Analysis of effect of nicotine on microbial community structure in sediment using PCR-DGGE fngerprinting
Ai-dong Ruan*,Chen-xiao Liu
State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering,Hohai University,Nanjing 210098,PR China
Analysis of effect of nicotine on microbial community structure in sediment using PCR-DGGE fngerprinting
Ai-dong Ruan*,Chen-xiao Liu
State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering,Hohai University,Nanjing 210098,PR China
Solid or liquid waste containing a high concentration of nicotine can pollute sediment in rivers and lakes,and may destroy the ecological balance if it is directly discharged into the environment without any treatment.In this study,the polymerase chain reaction(PCR)and denaturing gradient gel electrophoresis(DGGE)method was used to analyze the variation of the microbial community structure in the control and nicotinecontaminated sediment samples with nicotine concentration and time of exposure.The results demonstrated that the growth of some bacterial species in the nicotine-contaminated sediment samples was inhibited during the exposure.Some bacteria decreased in species diversity and in quantity with the increase of nicotine concentration or time of exposure,while other bacteria were enriched under the effect of nicotine,and their DGGE bands changed from undertones to deep colors.The microbial community structure,however,showed a wide variation in the nicotinecontaminated sediment samples,especially in the sediment samples treated with high-concentration nicotine.The Jaccard index was only 35.1% between the initial sediment sample and the sediment sample with a nicotine concentration of 0.030 μg/g after 28 d of exposure.Diversity indices showed that the contaminated groups had a similar trend over time.The diversity indices of contaminated groups all decreased in the frst 7 d after exposure,then increased until day 42.It has been found that nicotine decreased the diversity of the microbial community in the sediment. ©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Nicotine;Sediment;PCR-DGGE;Microbial community structure;Diversity index
1.Introduction
Nicotine(1-methyl-2-(3-pyridyl)-pyrrolidine,C10H14N2)is the principal alkaloid accumulated in mostNicotianaspecies, and the toxic aspects of nicotine are well known(Doolittle et al.,1995).Because of its harmfulness,nicotine has been classifed as a chemical in the Toxic Release Inventory by the U.S.Environmental Protection Agency since 1994(Blake,
This work was supported by the National Natural Science Foundation of China(Grants No.51378175 and 41323001),and the Special Fund of the State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering (Grant No.20145028212).
*Corresponding author.E-mail address:aidong_ruan@hhu.edu.cn(Ai-dong Ruan).
Peer review under responsibility of Hohai University. 1994).Moreover,waste in which the nicotine content exceeds 500 mg/kg dry weight(d.w.),is classifed as a toxic and hazardous waste inEuropean Union Regulationand laws in many countries.
Furthermore,the tobacco-manufacturing process and all the activities using tobacco often produce large amounts of solid or liquid waste containing nicotine.Nicotine in tobacco waste easily enters the aquatic environment,due to its high solubility in water(Kaiser et al.,1996;Wang et al.,2001).Nicotine concentration has been detected in U.S.estuaries at levels from 2.5×10-4to 6×10-4mg/L(Benotti and Brownawell, 2007);in Spanish rivers at levels up to 1.9×10-3mg/L (Valcˊarcel et al.,2011);and in U.K.groundwater at levels up to 8.1×10-3mg/L(Stuart et al.,2012).A global investigation showed an average nicotine concentration of 1.9×10-3mg/L in the drinking water of 30 cities(Boleda et al.,2011).Therefore,it should be noted that nicotine is widely distributed in water,and present in high concentrations.Nicotinecontaminated waste may become a considerable threat to the environment,imperiling the health of human beings and the ecological balance as well,if it is directly discharged into the environment without any treatment(Piotrowska-Cyplik et al., 2009).
China leads the world in tobacco production,with an annual production of 3.0 million tons of tobacco leaf and a planting area of 1.365 million hectares in 2010.Therefore,in China,a large quantity of tobacco waste,which has been estimated to be about 1.0 million tons every year,with an average nicotine content of 18 g/kg d.w.,is becoming a serious ecological problem(Zheng and Yu,2004).
