生物活性多甲氧基黄酮糖苷的合成及其结构表征*
汪秋安,王盛淳,李 悦,单 杨
(1. 湖南大学 化学化工学院,湖南 长沙 410082; 2. 湖南省农业科学院 农产品加工研究所,湖南 长沙 410125)
生物活性多甲氧基黄酮糖苷的合成及其结构表征*
汪秋安1†,王盛淳1,李 悦1,单 杨2
(1. 湖南大学 化学化工学院,湖南 长沙 410082; 2. 湖南省农业科学院 农产品加工研究所,湖南 长沙 410125)
为了提高多甲氧基黄酮类化合物的水溶性和药用价值,以2 种来源丰富且抗癌活性高的多甲氧基黄酮橘皮素和川陈皮素为底物,分别经过氧丙酮(DMDO)氧化得到多甲氧基黄酮醇9和10,然后,9和10分别与糖基供体α-溴代乙酰葡萄糖、半乳糖和鼠李糖在NaOH水溶液和氯仿体系中,进行相转移催化下的糖苷化反应及随后的脱乙酰化反应,首次合成了4种新的多甲氧基黄酮糖苷化合物1~4. 对所合成的化合物用1HNMR,13CNMR和MS等波谱法进行了结构表征. 合成方法原料易得、工艺简便、收率较高.
合成;多甲氧基黄酮;糖苷化反应;结构表征
多甲氧基黄酮(polymethoxyflavonoids, PMFs)是一类含有多个甲氧基、低极性、具有平面结构且生物活性显著的黄酮类天然产物[1]. 它们几乎专门来源于芸香科柑橘属,主要存在于陈皮、青皮、橘红、佛手和枳实等药材中. 目前已从该属植物中分离出40多种PMFs,以中国甜橙和柑橘果皮中的含量较高[2]. 橘皮素(tangeretin)和川陈皮素( nobiletin )是在柑橘(CitrusreticulateBlanaco)和甜橙(Citrussinensis)果皮中含量很高的多甲氧基黄酮[3-4],它们对HL-60白血病细胞、人乳腺癌细胞、小鼠皮肤癌和神经纤维瘤细胞等多种癌细胞具有非同寻常的抗癌活性[5-7]. 此外,这类化合物还具有良好的抗炎、抗病毒、抗诱变和抗高血压的作用[8-10]. 但橘皮素和川陈皮素等多甲氧基黄酮的水溶性差、对生物受体的亲和能力不强,限制了它们的进一步开发利用.
糖类化合物作为自然界中广泛存在的一大类典型的亲水性物质,在细胞识别、信号传导等诸多生命活动中扮演着重要角色[11]. 分子通过糖苷化修饰可以改变整个分子的构像,进一步改变其溶解性和导向性,增加对受体的亲和能力[12]. 例如半乳糖具有与肝细胞表面受体蛋白( ASGP-R )结合的特性,通过其抗肿瘤作用的靶向性而提高药效[13]. 为了提高多甲氧基黄酮类化合物的水溶性和药用价值,以橘皮素和川陈皮素为底物,分别经过氧丙酮(DMDO)氧化得到多甲氧基黄酮醇9和10. 然后9和10分别与糖基供体α-溴代乙酰葡萄糖、半乳糖和鼠李糖在NaOH水溶液和氯仿体系中,进行相转移催化下的糖苷化反应得化合物5~8, 随后经脱乙酰化反应, 首次合成了4种未见文献报道的多甲氧基黄酮糖苷1~4. 其合成路线如图1所示.

图1 多甲氧基黄酮糖苷1~4的合成路线
1 实验部分
1.1 仪器和试剂
熔点在 XRC-1 型显微熔点仪上测定(温度计未校正);NMR 用 Bruker AV-400 型核磁共振仪测定(溶剂 CDCl3或 DMSO-d6, TMS 为内标);MS 用 Agilent 1100 液-质联用仪或 ZAB-HS 型仪测定记录(ESI模式);薄层色谱和柱层析用硅胶均为青岛海洋化工厂产品;所用试剂和溶剂为化学纯和分析纯, 要求无水的溶剂均经去水和重蒸处理.
