Research of growth mechanism of ceramic coatings fabricated by micro-arc oxidation on magnesium alloys at high current mode
Wei-wei Chen,Ze-xin Wang,Lei Sun,Sheng Lu*
School of Materials Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang,212003 Jiangsu,China
Research of growth mechanism of ceramic coatings fabricated by micro-arc oxidation on magnesium alloys at high current mode
Wei-wei Chen,Ze-xin Wang,Lei Sun,Sheng Lu*
School of Materials Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang,212003 Jiangsu,China
Micro-arc oxidation(MAO)coatings of ZK60 magnesium alloys were formed in a self-developed dual electrolyte composed of sodium silicate and phosphate at the high constant current of 1.8 A(15 A/dm2).The MAO process and growth mechanism were investigated by scanning electron microscopy(SEM)coupled with an energy dispersive spectrometer(EDS),confocal laser scanning microscopy and X-ray diffraction(XRD).The results indicate that the growth process of MAO coating mainly goes through“forming→puncturing→rapid growth of micro-arc oxidation→large arc discharge→self-repairing”.The coating grows inward and outward at the same time in the initial stage,but outward growth of the coating is dominant later.Mg,Mg2SiO4and MgO are the main phases of ceramic coating.
ZK60 magnesium alloys;Micro-arc oxidation;High current;Growth mechanism
1.Introduction
Magnesium alloys have been paid enough attention to for many years due to the resource,performance,and price advantage,called as“the green structural materials in the 21st century”.However,the application potential and range are both limited by their susceptibility to corrosion and inferior wear performance.Therefore,the proper surface modifcation treatment can enhance the corrosion resistance of the existing magnesium alloy which has important practical signifcance [1,2].
Micro-arc oxidization(MAO)is a simple,friendly,effcient surface treatment method of forming ceramic coatings on Al, Ti,Mg and their alloys.The surface properties,such as wear and corrosion resistance,electrical insulation,adhesion to substrate,can be considerably improved by MAO[3–5].It is an incontrovertible fact that the growth mechanism of MAO process is extremely complex,including chemical,electrochemical,high temperature plasma reaction,etc.And no reasonable model can fully and precisely describe the theory at home and abroad.Chen et al.[6,7]thought the process is a submonolayer growth,which is a cyclic process experiencing the repeated course of“forming→breakdown→melting→liquating→ sintering→reforming”.Cellular structure composed of nanoparticles is growing layer-by-layer,whose dense layer is made up of MgO mainly.Ge et al.[8]studied the MAO process of wrought magnesium alloy in the silicate system. They found that dense layer is formed in the preliminary stage, and loose layer appears later,accounting for a large percentage of the total flm thickness.They also carried out quantitative analysis and measurement of the energy loss and impedance change of membrane layer in reaction.The researchers of Northwestern Polytechnical University[9–11]studied the effect from rare earth elements on the growth of membrane layer.They thought the MAO flm is a double-layer structure in silicate system.The dense layer is mainly composed of MgO, and the loose layer is mainly composed of MgSiO3.
Our group has completed the research on growth mechanism at low-current earlier[12].In this paper,combined with the testing results,the authors aim to make a further exploration at the high current in order to form a complete system on MAO process and growth mechanism at constant current mode.
2.Experimental
The substrate material used for investigation was wrought ZK60 magnesium alloy with a chemical composition(Zn 4.8–6.2%,Zr>0.45%,impurities≤0.30%,Mg balance). The samples with a size of 20 mm×20 mm×5 mm were successively ground to 1600 grit silicon carbide papers,cleanedby alcohol,and then dried in cold air.WHD-20 MAO system was employed for MAO treatment.The samples acted as anodes and a stainless steel container acted as cathode.The experiments were carried out in an optimized dual electrolyte composed of sodium silicate and phosphate under constant current. The temperature was kept in electrolyte under 40°C through circulating water cooling system.