Nicotine enters soil and sediment via eluviation,and infuences sediment texture and fertility owing to its heterocyclic structure(Civilini et al.,1997;Wang et al.,2005).Once into sediment,it infuences many groups of microorganisms through degradation and toxicological effects(Yuan et al., 2006).In bacteria,pyridine and pyrrolidine pathways are two common nicotine degradation pathways(Brandsch,2006; Tang et al.,2013).In addition,a variant of the pyridine and pyrrolidine pathway(the VPP pathway)also exists(Wang et al.,2012;Ma et al.,2014).Most previous research has concentrated on single types of nicotine-degrading bacteria. However,few studies have investigated the actual infuence of nicotine on microorganisms in sediment.
In this study,we explored the effect of nicotine pollution on the shift in the microbial community structure in the sediment from the aspects of nicotine concentration and time of exposure.Partial 16S rDNA genes were amplifed from the sediment microbial community DNA with a polymerase chain reaction(PCR),using primers that bind to evolutionarily conserved regions within these genes in the eubacteria.The diversity of PCR-amplifed products was transformed into genetic fngerprints using DGGE.
2.Materials and methods
2.1.Experimental sediment samples
The sediment samples were taken from the Nanjing section of the Yangtze River.After air-drying,the sediment samples were ground into smaller particles using a mill,and sieved with a 2 mm-size sieve to remove plant material,macrofauna, and stones.The pH values of sediment samples were measured in water suspension with a soil to solution ratio of 1:5(weight to volume ratio)using a PHS-2C pH meter with a glass electrode.The organic matter and total nitrogen contents in the sediment samples were measured with the K2Cr2O4and digestion-distillation standard methods,respectively(Sciubba et al.,2013).The NO-3-N content was detected with the spectrophotometric method using phenol disulfonic acid(Bai et al.,2010).The available P,Mg,K,and Ca contents in the sediment samples were analyzed using an atomic absorption spectrophotometer(Angino,2012).Table 1 lists some physical and chemical properties of the sediment samples.
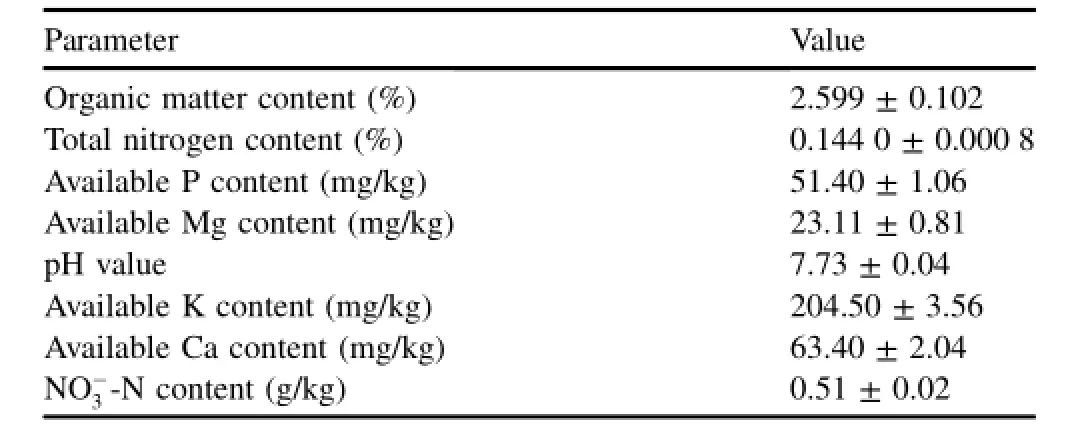
Table 1 Main physical and chemical properties of sediment samples.