溴代-2,3,4,6-四-O-乙酰基-α-D-吡喃葡萄糖、溴代-2,3,4,6-四-O-乙酰基-α-D-吡喃半乳糖和溴代-2,3,4-三-O-乙酰基-α-D-吡喃鼠李糖按文献方法合成[14-16]. 3-羟基橘皮素(5,6,7,8,4′-五甲氧基黄酮醇,9)和柚皮黄素(5,6,7,8,3′,4′-六甲氧基黄酮醇,10)按我们最近报道的方法合成[17].
1.2 5,6,7,8,4′-五甲氧基黄酮-3-O-β-乙酰基葡萄 糖苷5的合成
在装有磁子的100 mL 单口烧瓶中加入9 (100 mg, 0.26 mmol) 和15 mL 1.25 mol/L的NaOH溶液充分溶解搅拌10 min. 称取四丁基溴化铵 (TBAB, Bu4N+Br-) (20 mg,0.05 mmol) 和新制的溴代乙酰基葡萄糖 (123 mg,0.3 mmol) 溶于15 mL CHCl3中, 将该CHCl3溶液滴加到反应物溶液中, 60 ℃搅拌反应回流5 h左右. 薄层色谱(TLC)监测原料点消失, 反应结束. 加入20 mL CH2Cl2, 再加入20 mL 水, 静置分液, 水相再用CH2Cl2萃取 (20 mL×2), 合并有机相, 用水洗涤2次. 无水MgSO4干燥, 过滤, 浓缩, 经硅胶柱层析[洗脱剂:V石油醚/V乙酸乙酯=3∶1], 得到白色固体107 mg, 产率65%;1H NMR (400 MHz, CDCl3)δ7.79 (d,J= 8.9 Hz, 2H, H-2′ and H-6′) , 6.94 (d,J= 8.8 Hz, 2H, H-3′ and H-5′), 5.33 (d,J=7.0 Hz, 1H, H-1″), 5.28(m, 1H, 4″-H), 5.14~5.19 (m, 2H, 2″-H, 3″-H), 4.27~4.30 (m, 2H, 6″-H), 4.03 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 3.81~3.84 (m, 1H, 5″-H), 3.78 (s, 3H, OCH3), 2.12(s, 3H,COCH3), 2.07(s, 3H, COCH3), 2.06 (s, 3H, COCH3), 2.04 (s, 3H, COCH3). MS (ESI),m/z: 741.2 [M+Na]+.
1.3 5,6,7,8,4′-五甲氧基黄酮-3-O-β-乙酰基半乳 糖苷6的合成
6的合成方法同5, 以9和新制的溴代乙酰基半乳糖为原料, 得白色固体6, 产率 72%.1H NMR (400 MHz, CDCl3)δ8.19 (d,J= 9.1 Hz, 2H, H-2′ and H-6′), 7.02 (d,J= 9.1 Hz, 2H, H-3′ and H-5′), 5.49 (d,J=6.8 Hz, 1H, 1″-H), 5.46~5.49(m, 1H, 4″-H), 5.11~5.16 (m, 2H, 2″, 3″-H), 4.20~4.21 (m, 2H, 6″-CH2), 3.97~4.11 (m, 1H, 5″-H), 4.10 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 2.19 (s, 3H,COCH3), 2.11 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 2.02 (s, 3H, COCH3). MS (ESI),m/z: 741.2 [M+Na]+.
