicated that this excellent performance of Cu/MgO-SrO could be attributed to its strong alkalinity.
[1] Ma F, Hanna M A. Biodiesel production:a review [J].Bioresour.Technol.,1999, 70:1-15.
[2] Kouzu M, Tsunomori M, Yamanaka S, Hidaka J. Solid base catalysis of calcium oxide for a reaction to convert vegetable oil into biodiesel[J].Adv. Powder Technol., 2010, 21:488-494.
[3] King F, Kelly G J. Combined solid base/hydrogenation catalysts for industrial condensation reaction [J].Catal. Today, 2002, 73:75-81.
[4] Kabashima H, Tsuji H, Hattori H. Michael addition of methyl crotonate over solid base catalysts [J].Appl. Catal. A, 1997, 165:319-325.
[5] Yuan Z L, Wu P, Gao J, Lu X Y, Hou Z Y. Pt/solid-base:a predominant catalyst for glycerol hydrogenolysis in a base-free aqueous solution [J].Catal. Lett., 2009, 130:261-265.
[6] Yuan Z L, Wang J H, Wang L N, Xie W H, Chen P, Hou Z Y.Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts [J].Bioresour. Technol., 2010, 101:7088-7092.
[7] Yuan Z L, Wang L N, Wang J H, Xia S H, Chen P, Hou Z Y.Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts [J].Appl. Catal. B, 2011, 101:431-440.
[8] Xia S X, Yuan Z L, Wang L N, Chen P, Hou Z Y. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts preparedvialayered double hydroxides precursors [J].Appl. Catal. A, 2011, 403:173-182.
[9] Xia S X, Du W C, Zheng L P, Chen P, Hou Z Y. A thermally stable and easily recycled core-shell Fe2O3@CuMgAl catalyst for hydrogenolysis of glycerol [J].Catal. Sci. Technol., 2014, 4:912-916.
[10] Xia S X, Zheng L P, Nie R F, Chen P, Lou H, Hou Z Y. Trivalent metal ions M3+in M0.02Cu0.4Mg5.6Al1.98(OH)16CO3layered double hydroxide as catalyst precursors for the hydrogenolysis of glycerol [J].Chin. J. Catal.,2013, 34:986-992.
[11] Xia S, Zheng L P, Wang L N, Chen P, Hou Z Y. Hydrogen-free synthesis of 1,2-propanediol from glycerol over Cu-Mg-Al catalysts[J].RSC Adv., 2013, 3:16569-16576.
[12] Ma L, He D H. Influence of catalyst pretreatment on catalytic properties and performances of Ru–Re/SiO2in glycerol hydrogenolysis to propanediols [J].Catal. Today, 2010, 149:148-156.
[13] Zhu S H, Gao X Q, Zhu Y L, Zhu Y F, Xiang X M, Hu C X, Li Y W.Alkaline metals modified Pt-H4SiW12O40/ZrO2catalysts for the selective hydrogenolysis of glycerol to 1,3-propanediol [J].Appl.Catal. B,2013, 140/141:60-67.
[14] Li Y M, Liu H M, Ma L, He D H. Glycerol hydrogenolysis to propanediols over supported Pd-Re catalysts [J].RSC Adv.,2014, 4:5503-5512.
[15] Niu L, Wei R P, Yang H, Li X, Jiang F, Xiao G M. Hydrogenolysis of glycerol to propanediols over Cu-MgO/USY catalyst [J].Chin. J.Catal.,2013, 34:2230-2235.
[16] Gao J, Liang D, Chen P, Hou Z Y, Zheng X M.Oxidation of glycerol with oxygen in a base-free aqueous solution over Pt/AC and Pt/MWNTs catalysts [J].Catal. Lett., 2009, 130:185-191.
[17] Liang D, Gao J, Wang J H, Chen P, Hou Z Y, Zheng X M.Selective oxidation of glycerol in a base-free aqueous solution over different sized Pt catalysts [J].Catal. Commun., 2009, 10:1586-1590.
[18] Liang D, Gao J, Sun H, Chen P, Hou Z Y, Zheng X M. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts [J].Appl. Catal. B,2011, 106:423-432.