2.2.Experimental design and operation
The experimental setup consisted of plastic barrels,whose top diameter,bottom diameter,and height were 179,134,and 161 mm,respectively.A 10 cm-height conical gravel pile with a 25°slope was placed at the bottom of each barrel.Four layers of plastic woven meshes covered the gravel pile,and two polyethylene pipes(with an inner diameter of 1.0 cm and a length of 20 cm)passed through the plastic woven meshes and gravel to the bottom of each barrel at the center of the barrel.Then,1.5 kg of sediment was loaded,and 500 mL of deionized water was added through polyethylene pipes to saturate the system.Six barrels were divided into three groups (each group containing two biological repeats).After exposure at 28°C for two weeks,doses of 100 mL of solution with different nicotine concentrations were added to each barrel to attain fnal concentrations of 0,0.008,and 0.030 μg/g, respectively.A 10 mL well-mixed sample in each barrel was collected with the fve-point method(a 2 mL sample was collected at each point),without compensation,after 0,7,14, 28,and 42 d of exposure.The samples were stored at-80°C for molecular biological analysis.
2.3.DNA extraction and PCR-DGGE analysis
The DNA of the total sediment community was extracted using the method described by Duan and Min(2004).DNA purifcation was conducted using the Wizard DNA clean-up system(Promega,USA).The yield and quality of sedimentextracted DNA were assessed using a supermicro spectrophotometer(Kaiao,China).
The method used was based on that of Damiani et al. (1996)with some modifcations.A PCR mixture with a volume of 50 μL contained 1.0 μL of template DNA(or PCR products),0.2 μmol/L of each primer,5 U of Taq polymerase, 1.0 μL of 10×PCR buffer,200 μmol/L of dNTP,37.5 mmol/L of magnesium chloride, and sterile deionized water. The primers used for the frst reaction of nested PCR were BSF8/20(5′-AGAGTTTGAT CCTGGCTCAG-3′)and BSR1541/20(5′-AAGGAGGTGA TCCAG CCGCA-3′) (Devereux and Willis,1995).The touchdown PCR amplifcation included the initial denaturation at 95°C for 5 min, followed by nine touchdown cycles,at 95°C for 60 s,58°C (decreased by 0.5°C per cycle)for 50 s,and 72°C for 120 s, then 21 cycles of 95°C for 60 s,54°C for 50 s,and 72°C for 120 s,and,fnally,a fnal elongation at 72°C for 10 min.
Using the PCR products from the frst reaction as a template,the V3 variable region of 16S rDNA genes was amplifed using the primer pair 338F with a GC-clamp(5′-CGCCC GCCGCGCGCGGCGGGCGGGGCGGGGGCACG GGGGG ACTCC TACGG GAGGC A-3′)and 518R(5′-ATTAC CGCGG CTGCT GG-3′)(Òvreas et al.,1997).PCR amplifcation was performed at 95°C for 5 min,with ten touchdown cycles,at 95°C for 60 s,56°C(decreased by 0.5°C per cycle)for 60 s,and 72°C for 120 s,then an additional 15 cycles,at 94°C for 60 s,51°C for 60 s,and 72°C for 120 s, followed by a fnal elongation at 72°C for 10 min.DNA and PCR products were confrmed by 10 g/L(weight to volume ratio)agarose gel electrophoresis,followed by staining with ethidium bromide(Nalin et al.,1999).Strong bands of the V3 variable region of the 16S rDNA gene PCR products of approximately 240 bp were subjected to DGGE analysis.
DGGE analysis was conducted using a D-Code system (Bio-Rad,USA).The method has been described by Muyzer et al.(1993).Samples of PCR product were loaded onto 10%(weight to volume ratio)polyacrylamide gels with a denaturant gradient from 37%to 55%.The electrophoresis was run in a 1×TAE(Tris/Acetic acid/EDTA)buffer for 300 min at 150 V and 60°C.After electrophoresis,the gels were stained with silver.The silver-staining procedure was performed according to the method described by Bassam and Caetaneo-Anollˊes(1991).To obtain a clear image,the gel was photographed with a gel photo system(GelDoc,Bio-Rad, USA).
2.4.Analysis of DGGE patterns
Digitized DGGE images were analyzed with the Quantity One image analysis software(version 4.0,Bio-Rad,USA). This software identifes the bands occupying the same position in different lanes of a gel.The similarity of two samples was estimated with information in banding patterns with a Jaccard index(Cj)in each pair of samples(Eq.(1)):

wherejis the total number of bands in two DGGE gel profles, andaandbare the respective number of bands in the two DGGE gel profles.The similarity of sediment samples was examined using the unweighted pair-group method with arithmetic means(UPGMA)and PhyTools software.