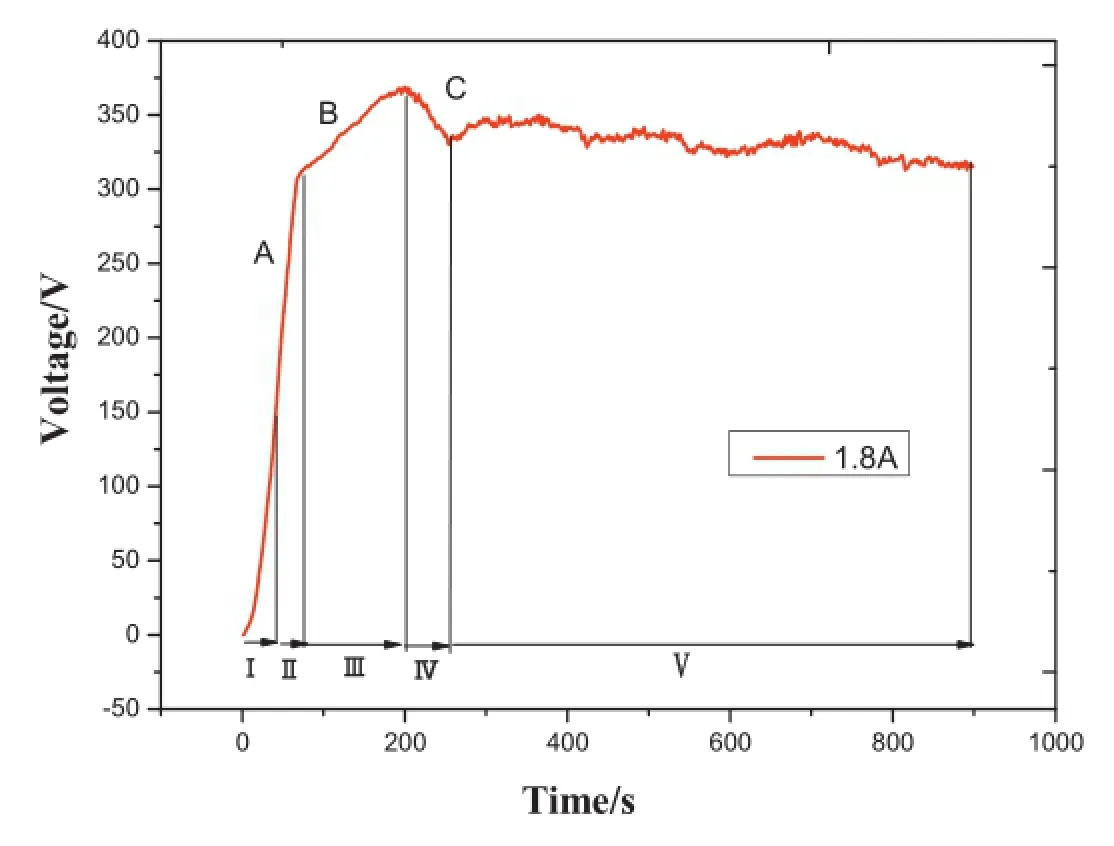
Fig.1.Voltage-time curve at the current of 1.8 A.
The micro-structural characteristics of coating,coating elements and phase constituent were investigated by scanning electron microscopy(SEM,JSM-6480),X-ray diffraction (XRD,Shimadzu XRD-6000)and Olympus confocal laser scanning microscopy(OLS4000).The micrometer was used to measure the thickness of the specimen before and after the MAO process.The MAO flm thickness was measured by the coating thickness measurement equipment(OXFORD CMI233).