1.4 5,6,7,8,4′-五甲氧基黄酮-3-O-α-乙酰基鼠李 糖苷7的合成
7的合成方法同5, 以9和新制的溴代乙酰基鼠李糖为原料, 得白色固体7, 产率 69%.1H NMR (400 MHz, CDCl3)δ8.16 (d,J= 8.8 Hz, 2H,H-2′ and H-6′), 7.04 (d,J= 8.8 Hz, 2H,H-3′ and H-5′), 5.32 (d,J= 2.3 Hz, 1H, 1″-H), 5.19 (s, 1H, sugar -H), 5.09 (s, 1H, sugar -H), 4.96~5.03 (m, 1H, sugar -H), 4.10 (s, 3H, OCH3), 4.05~4.09 (m, 1H, sugar -H), 4.01 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 2.09 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 1.93 (s, 3H, COCH3), 1.17 (d,J=5.5Hz, 3H, Rha-CH3). MS (ESI),m/z: 683.2 [M+Na]+.
1.5 5,6,7,8,3′,4′-六甲氧基-3-O-β-乙酰基半乳糖 苷8的合成
8的合成方法同5, 以10和新制的溴代乙酰基半乳糖为原料, 得白色固体8, 产率 70%.1H NMR (400 MHz, CDCl3)δ7.94 (d,J= 1.7 Hz, 1H, H-6′), 7.71 (dd,J= 8.6, 1.6 Hz, 1H, H-2′), 6.94 (d,J= 8.7 Hz, 1H, H-5′), 5.64 (d,J= 8.0 Hz, 1H,H-1″), 5.32~5.35 (m, 2H,sugar-H), 5.06 (dd,J=8.0, 3.4 Hz, 1H, sugar-H), 4.04 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.87 (s, 3H,OCH3,), 3.83 (dd,J= 6.2, 3.0 Hz, 2H, sugar-H), 3.79 (d,J= 5.8 Hz, 1H, sugar-H), 2.10 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 1.94 (s, 3H, COCH3), 1.85 (s, 3H, COCH3);13C NMR (100 MHz, CDCl3)δ172.60, 170.21, 170.06, 169.87, 169.80, 154.47, 151.30, 150.94, 147.98, 146.49, 143.75, 137.63, 135.21, 123.02, 121.62, 114.65, 112.36, 110.37, 99.18, 70.51, 68.86, 66.79, 62.09, 61.89, 61.66, 61.53, 60.30, 55.84, 55.79, 20.90, 20.44, 20.37. MS (ESI),m/z: 771.2 [M+Na]+.1.6 5,6,7,8,4′-五甲氧基黄酮-3-O-β-葡萄糖苷1 的合成
在100 mL单口圆底烧瓶中,用15 mL甲醇加热搅拌至溶解5,6,7,8,4′-五甲氧基黄酮-3-O-β-乙酰基葡萄糖苷5 (80 mg, 0.11 mmol),再加入5 mL甲醇钠溶液 (4.3 mg 钠和5 mL甲醇配置而成),回流搅拌反应3 h后,TLC跟踪反应,原料点消失,反应完成,将反应液冷却至室温,减压蒸馏除去溶剂,经硅胶柱层析[洗脱剂:乙酸乙酯及乙酸乙酯/乙醇的混合溶剂进行梯度洗脱],得51 mg化合物1, 白色固体,产率85%.1H NMR (400 MHz, DMSO-d6)δ8.01 (d,J= 8.9 Hz, 2H, H-2′ and H-6′), 7.15 (d,J= 9.0 Hz, 2H, H-3′ and H-5′), 5.19 (s, 1H, OH), 5.44 (d, 1H,J= 4.4 Hz, sugar-OH), 5.11 (d,J= 4.8Hz, sugar-OH), 5.41 (d,J=4.8 Hz, 1H, 2″-OH), 5.15 (d,J= 4.8 Hz, 1H, 3″-OH), 5.07 (d,J= 5.2 Hz, 1H, 4″-OH), 5.03 (d,J=7.6 Hz, 1H, 1″-H), 4.60 (t,J= 5.2 Hz, 1H, 6″-OH), 4.03 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 3.86 (s, 6H, 2OCH3), 3.78 (s, 3H, OCH3), 3.70~3.74 (1H, m, 3″-H), 3.42-3.48 (2H, m, 6″-CH2), 3.15~3.30 (3H, m, 2″, 4″, 5″-H );13C NMR (100 MHz, DMSO-d6)δ176.32, 161.25, 160.14, 150.34, 147.34, 146.70, 143.03, 137.05, 126.67,122.77, 113.85, 113.48, 105.65, 75.84, 74.74, 73.01, 69.84, 61.23, 61.01, 60.80, 60.63, 54.47. MS (ESI),m/z:573.2 [M+Na]+.
