Rapid screening and distribution of bioactive compounds in different parts ofBerberis petiolarisusing direct analysis in real time mass spectrometry☆
Awantika Singh,Vikas Bajpai,Mukesh Srivastava,Kamal Ram Arya, Brijesh Kumar,*
aSophisticated Analytical Instrument Facility(SAIF),CSIR-Central Drug Research Institute,Lucknow 226031,India
bAcademy of Scientifc and Innovative Research(AcSIR),New Delhi 110001,India
cBiometric and Statistics Division,CSIR-Central Drug Research Institute,Lucknow 226031,India
dBotany Division CSIR-Central Drug Research Institute,Lucknow 226031,India
Rapid screening and distribution of bioactive compounds in different parts ofBerberis petiolarisusing direct analysis in real time mass spectrometry☆
Awantika Singha,b,Vikas Bajpaia,b,Mukesh Srivastavac,Kamal Ram Aryab,d, Brijesh Kumara,b,*
aSophisticated Analytical Instrument Facility(SAIF),CSIR-Central Drug Research Institute,Lucknow 226031,India
bAcademy of Scientifc and Innovative Research(AcSIR),New Delhi 110001,India
cBiometric and Statistics Division,CSIR-Central Drug Research Institute,Lucknow 226031,India
dBotany Division CSIR-Central Drug Research Institute,Lucknow 226031,India
A R T I C L E I N F O
Article history:
Received 9 October 2014
Received in revised form
24 April 2015
Accepted 5 May 2015
Available online 12 May 2015Keywords:
Berberis petiolaris
Alkaloids
Profling
DART–TOF–MS
Statistical analysis
Berberis petiolarisWall.ex G.Don,an unexplored medicinal plant belonging to the family Berberidaceae, is a large deciduous shrub found in Western Himalaya between 1800–3000 m.Chemical profling of fruit, leaf,root and stem was done by direct analysis in real time mass spectrometry followed by multivariate analysis for discrimination among the plant parts.The bioactive compounds,including magnoforine, berberine,jatrorrhizine,thalifendine/berberrubine,demethyleneberberine,reticuline,8-oxoberberine, N-methyltetrahydroberberine,tetrahydropalmatine,tetrahydroberberine and palmatine,were identifed by their exact mass measurement and the corresponding molecular formula of each compound.A comparative study of distribution pattern for all these bioactive alkaloids showed qualitative and quantitative variations in different parts ofB.petiolaris.Principal component analysis clearly discriminated each part ofB.petiolarisplant.
©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http∶//creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Berberis petiolaris(B.petiolaris)is an unexplored deciduous shrub plant parts.Different parts of the plant such as leaf,stem,root and fruit were screened to study comparative detection of the bioactive compounds in different parts of the plant.Hence,it was decided to profle the chemical constituents using DART time-offight mass spectrometry(DART– TOF–MS)and to study their variations in different plant parts ofB.petiolaris. that belongs to the family Berberidaceae found in Western Himalaya between 1800–3000 m.This plant mainly contains different classes of alkaloids[1,2],but only berberine,jatrorrhizine and palmatine were previously identifed and isolated fromB.petiolaris[3].Decoction of root and stem of the plant is used for the treatment of malarial fever,diarrhea,conjunctivitis,and jaundice[4]. Recent developments in newer ionization methods have made it possible to obtain mass data directly from the plant parts without any sample preparation.Among the methods,direct analysis in real time(DART)is an ambient ionization technique requiring no sample preparation[5,6].It is,therefore,an appropriate technique for the rapid chemical profling of plant species[7,8].The aim of present work was to develop a method for identifcation of biologically active compounds inB.petiolarisdirectly from intact
2.Materials and methods
Plant samples ofB.petiolariswere collected from Pandukholi forest, Almora Uttarakhand(India)and identifed according to the fora of district Garhwal,northwest Himalaya and a forest fora of Kumaon. Herbarium specimen ofB.petiolaris(KRA 24410)was housed in the departmental herbarium,Central Drug Research Institute(Lucknow, India).For DART–TOF–MS analysis,intact plant parts were used directly as the samples.The plant samples were thoroughly washed with tap water and distilled water in order to remove any foreign particles attached to their surface and kept in oven to dry at 40°C.