3.Results and discussion
3.1.Analysis of voltage-time curve and MAO spark discharge phenomenon
The MAO coating growth process is monitored by the voltage-time curve,which can be divided into fve distinguishing stages as shown in Fig.1.At the frst stage,within 0–40 s, the substrate surface quickly formed a very thin layer,named anodic oxidation stage.The substrate surface appeared the frst spark(Fig.2(a)),which meant that the MAO process reached the striking voltage about 140 V at around 40 s.The second stage lasted from 40 s to 70 s;the anodic oxidation coating got punctured and MAO coating was formed.The third stage(70 s–200 s)corresponded to the main period of rapid growth of MAO,involving large numbers of white and bright sparks (Fig.2(b)),as a result of highlight plasma micro-arc discharge. The value of voltage reached a maximum of 370 V at about 200 s.In this stage,the porous flm solidifed rapidly because of the effect of high-temperature micro zone and“quenching”in the electrolyte.The thickness of coating increased by 25µm and the value of growth rate got to a maximum of 13.15µm/ min.After 200 s,plasma point discharge concentrated upon the places of edges or corners by the effects of high voltage and strong electric feld,which led to the breakdown,and even dissolution and desquamation of part of the membrane layer. Compared with the second stage and third stage,the growth rate decreases signifcantly after 200 s.The voltage curve of the fourth stage(200–250 s)presents a brief drop;the voltage value drops slightly.Through macroscopic observation,the number of sparks reduced,but single spark became larger.So this stage can be named local large arc discharge(Fig.2(c)).The last stage was characterized by a voltage plateau from 250 s to the end in which many micro-cracks and micro-pores were disappeared gradually and the surface also became smooth.According to the macroscopic experimental phenomena and microstructure,this stage played a key role to control the quality of the flm through self-repairing.
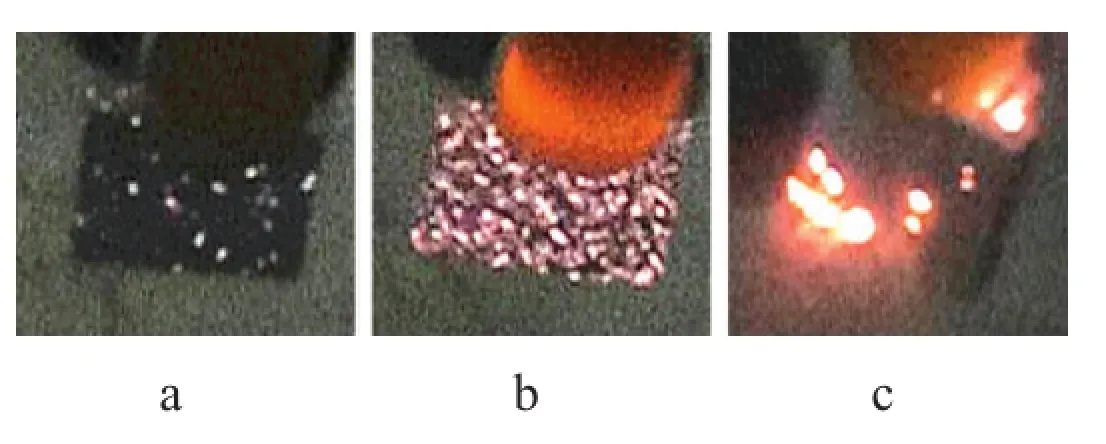
Fig.2.Spark discharge characteristics at different time points.
3.2.Analysis of microstructure
The surface and cross-section micro-morphologies of MAO coating at different stage are shown in Fig.3.At the time of 60 s,passive flm was punctured and large numbers of micro discharge channels appeared on the surface(Fig.3(a)).When oxidation time lasted for 180 s,the variations of surface morphologies show normal regularity that the quantity of the micro discharge channels decreased but the size of micro-pores increased sharply.Most of the pore diameters are in the range of 10 to 15µm.In addition,continuous micro-cracks and loose micro-pores both existed in the surface and cross-section of coating due to the effect of quenching stress(Fig.3(b)and Fig.3(e)).At the low-voltage self-repairing stage of 480 s,the number of micro-pores decreased to some degree because plenty of melt accumulated on the surface and covered parts of discharge channels as seen in Fig.3(c).The average pore diameter decreased to 4.3µm.At last,continuous micro-cracks of the coating disappeared gradually or transformed into discontinuous micro-cracks,and cross-section morphologies of the coating became smooth and dense(Fig.3(d)and(g)).The average thickness of the coating reached 65µm.