The Shannon-Wiener index(H′),richness(S),and evenness (E)were calculated to compare the change in bacterial diversity based on the DGGE band data.H′is calculated as

wherepiis the importance probability of bandiin a gel lane.Eis calculated asH′/lnS,andSis the total number of bands in a gel lane.
3.Results and discussion
3.1.Effect of nicotine on microbial community structure
DGGE profles of the V3 variable region of 16S rDNA genes from the sediment samples during different time periods are shown in Fig.1.The culture times of a through e were 0,7, 14,28,and 42 d,respectively;and a′through e′was the repetitions of a through e,respectively.Each of the distinguishable bands in the separation pattern represents an individual bacterial species(Luca et al.,2002).As shown in Fig.1,some bacterial species were inhibited in the nicotine-contaminated sediment samples.With the increase of culture time,the color of Band 1 and Band 2 in DGGE gel changed from a deep color to an undertone,and even disappeared in the sediment samples with a nicotine concentration of 0.030 μg/g.However, the color of Band 1 and Band 2 in DGGE gel changed from an undertone to a deep color in sediment samples with a nicotine concentration of 0.008 μg/g.This indicated that a low nicotine concentration might stimulate Band 1 and Band 2 bacterial species.However,when the nicotine concentration was 0.030 μg/g,it was harmful to them.In sediment samples with a nicotine concentration of 0.030 μg/g,Band 3 and Band 4 were present in the late stage of exposure.
3.2.Similarity analysis
Genetic similarity of microbialcommunity profles revealed that there were very large differences in the control and nicotine-contaminated sediment samples(Fig.2).The microbial community structure in the control sample had a similarity of more than 72%to the initial contaminated samples during 42 d of exposure(Fig.3).The microbial community structure,however,showed a large degree of variation in the nicotine-contaminated sediment samples,especially in the sediment samples with high nicotine concentrations.The Jaccard index was only 61.4%between the initial sample and the sample with a nicotine concentration of 0.008 μg/g after 42 d of exposure.The sediment sample on day 28 with a nicotine concentration of 0.030 μg/g only had a similarity of 35.1%to the initial sample.The variation of the microbialcommunity structure in the sediment samples mostly occurred during the frst week of exposure.As compared with the initial sediment samples,the Jaccard index decreased by 33.2%in the sample with a 0.008 μg/g nicotine concentration and 48.9%in the sample with a 0.030 μg/g nicotine concentration, respectively,while the control sample only decreased by 13.4%.In the nicotine-contaminated sediment samples,the Jaccard index decreased until day 28,and then increased a little on day 42.Linear regression showed that there was not a signifcant time-effect relationship between the nicotine concentration and microbial community structure in the experimental sediment samples.
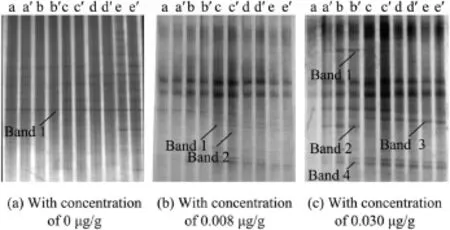
Fig.1.DGGE patterns of V3 variable region of 16S rDNA genes from sediment samples with different nicotine concentrations at different culture times.
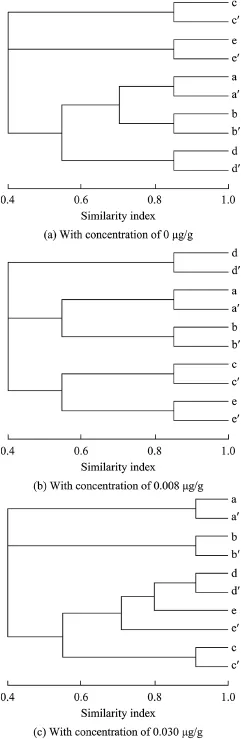
Fig.2.Genetic similarity of microbial community profles obtained with PCR-DGGE from sediment samples with different nicotine concentrations at different culture times.