The mass spectrometer was a Jeol the Accu TOF JMS-T100LC atmospheric pressure ionization time-of-fight mass spectrometer(Jeol, Tokyo,Japan)ftted with a DART ion source.The mass spectrometer was operated in positive-ion mode with a resolving power of 6000(full-width at half-maximum).The ori f ce 1 potential was set to 28 V, resulting in minimal fragmentation.The ring lens and ori f ce 2 potential were set to 13 and 5 V,respectively.Ori f ce 1 was set to a temperature of 100°C.The RF ion guide potential was 300 V.The DART ion source was operated with helium gas f owing at approximately 4.0 L/min.The gas heater was set to 300°C.The potential on the discharge needle electrode of the DART source was set to 3000 V; electrode 1 was 100 V and the grid was at 250 V.Freshly cuted pieces of plant parts were positioned in the gap between the DART source and mass spectrometer for measurements.Data acquisition was fromm/z10 to 1050.Exact mass calibration was accomplished by including a mass spectrum of neat polyethylene(PEG)glycol(1∶1mixture of PEG 200 and PEG 600)in the data f le.The mass calibration was accurate within±0.002 u.Using the Mass Centre Main software(version 1.3.m;JEOL Japan),the elemental composition could be determined on selected peaks.Principal component analysis (PCA)analysis was carried out using Statistica windows version 7.0 (Stat Soft Inc.,USA)statistical analysis software.This software normalizes observations with respect to mean and variance followed by PCA.
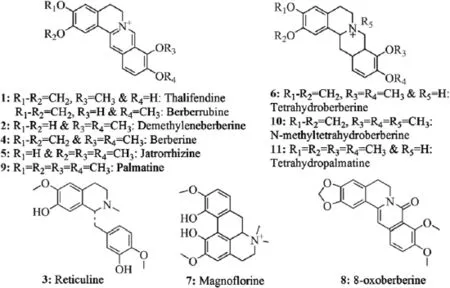
Fig.1.Chemical structure of indenti f ed compounds.
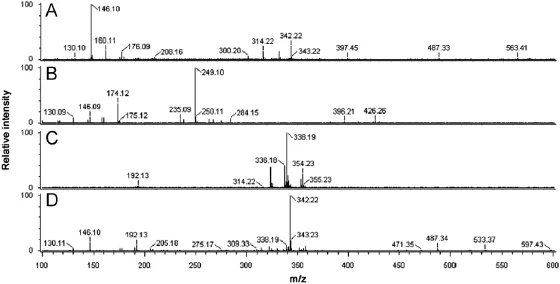
Fig.2.DART mass spectra of(A)fruit,(B)leaf,(C)root and(D)stem.

Table 1Exact mass data from the DART mass spectra ofB.petiolaris(R=root;S=stem;F=fruit;L=leaf).

Fig.3.(A)PCA plot discriminating plant parts ofBerberis petiolaris.(B)Score plot discriminating plant parts ofBerberis petiolaris.
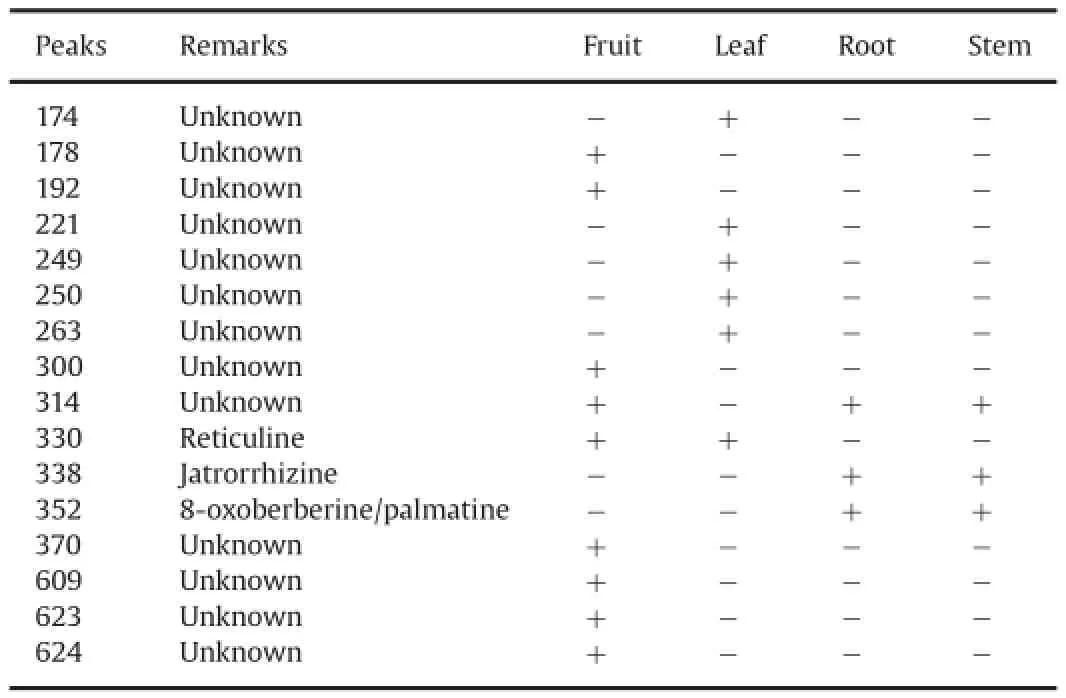
Table 2Identi f ed peaks which discriminated plant parts ofB.petiolaris.