3.3.Analysis of growth characteristic
For growth characteristic of MAO coating by high-current mode,Fig.4 shows the dynamic changing process of coating thickness in two sides.It can be found that the growth rate of the two sides is basically synchronous without huge difference.The growth rate is nearly close to 0 before 70 s,namely,only anultrathin layer is formed on the surface which is hard to measure accurately.At the rapid growth of MAO stage from 70 to 200 s,the flm thickness of two sides both increased signifcantly and the growth rate also reach their extreme value of 13.43µm/min and 12.87µm/min,respectively.Compared with the previous stage,the growth rate reduces to a certain degree after 200 seconds,but the coating thickness all sides,so the two sides of the coating can grow together basically.However,the discharge concentrated on one side and stimulated the continued increase up to the end at a steady rate of 3.30µm/min. Finally,the average coating thickness is 66µm.It can be speculated from the above laws that the available energy budget provided by the high-current is suffcient to meet the discharge requirement in growth of flm at the same side by low-current MAO.Consequently,the two sides of coating fabricated by low-energy MAO grew together in the initial of MAO process, but evolved asynchronously in the late process of MAO,and consistently in the end[12].

Fig.3.Surface and cross-section micromorphologies of MAO coating at the current of 1.8 A.
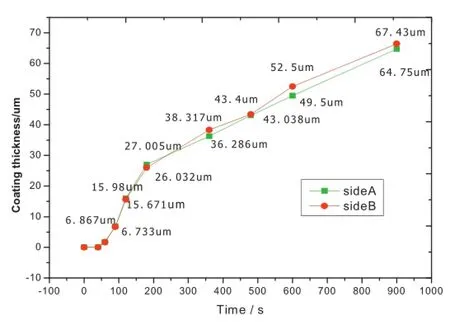
Fig.4.Growth rate of MAO coating at the current of 1.8 A.
The rule of dynamic process in the growth of MAO coating shows that the coating thickness is controllable by adjusting the process parameters to meet the different needs in engineering.
3.4.Analysis of inward growth and outward growth of the coating
The growth schematic diagram of MAO coating on Mg alloy is presented in Fig.5.The dashed lines show the original surface of magnesium alloy,h means the coating’s total thickness,a means the thickness of outward growth part and b means the thickness of inward growth part[15].H1 means the sample total thickness after MAO.H0 means the blank sample thickness.H1 and H0 were both measured by the micrometer. According to Eq.(1)and Eq.(2),a and b can be:

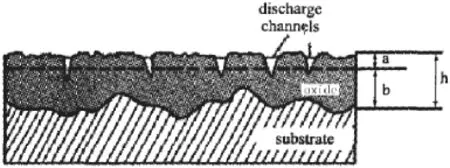
Fig.5.Schematic diagram of micro-arc oxidation coating on Mg alloy.
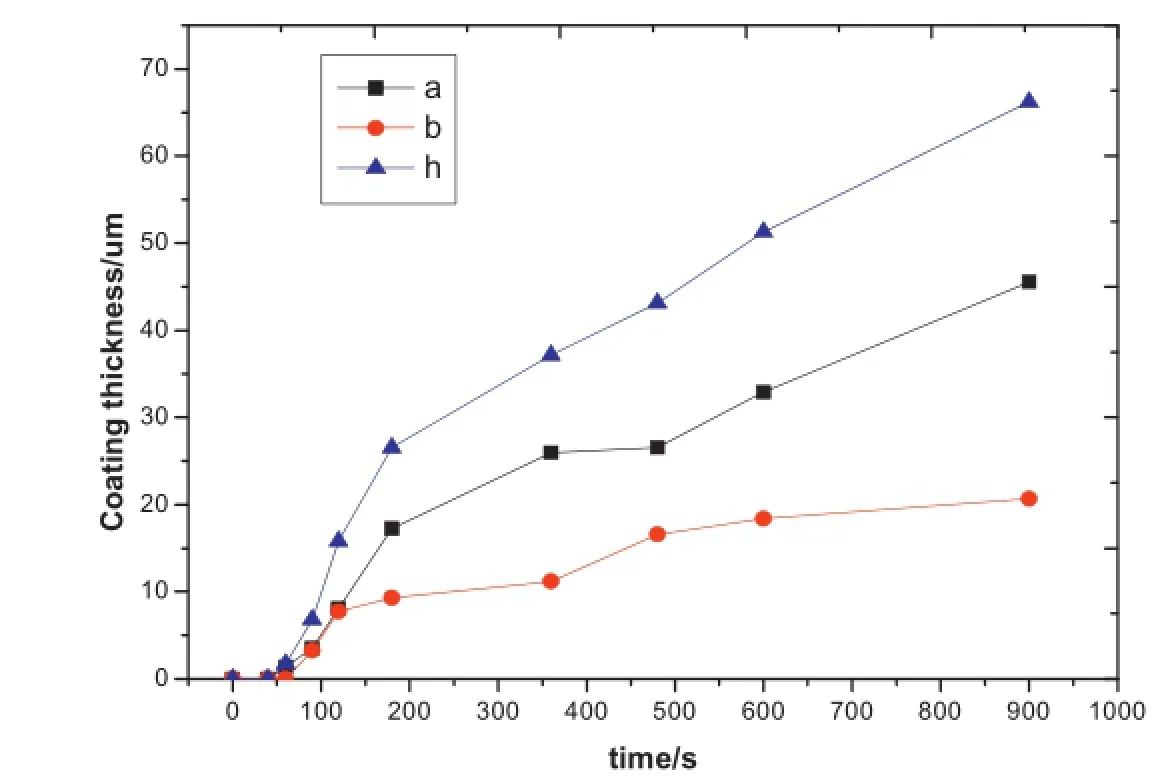
Fig.6.The growth characteristic of inward and outward micro-arc oxidation coating.
