2014年全球新药研发报告
——第一部分:新药和生物制品(Ⅲ)
Graul A I,Cruces E,Stringer M
·全球药讯·
GLOBAL PHARMACEUTICAL INFORMATION
2014年全球新药研发报告
——第一部分:新药和生物制品(Ⅲ)
Graul A I,Cruces E,Stringer M

编者按:本刊自2013年起连续2年分期译载了汤森路透公司独家授权的“全球新药研发报告”,该报告一经刊出,就因内容全面、资料权威、视角独到、数据翔实、时效性强广受好评。读者纷纷来函索要单行本,众多药企高层对该报告也高度关注。
本期“全球药讯”栏目继续刊登“2014年全球新药研发报告”第一部分。
2014的新药批准和上市年终报告显示医药行业的活跃性持续保持在高位。截至2014年12月23日,共有55个新药和生物制品首次上市。此外,29个重要的延伸性新药(新处方、新复方或已上市药物的新适应证)也在2014年上市。在这些新上市的药物中,最多的是抗感染药物,有11个新药和生物制品。它们大多用于多药耐药菌引发的感染或丙肝的治疗。美国再一次成为这些新上市药物最青睐的市场,该国是2014年半数以上新上市药物的首选地区。不过,日本在2014年开发上市新药的能力显著增强,多年来首次超越欧盟。另一重要成果是:2014年上市的新药和生物制品中有15个获得罕见病用药资格,5个获得突破性治疗药物资格,以及3个获得合格传染病产品(QIDP)资格。另外,2014年还有19个产品首度获批,将于2015年初上市。
上市新药;新药批准;延伸性新药;首创药物
表2列出了2014年首次上市的新药、生物制品及 其延伸性产品,表3为这些新药的市场前景。