1.7 5,6,7,8,4′-五甲氧基-3-O-β-半乳糖苷2的合成
2的合成方法同1, 以6为原料得白色固体2, 产率80%;1H NMR (400 MHz, DMSO-d6)δ7.94 (d,J= 8.8 Hz, 2H, H-2′ and H-6′), 7.02 (d,J= 8.9 Hz, 2H, H-3′ and H-5′), 5.30 (d,J= 4.8 Hz, 1H, 2″-OH), 4.99 (d,J= 8.0 Hz, 1H, 1″-H), 4.73 (d, 1H,J= 5.5 Hz, 4″-OH), 4.61 (t,J= 7.2 Hz, 1H, 6″-OH ), 4.02 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 3.42~3.71 (m, 6H, sugar-H);13C NMR (100 MHz, DMSO-d6)δ174.57, 159.51, 158.40, 148.60, 145.60, 144.96, 141.29, 135.31, 124.93, 121.03, 112.11, 111.74, 103.90, 75.84, 74.74, 73.01, 69.84, 59.49, 59.27, 59.06, 58.89, 52.73. MS (ESI),m/z: 573.2 [M+Na]+.1.8 5,6,7,8,4′-五甲氧基黄酮-3-O-α-鼠李糖苷3 的合成
3的合成方法同1, 以7为原料得白色固体3, 产率83%;1H NMR (400 MHz, CDCl3)δ7.94 (d,J= 8.8 Hz, 2H, H-2′ and 6′), 7.04 (d,J= 8.8 Hz, 2H, H-3′ and 5′), 6.34 (d,J= 2.3 Hz, 1H, H-1″), 4.27 (dd,J= 6.1, 2.3 Hz, 1H, H-2″), 3.89 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.74 (m, 2H, H-3″ and 4″), 3.71 (s, 9H, 3OCH3), 3.29 (m, 1H, H-5″), 1.78 (s, 1H, 3″-OH), 1.70 (s, 1H, 2″-OH), 1.58 (s, 1H, 4″-OH), 1.13 (d,J= 6.5 Hz, 3H, CH3-5″).13C NMR (100 MHz, CDCl3)δ173.44, 161.56, 157.51, 152.21, 146.41, 145.25, 138.32, 136.88, 136.42, 130.54, 123.74, 114.27, 113.65, 100.60, 74.37, 73.41, 73.01, 70.24, 60.70, 56.08, 17.66. MS (ESI),m/z: 557.2 [M+Na]+.
1.9 5,6,7,8,3′,4′-六甲氧基-3-O-β-半乳糖苷4的 合成
4的合成方法同1, 以8为原料得白色固体4, 产率82%;1H NMR (400 MHz, DMSO-d6)δ7.50 (dd,J= 8.5,1.9 Hz, 1H, H-6′), 7.34 (s, 1H, H-2′), 6.92 (d,J= 8.5 Hz, 1H, H-5′), 5.30 (d,J= 4.8 Hz, 1H, 2″-OH), 4.99 (d,J= 8.0 Hz, 1H, 1″-H), 4.73 (d,J= 5.5 Hz, 1H, 4″-OH), 4.61 (t,J= 7.2 Hz, 1H, 6″-OH ), 4.03 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 3.88 (s, 6H, 2OCH3), 3.42~3.71 (m, 6H, sugar-H);13C NMR (100 MHz, DMSO-d6)δ177.24, 153.79, 150.87, 149.55, 147.13, 146.82, 142.84, 136.72, 134.12, 130.77, 120.98, 120.33, 109.08, 109.00, 105.43, 75.84, 74.74, 73.01, 69.84, 61.92, 60.02, 59.70, 59.13, 58.10, 54.00, 53.91. MS (ESI),m/z: 603.2 [M+Na]+.