At present,different opinions about the growth mechanism theory still exist[8–10,13].In this paper,the inward and outward growth problem at the high current mode is investigated appropriately.Fig.6 shows that the thickness values of inward and outward growth are both low in the frst and second stages,and only a very thin flm is formed in the substrate surface.At the rapid growth stage of MAO(70–200 s),the thickness of both the inward and outward growth change signifcantly.And the inward and outward growth rate both get maximum values that are 7.76µm/min and 4.66µm/min respectively.It can be speculated that the ions of Mg alloy substrate and electrolyte reacted rapidly in the discharge channel due to the enormous energy releasing from the spark discharge of micro-arc oxidation under high current and temperature,then the flm formed.The inward and outward growth rates both decrease after 200 s when the MAO process enters into the self-repairing stage.The two sides of the coating mainly grow outward later,and the inward growth rate reduces to 0.94µm/min.This is because only small amount of electrolyte can go through the micro discharge channels and react with the inward substrate;more electrolyte is involved in the outward reaction.Finally,the thickness of the outward growth part takes a larger part of the total thickness.
In order to further testify the conclusion above,there is a way to make half of one sample under MAO;the other half was sealed with resin.The microstructure of inward and outward of micro-arc oxidation coating is presented in Fig.7.It can be seen that the thickness appears both on the inward and outward growth at 90 s and 180 s.With the extension of oxidation time, the outward growth part of the thickness gradually takes a larger part of the total thickness at the point of 300 s.
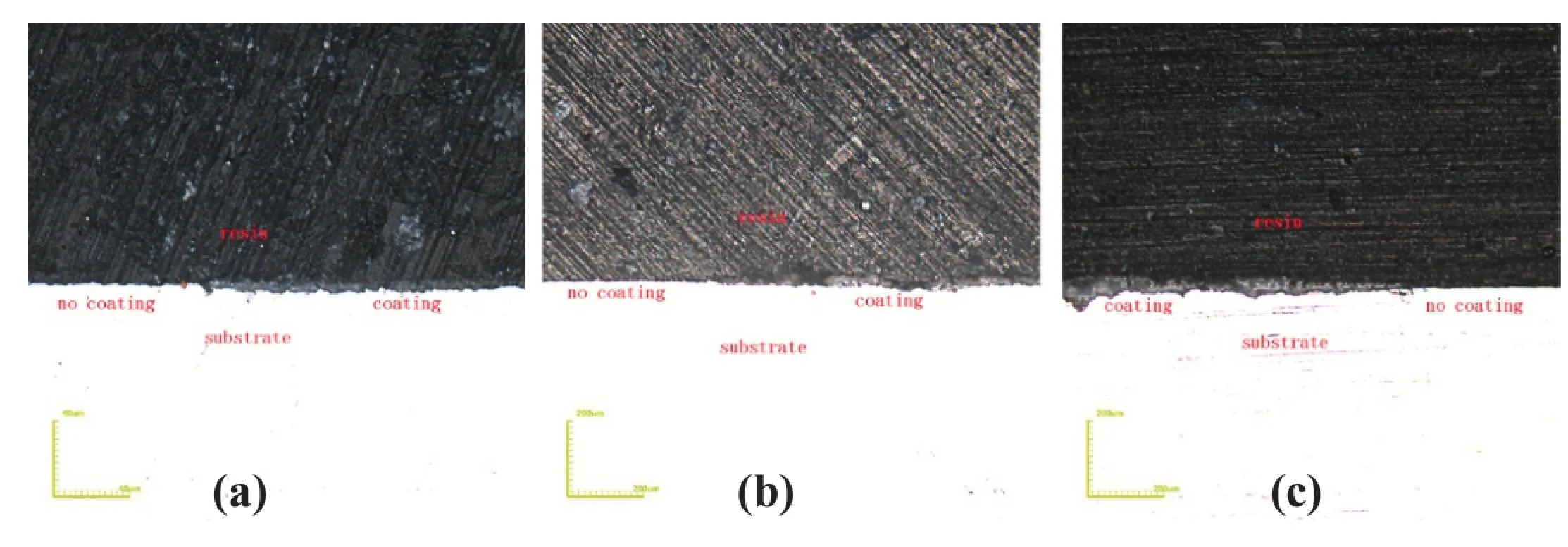
Fig.7.Microstructure of inward and outward of micro-arc oxidation coating.
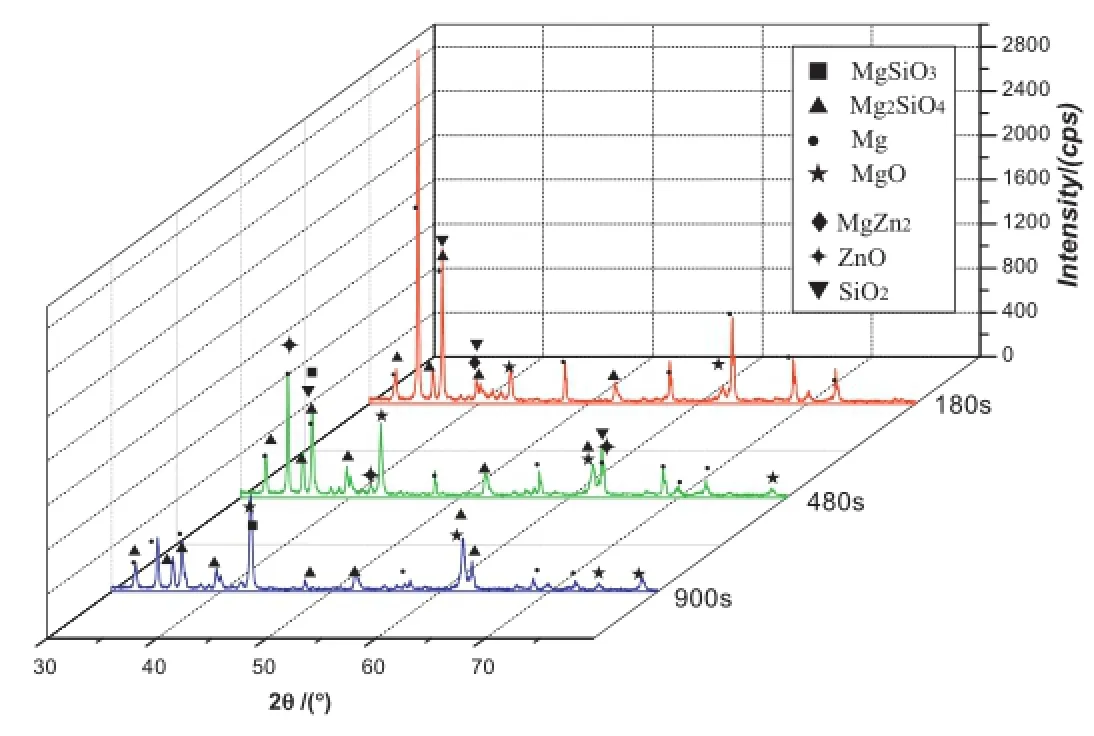
Fig.8.Phases of MAO coating at the current of 1.8 A.