续表2

续表2
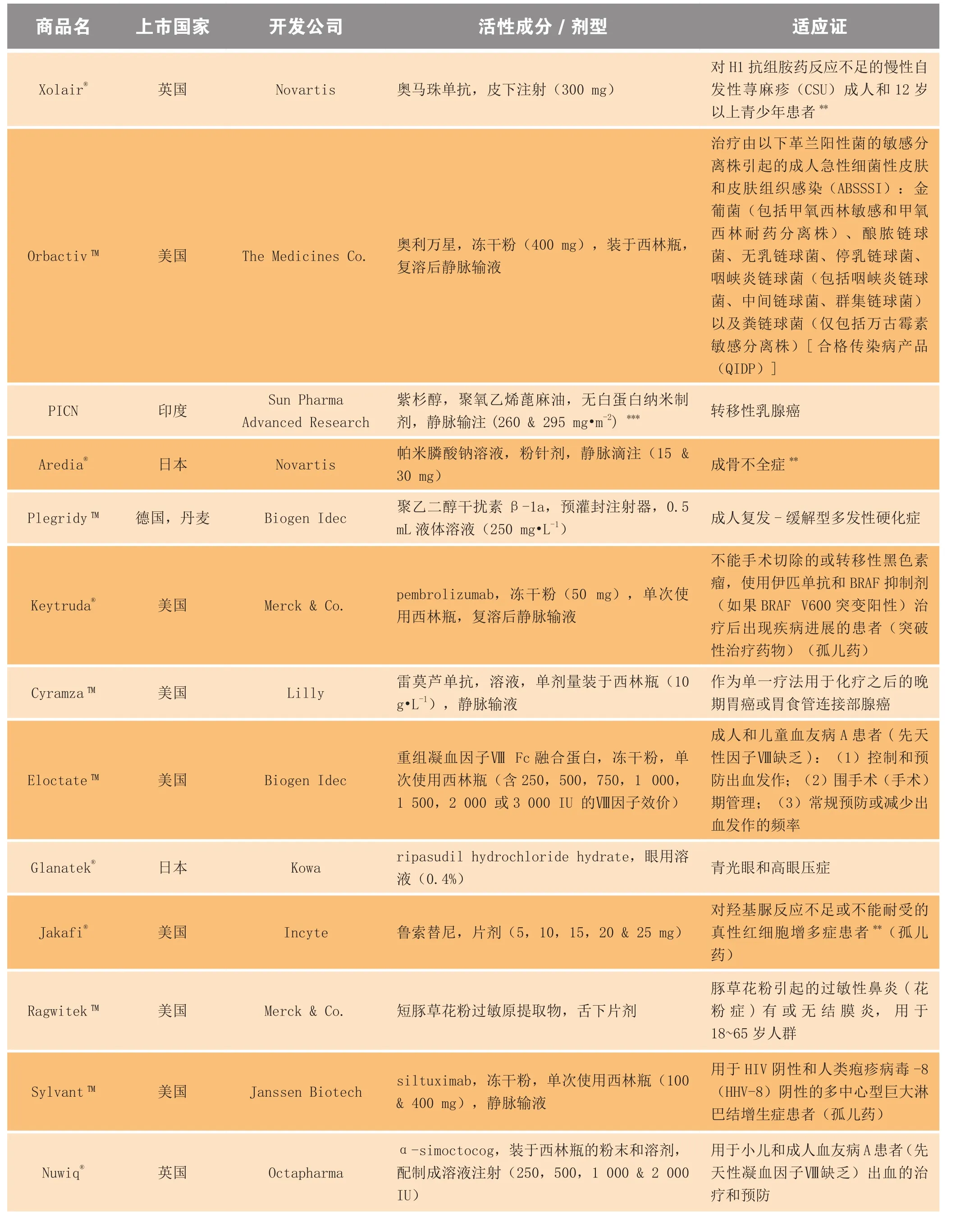
续表2

表3 2014年上市的部分新药、生物制品及延伸性新药的预估市场规模Table3 Estimated market size for selected new drugs and biologics and new line extensions launched in 2014