2 结果与讨论
2.1 合成方法
采用高氯酸-红磷法制备溴代乙酰糖类化合物. 将葡萄糖、半乳糖和鼠李糖乙酰化后,在红磷和溴同时存在下,将乙酰基葡萄糖、半乳糖和鼠李糖的异头碳溴化,得到溴代乙酰基葡萄糖、半乳糖和鼠李糖. 对于糖苷化反应曾尝试采用如下方法:1) 四丁基溴化铵(TBBA)为相转移催化剂, 以无水丙酮为溶剂, 同时存在弱碱 (K2CO3)的体系. 2) 氧化银/无水二氯甲烷体系下室温反应. 3) 四丁基溴化铵 (TBBA)为相转移催化剂, 在稀氢氧化钠碱性条件下的水/三氯甲烷体系中. 经过比较这3种糖苷合成方法和底物的酚羟基活性选择了一条操作简单且产率较高的有效的糖苷合成路线, 即以稀碱氢氧化钠水溶液为碱性缩合剂, 采用双相体系即CHCl3/H2O 体系, 以四丁基溴化铵(TBBA)为相转移催化剂, 在60 ℃的反应温度下, 分别将底物与溴代乙酰基葡萄糖、半乳糖和鼠李糖在碱性条件下进行缩合反应合成多甲氧基黄酮糖苷化合物5~8, 值得注意的是,控制反应温度和反应时间,可以有效抑制端基碳的异构化.在相转移催化剂条件下,可减少溴代糖水解副反应的发生.在脱去乙酰基糖苷的乙酰基保护时, 采用了简单实用的甲醇钠/甲醇体系, 最终合成了一系列多甲氧基黄酮糖苷化合物1~4. 1~4及化合物5~8均为未见文献报道的新化合物. 该合成方法具有反应条件温和、后处理方便、产率较高和立体选择性强的优点,对具有重要生物活性的多甲氧基黄酮苷的合成具有较大的应用价值.
2.2 结构表征
利用核磁共振氢谱中糖的端基质子H-1″与H-2″的偶合常数(J值)判断苷键的构型是目前最常用的方法[18]. 在葡萄糖和半乳糖的六元环中,β-型糖的H-1″和H-2″都处于a键,它们之间的偶合常数J1, 2= 7~10 Hz,而α-型糖的H-1″和H-2″分别处于e键和a键,它们之问的偶合常数J1, 2= 2~6 Hz,所以由此可以大概了解它们的构型. 溴代乙酰基葡萄糖、半乳糖和鼠李糖中相应的J1, 2为2~4 Hz,为α-型. 在糖苷化反应中多甲氧基黄酮3-羟基位的氧负离子一般从背面进攻溴代乙酰基糖的C1-Br 键,该反应属于SN2反应机理。由于2-乙酰基的邻基参与作用, 糖苷化反应得到1,2-反式产物,若 H-1″和H-2″都处于a键,得到β-型糖苷化合物. 通过用1H NMR对目标黄酮苷化合物1~8进行结构表征,化合物1, 2, 4~6, 8的端基质子H-1″与H-2″相应的J1, 2为7.6~8.0 Hz,可确证为β-苷键,而对于鼠李糖苷3, 7的H-1″和H-2″都处于e键上,相应的J1, 2为2.3 Hz,属于α-苷键.
以5,6,7,8,3′,4′-六甲氧基黄酮-3-O-β-乙酰基半乳糖苷8和5,6,7,8,3′,4′-六甲氧基黄酮-3-β-D-半乳糖苷4为例,通过谱图分析进行结构表征.化合物8和4的结构式如图2所示.