[19] Nie R, Liang D, Shen L, Gao J, Chen P, Hou Z Y. Selective oxidation of glycerol with oxygen in base-free solution over MWCNTs supported PtSb alloy nanoparticles [J].Appl. Catal. B, 2012, 127:212-220.
[20] Zhang M Y, Nie R F, Wang L N, Shi J J, Du W C, Hou Z Y. Selective oxidation of glycerol over carbon nanofibers supported Pt catalysts in a base-free aqueous solution [J].Catal. Commun.,2015, 59:5-9.
[21] Gandarias I, Arias P L, Requies J, EI Doukkali M, Güemez M B.Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source [J].J. Catal.,2011, 282:237-247.
[22] Zhu S H, Gao X Q, Zhu Y L, Fan W B, Wang J G, Li Y W. A highly efficient and robust Cu/SiO2catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol [J].Catal. Sci. Technol.,2015, 5:1169-1180.
[23] Hu J Y, Liu X Y, Fan Y Q, Xie S H, Pei Y, Qiao M H, Fan K, Zhang X,Zong B. Physically mixed ZnO and skeletal NiMo for one-pot reforming-hydrogenolysis of glycerol to 1,2-propanediol [J].Chin. J.Catal.,2013, 34:1020-1026.
[24] Feng J, Fu H Y, Wang J B, Li R X, Chen H, Li X J. Hydrogenolysis of glycerol to glycols over ruthenium catalysts:effect of support and catalyst reduction temperature [J].Catal. Commun., 2008, 9:1458-1464.
[25] Maris E P, Davis R J. Hydrogenolysis of glycerol over carbon-supported Ru and Pt catalysts [J].J. Catal., 2007, 249:328-337.
[26] Lahr D G, Shanks B H. Effect of sulfur and temperature on ruthenium-catalyzed glycerol hydrogenolysis to glycols [J].J. Catal.,2005, 232:386-394.
[27] Runeberg J, Baiker A, Kijenski J. Copper catalyzed amination of ethylene glycol [J].Appl. Catal., 1985, 17:309-319.
[28] Huang Z W, Cui F, Kang H X, Chen J, Zhang X Z, Xia C G.Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method:a simple but efficient and stable catalyst for glycerol hydrogenolysis [J].Chem. Mater., 2008, 20:5090-5099.
[29] Huang Z W, Cui F, Kang H X, Chen J, Xia C G.Characterization and catalytic properties of the CuO/SiO2catalysts prepared by precipitation-gel method in the hydrogenolysis of glycerol to 1,2-propanediol:effect of residual sodium [J].Appl. Catal. A, 2009,366:288-298.
[30] Wang S, Liu H C. Selective hydrogenolysis of glycerol to propylene glycol on Cu-ZnO catalysts [J].Catal. Lett.,2007, 117:62-67.
[31] Gandarias I, Arias P L, Requies J, Güemez M B, Fierro J L G.Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst:the role of acid and metal sites on product selectivity and the reaction mechanism [J].Appl. Catal. B, 2010, 97:248-256.
[32] Wang S, Zhang Y C, Liu H C. Selective hydrogenolysis of glycerol to propylene glycol on Cu-ZnO composite catalysts:structural requirements and reaction mechanism [J].Chem.-Asi. J., 2010, 5:1100-1111.
[33] Anderson J A, Márquez-Alvarez C, López-Munõz M J, Rodríguez-Ramos I, Guerrero-Ruiz A. Reduction of NOxin C3H6/air mixtures over Cu/Al2O3catalysts[J].Appl. Catal. B, 1997, 14(3/4):189-202.
[34] Carniti P, Gervasini A, Modica V H, Ravasio N. Catalytic selective reduction of NO with ethylene over a series of copper catalysts on amorphous silicas [J].Appl. Catal. B, 2000, 28:175-185.
[35] Suh Y-W, Moon S-H, Rhee H-K. Active sites in Cu/ZnO/ZrO2catalysts for methanol synthesis from CO/H2[J].Catal. Today, 2000,63:447-452.