续表3

续表3
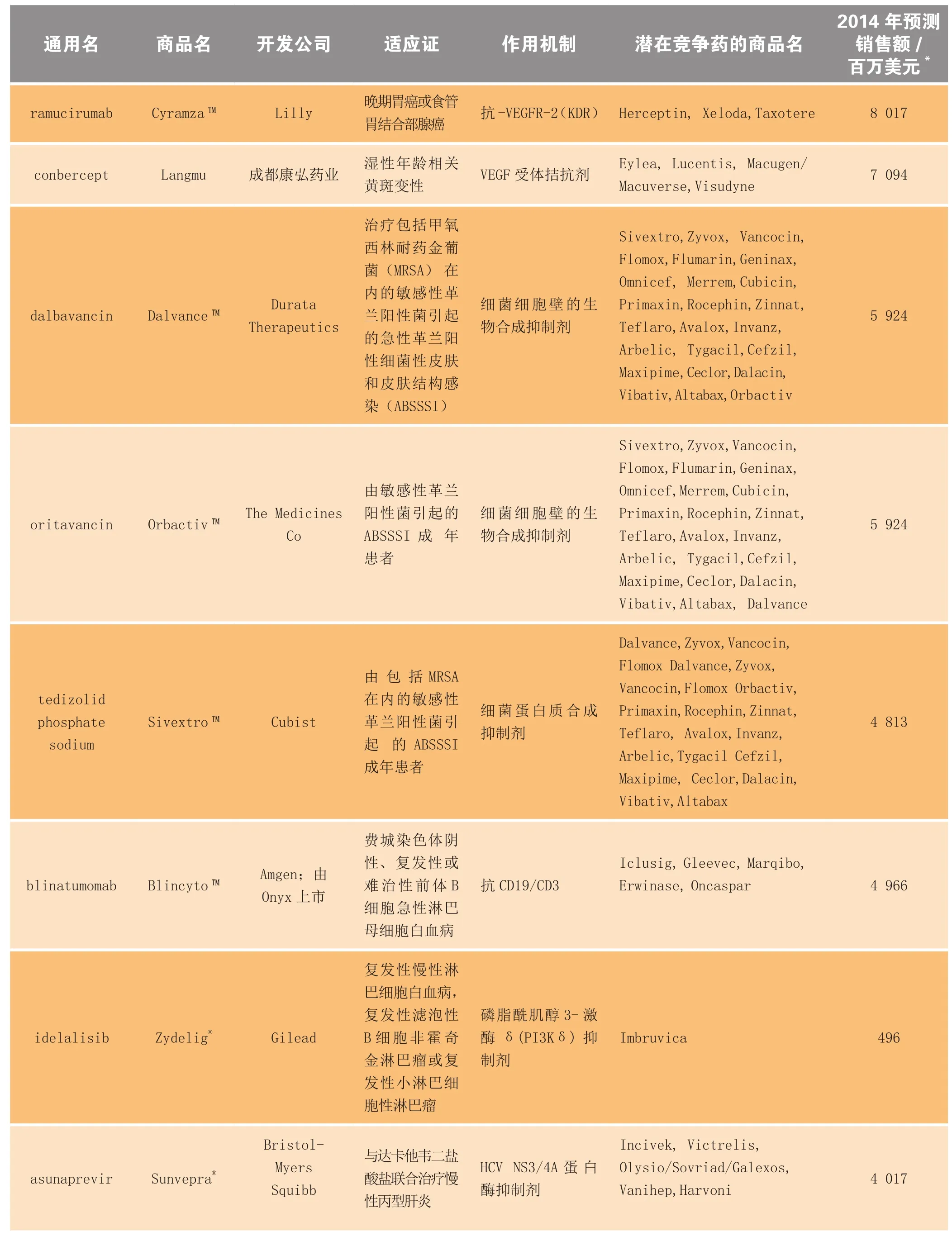
续表3

续表3
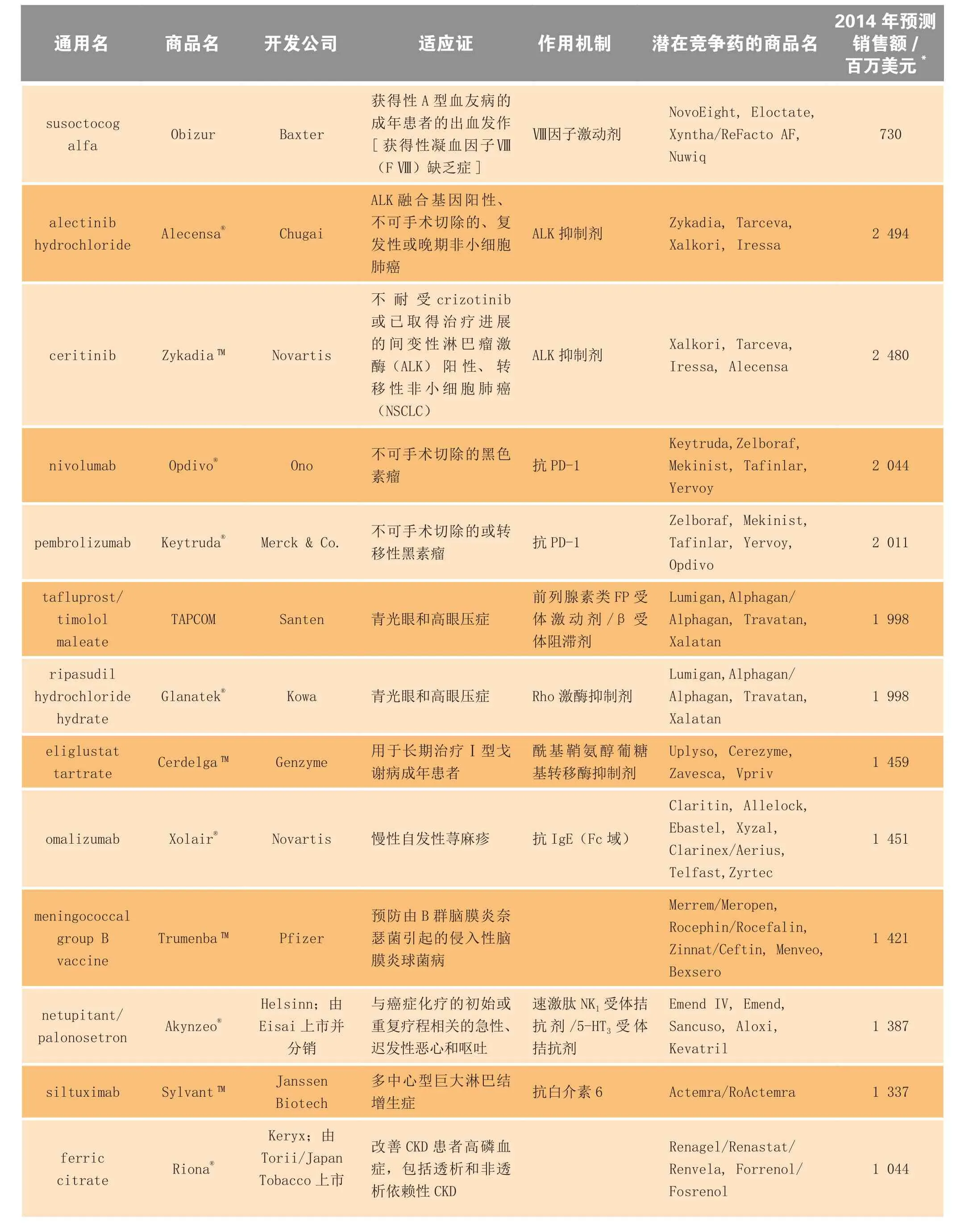
续表3

续表3
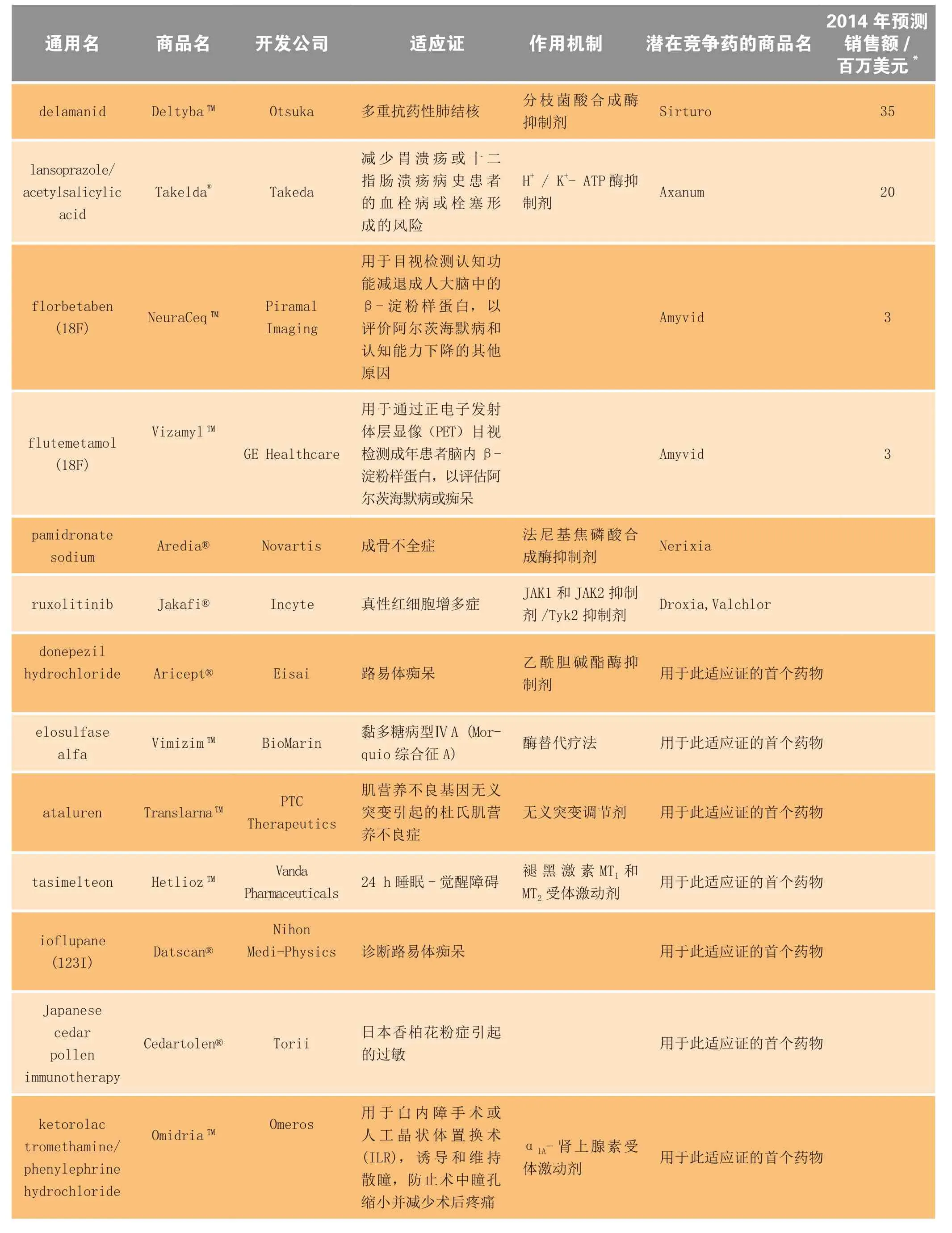
续表3

续表3
[1]Morgenthaler T I, Lee-Chiong T, Alessi C,et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders.An American Academy of Sleep Medicine report[J].Sleep, 2007, 30(11): 1445-1459.
[2]Yamada T, Saito H, Fujieda S.Present state of Japanese cedar pollinosis: the national affliction[J].J Allergy Clin Immunol,2014, 133(3): 632-639.
[3]Lozano R, Naghavi M, Foreman K,et al.Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010[J].Lancet,2012, 380(9859):2095-2128.
[4]Coyne K S, LoCasale R J,,et al.Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany,and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review[J].Clinicoecon Outcomes Res,2014, 6: 269-281.
[5]IDF Diabetes Atlas, 6th edition (international Diabetes Federation, 2013)[EB/OL].http://www.idf.org/sites/default/fles/ eN_6e_Atlas_Full_0.pdf.