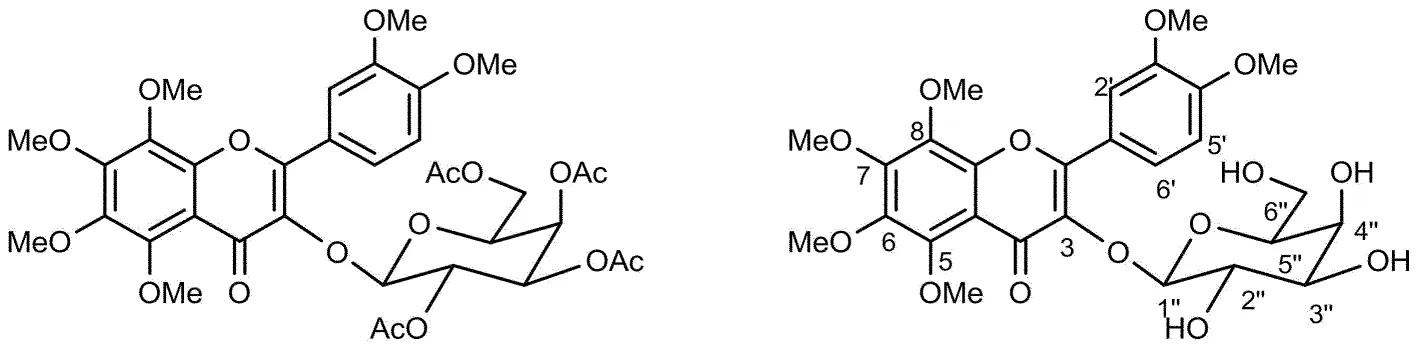
图2 化合物8和化合物4的结构式
5,6,7,8,3′,4′-六甲氧基黄酮-3-O-β-乙酰基半乳糖苷8的1HNMR谱图分析如下,在δH7.50处积分为1的双峰可归属为B环6′位的氢,6′位的氢和5′位的氢有较强的邻位偶合,和2′位的氢有较弱的间位偶合,偶合常数(J)分别为8.6 Hz和1.6 Hz;δH7.71处的双峰可归属为B环2′位的氢;δH7.71处的双峰可归属为5′位的氢,偶合常数为8.7 Hz;δH5.64处的双峰为半乳糖基1″的氢,偶合常数为8.0 Hz;δH5.32~5.39处的多重峰可归属为半乳糖基3″和4″的氢;δH5.06处的两个双峰可归属为半乳糖基2″的氢,偶合常数为8.0 Hz和3.4 Hz,由H-1″和H-2″的耦合常数J> 6 Hz,由此判断H-1″和H-2″为均为a键,乙酰半乳糖苷为β构型;δH3.87~4.04处6个单峰可归属为黄酮6个甲氧基的氢;δH3.83处两个双峰为半乳糖基6″的亚甲基氢,偶合常数为6.2 Hz和3.0 Hz;δH3.79处双峰为半乳糖基5″的氢,偶合常数为5.8 Hz;δH1.85~2.10处4个单峰可归属为半乳糖基4个乙酰基氢. 5,6,7,8,3′,4′-六甲氧基黄酮-3-O-β-乙酰基半乳糖苷8的13C NMR谱图解析如下,δC62.09, 61.89, 61.66, 61.53, 60.30, 55.84为黄酮环上6个甲氧基的碳信号;δC172.60, 170.21, 170.06, 169.87, 169.80, 154.47, 151.30, 150.94, 147.98, 146.49, 143.75, 137.63, 135.21, 123.02, 121.62, 114.65归属于黄酮母核环上碳信号;δC112.36, 110.37, 99.18, 70.51, 68.86, 66.79 归属于β-D-吡喃半乳糖苷碳信号;δC55.79, 20.90, 20.44, 20.37归属于半乳糖苷上4个乙酰基上的甲基碳信号.