3.Results and discussion
The identi f cation from direct analysis of pharmaceutical products without any sample preparation is an ultimate achievement in pharmaceutical analysis for quality control and assurance of pharmaceutical products and herbal materials.Screening of bioactive constituents for identi f cation of right selection of plant/parts for better ef f cacy is important for quality control of herbal products. DART–TOF–MS pro f les or f ngerprints of different plant parts such as fruit,leaf,root and stem ofB.petiolariswere obtained after analysis in the present study.All these plant parts were subjected to DART–TOF–MS analysis under the same conditions.On the basis of literature report[9,10]and our f ndings described below,some of the expected phytochemical components inB.petiolarisare shown in Fig.1.These components are directly ionized from the plant part during analysis as molecular species in the resulting spectra.For instance,the DART mass spectra of the fruit,leaf,root and stem ofB.petiolarisare given in Fig.2.Peaks corresponding to the molecular species of thalifendine/berberrubine 1(m/z322),demethyleneberberine 2(m/z324),reticuline 3(m/z330),berberine 4(m/z336),jatrorrhizine 5(m/ z338),tetrahydroberberine 6(m/z339),magno f orine 7(m/z342), 8-oxoberberine 8(m/z352),palmatine 9(m/z352),N-methyltetrahydroberberine 10(m/z354)and tetrahydropalmatine 11(m/z355)were observed in the DART mass spectra.Thalifendine and berberrubine 1 have the same molecular formula and exact mass. Therefore,they cannot be distinguished on the basis of high resolution mass spectrometry(HRMS).Measured mass,calculated mass[2] and molecular formula[1]obtained from all the above constituents are reported in Table 1.Reticuline 3(m/z330)was not detected inB. petiolarisroot or stem but detected in fruit and leaf.However,berberine 4(m/z336)was identi f ed only in root and stem according to literature reports from otherBerberisspecies[11].Similarly,magnof orine 7 was present in fruit,root and stem but absent in leaf.A peak atm/z352[M+H]+corresponding to 8-oxoberberine 8 and a peak atm/z352[M]+corresponding to palmatine 9 were observed in stem and root but not in leaf or fruit.N-methyltetrahydroberberine 10(m/z354)was also absent in the fruit,leaf and stem ofB.petiolarisbut showed signi f cant presence in the root.DART–TOF–MS analysis of the fruit,leaf,root and stem ofB.petiolarisshowed differences in their spectra.Maximum abundance of compounds thalifendine/berberrubine 1(m/z322),berberine 4(m/z336),jatrorrhizine 5(m/z338)and N-methyltetrahydroberberine 10(m/z354)was observed in root,whereas magno f orine 7(m/z342)showed its maximum abundance in fruit followed by stem.These observations con f rmed that results obtained from DART–TOF–MS data were good,and it was the instrument of choice for the screening of natural products.