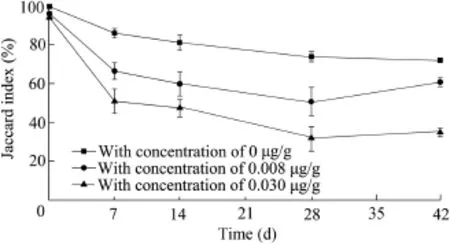
Fig.3.Jaccard index of microbial communities for sediment samples with different nicotine concentrations at different culture times.
3.3.Analysis of diversity indices
Diversity indices are useful for investigating the diversity of microbial communities.The higher the Shannon-Wiener index is,the greater the diversity of microbial communities is.Two components contributed to the diversity indices:(1)the total numbers of species present or species richness(S),and(2)the distribution of the number of individuals among those different species,or species evenness(E).
The diversity indices of microbial communities in nicotinecontaminated groups are shown in Table 2.Group 1 and group 2 are the experimental group and its biological repeat, respectively.The diversity indices between group 1 and group 2 are extremely similar at the same time and at the same concentration,demonstrating excellent repeatability.Before the exposure,the groups with the same concentration showed homogeneity:H′was between 1.52 and 1.58,Swas between 12 and 13,andEwas between 0.59 and 0.63.It can be seen that,before contamination,each system had an essential homogeneity with stable microbial community structure and activities.However,the diversity indices of the control group increased slightly with time.In addition,the contaminated groups had a similar trend over time,and the diversity indicesall decreased after 7 d of exposure,then increased until day 42. After 7 d,the group with the nicotine concentration of 0.030 μg/g reached the minimum diversity indices of the experimental groups.These results reveal thathighconcentration nicotine exposure has a stronger infuence on the diminishment of the microbial community in the frst 7 d than low-concentration nicotine exposure.After 28 d,the diversity indices of microbial communities in contaminated groups were much greater than those of the control groups, indicating that the increase of diversity of contaminated groups occurs over a long period of time,nearly three weeks. Moreover,after exposure,some microbial species quickly decreased,and,with time,the growth of new species was promoted,enriching the community structure.

Table 2 Diversity indices of microbial communities in groups with different nicotine concentrations.
4.Conclusions
The PCR-DGGE method was used to investigate the microbial community structure in nicotine-contaminated sediment samples.The contamination of nicotine can greatly impact the microbial activity and function.Profles of DGGE showed that,with the increase of nicotine concentration and culture time,the toxicity of nicotine to sediment microorganisms increased,resulting in the variation of sediment bacteria diversity.The Jaccard index showed that the changes in sediment bacteria community were slight in control groups but signifcant in nicotine-contaminated groups,especially in groups with high nicotine concentration.In addition,it was revealed by the diversity indices that the diversity of community structure in nicotine-contaminated groups,decreased in the frst 7 d after exposure,then increased gradually until day 42.The results also indicated that new species of nicotineresistant microbial organisms were enriched in the nicotinecontaminated sedimentenvironment.The PCR-DGGE method can provide detailed information about the shift and diversity in microbial community structure in the sediment environment.These results will beneft the assessment and remediation of nicotine-contaminated sediment.
Angino,E.,2012.Atomic Absorption Spectrometry in Geology.Elsevier, Amsterdam.
Bai,J.H.,Wang,Q.G.,Gao,H.F.,Xiao,R.,Deng,W.,Cui,B.S.,2010.Spatial and temporal distribution patterns of nitrogen in marsh soils from an inland alkaline wetland:A case study of Fulaowenpao wetland,China.Acta Ecol. Sin.30(4),210-215.http://dx.doi.org/10.1016/j.chnaes.2010.06.004.
Bassam,B.J.,Caetaneo-Anollˊes,G.,1991.Fast and sensitive silver staining of DNA in polyacrylamide gels.Anal.Biochem.196(1),80-83.http:// dx.doi.org/10.1016/0003-2697(91)90120-I.