5,6,7,8,3′,4′-六甲氧基黄酮-3-O-β-半乳糖苷4 的1H NMR 谱图分析如下,在δH7.50处积分为1的双峰可归属为B环6′位的氢,偶合常数为8.5 Hz和1.9 Hz;δH7.34处的双峰可归属为B环2′位的氢;δH6.92处的双峰可归属为5′位的氢,偶合常数为8.5 Hz;δH5.30 处的双峰为吡喃半乳糖基2″位的羟基氢,偶合常数为8.0和4.8 Hz;δH4.99处的双峰可归属为吡喃半乳糖基1″位的氢,偶合常数为8.0 Hz;δH4.73处的双峰可归属为吡喃半乳糖基4″位的羟基氢,偶合常数为5.5 Hz;δH4.61处的多重峰为吡喃半乳糖基6″位的羟基氢,偶合常数为7.2 Hz;δH3.88~4.03处6个单峰可归属为黄酮6个甲氧基的氢信号;δH3.42~3.71处的多重峰可归属为吡喃半乳糖环上的6个氢信号.5,6,7,8,3′,4′-六甲氧基黄酮-3-O-β-半乳糖苷4的13C NMR谱图解析如下,δC60.02, 59.70, 59.13, 58.10, 54.00, 53.91为黄酮环上6个甲氧基的碳信号;δC105.43, 75.84, 74.74, 73.01, 69.84, 61.92 归属于β-D-吡喃半乳糖苷碳信号;其余为黄酮母核环上碳信号.
3 结 论
以多甲氧基黄酮橘皮素和川陈皮素为原料,分别经过氧丙酮(DMDO)氧化得到2种多甲氧基黄酮醇-3-羟基-5,6,7,8,4′-五甲氧基黄酮9和3-羟基-5,6,7,8,3′,4′-六甲氧基黄酮10,然后分别与溴代乙酰葡萄糖、溴代乙酰半乳糖和溴代乙酰鼠李糖等3种糖基供体进行糖苷化反应,首次合成了4种未见文献报道的多甲氧基黄酮糖苷化合物1~4. 在多甲氧基黄酮糖苷合成过程中,采用NaOH水溶液/CHCl3反应体系,TBAB作相转移催化剂. 该合成方法具有反应条件温和、后处理方便、产率较高和立体选择性强的优点,对具有重要生物活性的多甲氧基黄酮苷的合成具有较高的应用价值.
[1] TRIPOLE E, GUARDIA M L, GIAMMANCO S,etal. Citrus flavonoids: molecular structure, biological activity and nutritional properties: A review [J]. Food Chemistry, 2007, 104 (2): 466-479.
[2] OOGHE W C, OOGHE S J, DETAVERNIER C M,etal. Characterization of orange juice (Citrussinensis) by polymethoxylated flavones [J]. Journal of Agricultural and Food Chemistry, 1994, 42 (10): 2191-2195.
[3] LEWIN G, MACIUK A, THORET S,etal. Semisynthesis of natural flavones inhibiting tubulin polymerization, from hesperidin [J]. Journal of Natural Products, 2010, 73 (4): 702-706.
[5] LI S, PAN M H, LAI C S,etal. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines [J]. Bioorganic and Medicinal Chemistry, 2007, 15 (10): 3381-3389.
[6] MANTHEY J A, GUTHRIE N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines [J]. Journal of Agricultural and Food Chemistry, 2002, 50 (21): 5837-5843.
[7] PAN M H, CHEN W J, LIN-SHIAU S Y,etal. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells [J]. Carcinogenesis, 2002, 23(10):1677-1684.
[8] HUANG Y S,HO S U.Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel [J]. Food Chemistry, 2010, 119(3): 868-873.
[9] LI S, SANG S, PAN M H,etal. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse [J]. Bioorganic and Medicinal Chemistry Letters, 2007, 17(18): 5177-5181.