[6]Antimicrobial resistance: tackling a crisis for the health and wealth of nations.The review on antimicrobial resistance-chaired by Jim O’Neill.December 2014[EB/OL].http://amr-review.org/sites/default/files/ AMr%20 review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20 health%20and%20wealth%20of%20nations_1.pdf.
[7]Hepatitis C.Fact sheet Nº164(world Health Organization,updated April 2014)[EB/OL].http://www.who.int/mediacentre/factsheets/fs164/en/.
[8]Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 (international Agency for research on Cancer)[EB/ OL].http://globocan.iarc.fr/Default.aspx.
[9]Polycythemia vera facts(Leukemia & Lymphoma Society, revised June 2012)[EB/OL].http://www.lls.org/content/ nationalcontent/ resourcecenter/freeeducationmaterials/mpd/pdf/polycythemiavera.pdf.
(待续)
原文来源:Drugs of Today,2015,51(1):37-87
A Report of New Drugs Research and Development in 2014——Part I:New Drugs & Biologics (III)
Graul A I, Cruces E, Stringer M
A year-end wrap-up of new drug approvals and launches reveals that activity in the pharmaceutical industry continues at a high level, with 55 new drugs and biologics introduced on their frst markets in 2014 (as of December 23,2014).Additionally, 29 important new line extensions (new formulations, new combinations or new indications for previously marketed products) also reached their frst markets during the year.The most active therapeutic group in terms of new launches was anti-infective therapies, with 11 new drugs and biologics launched, most for the treatment of multidrug-resistant bacterial infections or hepatitis C.The most active market for new launches was again the U.S., site of more than half of all new launches in 2014.However new launch activity increased considerably last year in Japan, which actually pulled ahead of the E.U.For the frst time in many years.In another important new development,15 of the new drugs and biologics launched last year had orphan drug status, 5 had breakthrough therapy designation and 3 had Qualifed Infectious Disease Product (QIDP) status.Another 19 products were approved for the frst time during the year but not yet launched by close of this article, most are slated for launch in the frst months of the new year.
new drug launch; new drug approval; line extension; frst-in-class drug
R97
A
1001-5094(2015)04-0305-16