Benotti,M.J.,Brownawell,B.J.,2007.Distributions of pharmaceuticals in an urban estuary during both dry-and wet-weather conditions.Environ.Sci. Technol.41(16),5795-5802.http://dx.doi.org/10.1021/es0629965.
Blake,D.M.,1994.Bibliography of Work on the Heterogeneous Photocatalytic Removal of Hazardous Compounds from Water and Air.National Renewable Energy Laboratory,Golden.
Boleda,M.R.,Huerta-Fontela,M.,Ventura,F.,Galceran,M.T.,2011.Evaluation of the presence of drugs of abuse in tap waters.Chemosphere 84(1), 1601-1607.http://dx.doi.org/10.1016/j.chemosphere.2011.05.033.
Brandsch,R.,2006.Microbiology and biochemistry of nicotine degradation. Appl.Microbiol.Biotechnol.69(5),493-498.http://dx.doi.org/10.1007/ s00253-005-0226-0.
Civilini,M.,Domenis,C.,Sebastianutto,N.,Bertoldi,M.D.,1997.Nicotine decontamination of tobacco agro-industrial waste and its degradation by micro-organisms.Waste Manag.Res.15(4),349-358.http://dx.doi.org/ 10.1177/0734242X9701500403.
Damiani,G.,Amedeo,P.,Bandi,C.,Fani,R.,Bellizzi,D.,Sgaramella,V., 1996. Bacteria identifcation by PCR-based techniques. In: Adolph,K.W.ed.,Microbial Genome Methods.CRC Press,Boca Raton,pp.167-173.
Devereux,R.,Willis,S.G.,1995.Amplifcation of ribosomal RNA sequences. Mol.Microb.Ecol.Man.33,277-287.http://dx.doi.org/10.1007/978-94-011-0351-0_19.
Doolittle,D.J.,Winegar,R.,Lee,J.K.,Caldwell,W.S.,Wallace,A.,Hayes,J., Bethizy,J.D.,1995.The genotoxic potential of nicotine and its major metabolites.Mutat.Res.344(3-4),95-102.http://dx.doi.org/10.1016/ 0165-1218(95)00037-2.
Duan,X.J.,Min,H.,2004.Diversity of microbial genes in paddy soil stressed by cadminum using DGGE.Environ.Sci.25(5),122-126(in Chinese).
Kaiser,J.P.,Feng,Y.,Bollag,J.M.,1996.Microbial metabolism of pyridine, quinoline,acridine,and their derivatives under aerobic and anaerobic conditions.Microbiol.Rev.60(3),483-498.
Luca,C.,Daniele,A.,Marisa,M.,Carlo,C.,Giuseppe,C.,2002.An application of PCR-DGGE analysis to profle the yeast population in raw milk. Int.Dairy J.12(5),407-411.http://dx.doi.org/10.1016/S0958-6946(02) 00023-7.
Ma,Y.,Wei,Y.,Qiu,J.G.,Wen,R.T.,Hong,J.,Liu,W.P.,2014.Isolation, transposon mutagenesis,and characterization of the novel nicotinedegrading strainShinellasp.HZN7.Appl.Microbiol.Biotechnol.98(6), 2625-2636.http://dx.doi.org/10.1007/s00253-013-5207-0.
Muyzer,G.,Waal,E.C.,Uitterlinden,A.G.,1993.Profling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplifed genes coding for 16S rRNA.Appl. Environ.Microbiol.59(3),695-700.
Nalin,R.,Simonet,P.,Vogel,T.M.,Normand,P.,1999.Rhodanobacter lindaniclasticusgen.nov.,sp.nov.,a lindane-degrading bacterium.J.Syst. Bacteriol.49(1),19-23.http://dx.doi.org/10.1099/00207713-49-1-19.
Òvreas,L.,Forney,L.,Daàe,F.L.,1997.Distribution of bacterioplanton in meromictic Lake Saelevannet,as determined by denaturing gradient gel electrophoresis of PCR-amplifed gene fragments coding for 16S rRNA. Appl.Environ.Microbiol.63(9),3367-3373.