[10]XU J J, WU X, LI M M,etal. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of Citrus reticulata ‘Chachi’ [J]. Journal of Agricultural and Food Chemistry, 2014, 62(10): 2182-2189.
[11]汪钢强, 陈玉玲, 韩明松, 等. 具有生物活性的紫檀芪和3'-甲氧基紫檀芪及其糖苷类化合物的合成 [J]. 有机化学, 2011, 31(12): 2114-2120.
WANG Gang-qiang, CHEN Yu-ling, HAN Ming-song,etal. Synthesis and biological activity of pterostilbene and 3′-methoxy pterostilbene and their glucosides derivatives [J]. Chinese Journal of Organic Chemistry, 2011, 31(12): 2114-2120. (In Chinese)
[12]PARK S H, KIM H J, YIM S H,etal. Delineation of the role of glycosylation in the cytotoxic properties of quercetin using novel assays in living vertebrates [J]. Journal of Natural Products, 2014, 77(11): 2389-2396.
[13]ZHANG X, SIMMONS C G, COREG D R. Liver cell specific targeting of peptide nucleic acid oligomers [J]. Bioorganic and Medicinal Chemistry Letters, 2001, 11(10):1269-1272.
[14]FURNISS B S, HANNAFORD A J, SMITH P W G A R. Textbook of practical organic chemistry [M]. 5th ed.New York:With John Wiley & Sons,1989:647.
[15]LIU J D, CHEN L, CAI S L. Semisynthesis of apigenin and acacetin-7-O-β-D-glycosides from naringin and their cytotoxic activities [J]. Carbohydrate Research, 2012, 357: 41-46.
[16]BEBAULT G M, DUTION G G S, WARFIED C K. Synthesis of 4-O-α-L-rhamnopyranosyl-L-rham-nopyranose [J]. Carbohydrate Research, 1974, 34(1):174-179.
[17]LI Y, CAI S L, HE K L,etal. Semisynthesis of polymethoxyflavonoids from naringin and hesperidin [J]. Journal of Chemical Research., 2014, 38(5):287-290.
[18]WU Z, FU X L, YANG N,etal. Synthesis and fluorescence properties of coumarin glycosides and triazoylglycosides [J]. Chemical Research in Chinese Universities, 2013, 29 (3): 460-465.
Synthesis and Structure Characterization of Bioactive Polymethoxyflavonoids Glycosides
WANG Qiu-an1†,WANG Sheng-chun1, LI Yue1, SHAN Yang2
(1.College of Chemistry and Chemical Engineering, Hunan Univ, Changsha, Hunan 410082, China;2. Institue of Agricultural Product Processing, Hunan Academy of Agricultural Science, Changsha, Hunan 410125, China)
Two most abundant sources with the highest anticancer activity of natural polymethoxyflavonoids were oxidated by dimethyldioxirane ( DMDO ) to polymethoxy flavonoids 9 and 10 respectively. 9 or 10 were condensed withα-acetylglucosyl bromide, α-acetylgalactosyl bromide or α-acetylrhamnosyl bromide in dilute NaOH (aq)/CHCl3system through phase transfer catalytic glycosation reaction, and followed by deacetylation, four new polymethoxyflavonoids glycosides 1~4 were synthesized. The structures of all the synthesized compounds were characterized by1HNMR,13CNMR and MS spectra. This synthetic method has the advantages of easy availability of starting materials,simple operation.
synthesis; polymethoxyflavonoids; glycosation reaction; structure characterization
2014-12-11
国家自然科学基金资助项目(J1210040),National Natural Science Foundation of China(J1210040) ;“十二五”农村领域国家科技计划项目(2012BAD31B02)
汪秋安(1962-),男,湖南常德人,湖南大学教授
†通讯联系人,E-mail:WangQA@hnu.edu.cn
1674-2974(2015)12-0053-06
O625.4
A