3.5.Analysis of the coating phase
Fig.8 demonstrates the X-ray diffraction results of coatings produced at different stages,respectively.
180 s:When it is at 180 s,the coating in rapid growth stage is mainly composed of Mg,MgO magnesia,MgZn2,SiO2 and Mg2SiO4.Among them,magnesium and MgZn2 are from substrate.Mg transforms into the Mg2+ions at the high volatge and temperature at the frst stage of process and this magnesium ions formed during MAO process react with other ions such as OH−and SiO32−in the micro discharge channels.Mg2SiO4is formed by outward migration of Mg2+from substrate metal to discharge channels and inward migration of SiO32−from electrolyte to discharge channels at high temperature.The main reactions are shown below(Eqs(3–6)):
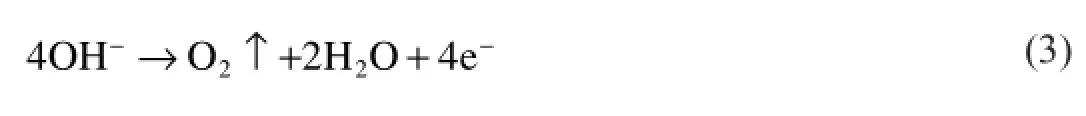
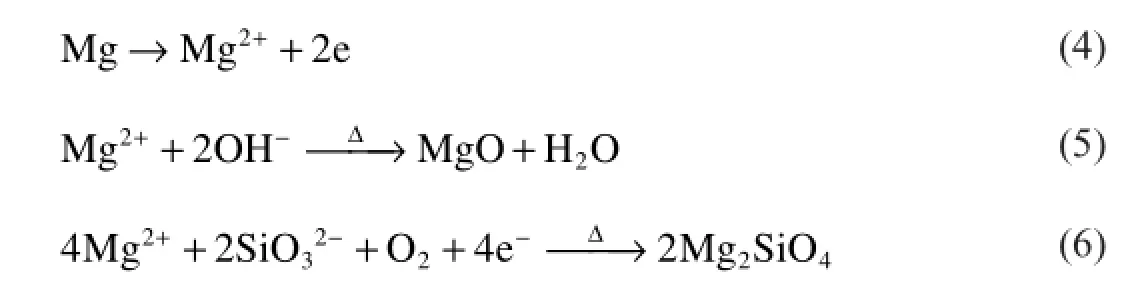
480 s:Compared with the previous stage,new phase appears in the coating in self-repairing stage at 480 s,such as MgSiO3 and ZnO.Zn in the substrate and oxygen in the electrolyte react into ZnO.MgSiO3 phase can be formed in two different reactions during MAO process.At the frst reaction type,the Mg2−ions react with the SiO32−directly owing to the decreasing amount of O2.Later,the reaction between MgO and SiO2 occurs during process at high temperature and MgSiO3 is created.The two reactions are shown in(Eq(7,8)):
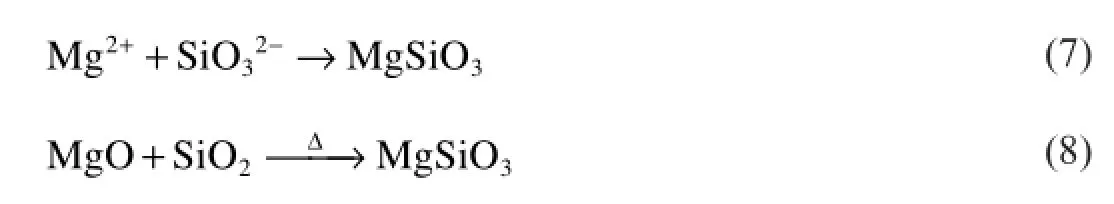
900 s:It is a whole MAO process at 900 s.Major phases are found to be Mg,MgO periclase,Mg2SiO4forsterite and MgSiO3.Due to the drastic discharge of sparks and severe erosion in the last stage,the MgO magnesia and Mg2SiO4transform into MgO periclase and Mg2SiO4forsterite under the effect of high temperature and liquating,which has a signifcant effect on improving the comprehensive performance of the coating.