Piotrowska-Cyplik,A.,Olejnik,A.,Cyplik,P.,Dach,J.,Czarnecki,Z.,2009.The kineticsofnicotinedegradation,enzymeactivitiesandgenotoxicpotentialin the characterization of tobacco waste composting.Bioresour.Technol. 100(21),5037-5044.http://dx.doi.org/10.1016/j.biortech.2009.05.053.
Sciubba,L.,Cavani,L.,Marzadori,C.,Ciavatta,C.,2013.Effect of biosolids from municipal sewage sludge composted with rice husk on soil functionality.Biol.Fertil.Soils 49(5),597-608.http://dx.doi.org/10.1007/ s00374-012-0748-4.
Stuart,M.,Lapworth,D.,Crane,E.,Hart,A.,2012.Review of risk from potential emerging contaminants in UK groundwater.Sci.Total Environ. 416(2),1-21.http://dx.doi.org/10.1016/j.scitotenv.2011.11.072.
Tang,H.Z.,Wang,L.J.,Wang,W.W.,Yu,H.,Zhang,K.Z.,Yao,Y.X.,Xu,P., 2013.Systematic unraveling of the unsolved pathway of nicotine degradation in Pseudomonas.PLOS Genet.9(10),e1003923.http://dx.doi.org/ 10.1371/journal.pgen.1003923.
Valcˊarcel,Y.,Alonso,S.G.,Rodriguez-Gil,J.L.,Gil,A.,Catala,M.,2011. Detectionofpharmaceuticallyactivecompoundsintheriversandtapwaterof the Madrid region(Spain)and potential ecotoxicological risk.Chemosphere 84(10),1336-1348.http://dx.doi.org/10.1016/j.chemosphere.2011.05.014.
Wang,S.J.,Wang,Z.M.,Chen,Q.F.,Liu,G.Q.,2001.The countermeasure of the problems in fertilizer use and tobacco quality in tobacco growth area in Southern Anhui.J.Anhui Agric.Sci.29(3),366-367(in Chinese).
Wang,S.N.,Xu,P.,Tang,H.Z.,Meng,J.,Liu,X.L.,Ma,C.Q.,2005.“Green”route to 6-hydroxy-3-succinoyl-pyridine from(S)-nicotine of tobaccowaste by whole cells of aPseudomonassp.Environ.Sci.Technol.39(17), 6877-6880.http://dx.doi.org/10.1021/es0500759.
Wang,S.N.,Huang,H.Y.,Xie,K.B.,Xu,P.,2012.Identifcation of nicotine biotransformation intermediates byAgrobacterium tumefaciensstrain S33 suggests a novel nicotine degradation pathway.Appl.Microbiol.Biotechnol.95(6),1567-1578.http://dx.doi.org/10.1007/s00253-012-4007-2.
Yuan,Y.J.,Lu,Z.X.,Huang,L.J.,Bie,X.M.,Lu¨,F.X.,Li,Y.,2006.Optimization of a medium for enhancing nicotine biodegradation byOchrobactrum intermediumDN2.J.Appl.Microbiol.101(3),691-697.http:// dx.doi.org/10.1111/j.1365-2672.2006.02929.x.
Zheng,K.L.,Yu,D.M.,2004.A summary of the comprehensive utilizations of discarded tobacco leaves.J.Chongqing Univ.27(3),61-64(in Chinese).
Received 25 December 2014;accepted 12 October 2015
Available online 1 December 2015
http://dx.doi.org/10.1016/j.wse.2015.11.003
1674-2370/©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Water Science and Engineering2015年4期
Water Science and Engineering2015年4期
- Water Science and Engineering的其它文章
- Temporal and spatial distribution characteristics of water resources in Guangdong Province based on a cloud model
- Analysis and prediction of reference evapotranspiration with climate change in Xiangjiang River Basin,China
- Analysis of spatio-temporal evolution of droughts in Luanhe River Basin using different drought indices
- Effects of temporal variability on HBV model calibration
- Impacts of water quality variation and rainfall runoff on Jinpen Reservoir, in Northwest China
- Numerical simulation of fow past twin near-wall circular cylinders in tandem arrangement at low Reynolds number