4.Conclusion
The MAO process and growth mechanism of micro-arc oxidation coating on magnesium alloy at the single constant current mode are systematically investigated.The main results are shown as follows:
1.According to the voltage-time curve,the MAO process can be divided into fve parts,named anodic oxidation stage,passive flm puncturing stage,rapid growth of micro-arc oxidation stage,large arc discharge stage and self-repairing stage.Among them,the third stage plays a key role in the thickness of coating,and the last stage plays a main role in the quality of flm.
2.The analysis of the growth characteristic shows that the growth rate of the two sides is basically synchronous,and the coating grows inward and outward at the same time in the early stage,but outward growth of the coating is dominant later.
3.The coating is mainly composed of Mg,MgO periclase, Mg2SiO4forsterite and MgSiO3.The comprehensive performance of coated ZK60 alloy might be enhanced due to the main phases MgO and Mg2SiO4with high-meltingpoint,high strength and better wear resistance.
[1]R.O.Hussein,D.O.Northwood,WIT Transactions on the Built Environment 137(2014)531–544.
[2]X.D.Chen,Q.Z.Cai,Z.W.Deng,L.S.Yin,Appl.Mechan.Mater.496 (2014)63–70.
[3]R.Arrabal,A.Pardo,M.C.Merino,M.Mohedano,P.Casajús,E. Matykina,et al.,Corros.Sci.52(2010)3738–3749.
[4]D.Veys-Renaux,C.-E.Barchiche,E.Rocca,Surf.Coat.Technol.251(25) (2014)232–236.
[5]E.E.Demirci,E.Arslan,K.V.Ezirmik,Ö.Baran,Y.Totik,I˙.Efeoglu,Thin Solid Films 528(15)(2013)116–122.
[6]X.-M.Chen,C.-P.Luo,J.-W.Liu,Mater.Sci.Forum 650(2010)228–233.
[7]X.-M.Chen,C.-P.Luo,J.-W.Liu,J.Funct.Mater.41(12)(2010) 2075–2079.
[8]Y.-F.Ge,B.-L.Jiang,C.-J.Wang,Y.Long,Trans.Mater.Heat Treat.35 (12)(2014)190–194.
[9]Y.-C.Guo,J.-P.Li,J.-S.Li,P.Wang,Adv.Mater.Res.189(2011) 891–896.
[10]P.Wang,J.-P.Li,Y.-C.Guo,Z.Yang,Journal of Rare Earths 28(5)(2010) 798–802.
[11]P.Wang,D.Liu,J.Li,Z.Yang,Y.Guo,Rare Metal Mater.Eng.40(6) (2011)995–999.
[12]X.Z.Jiang,The study of growth mechanism of coating fabricated by micro-arc oxidation on magnesium alloys at constant current mode.Jiansu University of Science and Technology,32–33,2014.
[13]Y.-S.Huang,H.-W.Liu,J.Mater.Eng.Perform.20(3)(2011)463–467.
Received 27 May 2015;revised 4 July 2015;accepted 20 July 2015
*Corresponding author.School of Materials Science and Engineering, Jiangsu University of Science and Technology,Zhenjiang,212003 Jiangsu, China.Tel.:+86 15751012979.
E-mail address:lusheng88168@qq.com(S.Lu).
http://dx.doi.org/10.1016/j.jma.2015.07.003
2213-9567/©2015 Production and hosting by Elsevier B.V.on behalf of Chongqing University.
©2015 Production and hosting by Elsevier B.V.on behalf of Chongqing University.
 Journal of Magnesium and Alloys2015年3期
Journal of Magnesium and Alloys2015年3期
- Journal of Magnesium and Alloys的其它文章
- GUIDE FOR AUTHORS
- Infuence of sulfate ion concentration and pH on the corrosion of Mg-Al-Zn-Mn(GA9)magnesium alloy
- Infuence of solution treatment on microstructure,mechanical and corrosion properties of Mg-4Zn alloy
- Effect of experimental parameters on the micro hardness of plasma sprayed alumina coatings on AZ31B magnesium alloy
- Electrochemical deposition of Mg(OH)2/GO composite flms for corrosion protection of magnesium alloys
- Effect of temperature and strain rate on compressive response of extruded magnesium nano-composite