In metabolic pro f ling,identi f cation of metabolite concentration changes by visual inspection of data is cumbersome and almost impractical for large sample sizes.Therefore,it is necessary to resort to multivariate techniques such as PCA,factor analysis,and partial least squares,which are important and proven techniques for complex data analysis[12].We selected PCA for dimensionality reduction in an attempt to distinguish characteristic pro f les from the DART–TOF–MS data.Accordingly,Fig.3A shows the PCA plot which discriminates plant parts ofB.petiolarisand their score plot(PC1 vs.PC2)obtained is given in Fig.3B.It can be seen in Fig.3A that the fruit,leaf,root and stem ofB.petiolarisshow clustering of the data according to the parts. Fig.3A shows the distinct collection of PC scores in the biplot.The clustering of scores clearly shows the position of each plant part with a reasonable distance.This indicates that the f rst two PCs can easily discriminate the plant parts.Similar clustering and differentiation areclearly observed for the fruit,leaf,root and stem in Fig.3B.Totally 16 peaks(m/z178,174,192,221,249,250,263,300,314,330,338,352, 370,609,623 and 624)were selected to study PCA for all the plant parts relying on percent(%)ionization of peaks(Table 2).PCA score plot clearly brings out relationship among all the plant parts.On the basis of eigen values,it was observed that the whole information of data matrix(81.44%)has been explained by two principal components. The contribution of PC1 was 53.45%and that of PC2 was 27.99%.Different parts of the same plant could be discriminated effectively by this technique and the presence and absence of peaks(as shown in Table 2)are complementary to PCA results.Peaks atm/z338 and 352 were identi f ed as markers for root and stem,whilem/z174,221,249, 250 and 263 for leaf andm/z178,192,300,314,330,370,609,623,624 for fruit.It is,therefore,clear that DART–TOF–MS followed by PCA is an appropriate method for the clear differentiation of plant parts and rapid identi f cation of compounds.The DART mass spectrometric technique has been applied for the f rst time for the pro f ling of alkaloids inB.petiolaris.Chemical pro f ling of fruit,leaf,root and stem was done successfully.It was observed that there were signi f cant differences in mass spectra obtained from the fruit,leaf,root and stem ofB.petiolaris.PCA analysis showed that DART–TOF–MS data could be used to differentiate these alkaloids containing plant species and to distinguish the plant parts.Peaks atm/z338[M]+of jatrorrhizine,m/z352[M+H]+of 8-oxoberberine andm/z352[M]+of palmatine were identi f ed as markers for root and stem.This will help with identi fcation and characterization of genuine parts used for drug preparation and the effective quality control and ef f cacy of drug candidates in future.
Acknowledgments
Grateful acknowledgment is made to the Department of Science and Technology,India for Grant SB/EMEQ-095.Authors are also thankful to SAIF-CDRI,Lucknow,where all the mass spectral studies were carried out.Awantika Singh and Vikas Bajpai are also thankful to UGC and CSIR,respectively,for providing Senior Research Fellowship.CDRI communication number is 8994.
[1]A.Singh,V.Bajpai,M.Srivastava,et al.,Rapid pro f ling and structural characterization of bioactive compounds and their distribution in different parts ofBerberis petiolarisWall.ex G.Don applying hyphenated mass spectrometric techniques,Rapid Commun.Mass Spectrom.28(2014) 2089–2100.
[2]V.Bajpai,A.Singh,K.R.Arya,et al.,Rapid screening for the adulterants ofBerberis aristatausing direct analysis in real-time mass spectrometry and principal component analysis for discrimination,Food Addit.Contam.Part A 32(2015)799–807.
[3]D.Bhardwaj,N.Kaushik,Phytochemical and pharmacological studies in genus Berberis,Phytochem.Rev.11(2012)523–542.
[4]A.Karimov,Berberis alkaloids,Chem.Nat.Compd.29(1993)415–438.
[5]V.Bajpai,D.Sharma,B.Kumar,et al.,Pro f ling ofPiper betlelinn cultivars by direct analysis in real time mass spectrometric technique,Biomed.Chromatogr.24(2010)1283–1286.
[6]R.B.Cody,J.A.Laramee,H.D.Durst,Versatile new ion source for the analysis of materials in open air under ambient conditions,Anal.Chem.77(2005) 2297–2302.
[7]S.W.Kim,H.J.Kim,J.H.Kim,et al.,A rapid,simple method for the genetic discrimination of intactArabidopsis thalianamutant seeds using metabolic pro f ling by direct analysis in real-time mass spectrometry,Plant Methods 7 (2011)14–24.
[8]H.J.Kim,E.H.Jee,K.S.Ahn,et al.,Identi f cation of marker compounds in herbal drugs on TLC with DART-MS,Arch.Pharm.Res.33(2011) 1355–1359.
[9]L.Grycova,J.Dostal,R.Marek,Quaternary protoberberine alkaloids,Phytochemistry 68(2007)150–175.
[10]M.S.Arayne,N.Sultana,S.S.Bahadur,The Berberis story-Berberis vulgarisin therapeutics,Pak.J.Pharm.Sci.20(2007)83–92.
[11]B.T.Cromwell,Experiments on the origin and function of Berberine inBerberis darwini,Biochem.J.27(1993)860–872.
[12]M.Li,X.Zhou,Y.Zhao,et al.,Quality assessment ofCurcuma longaL.by gas chromatography mass spectrometry f ngerprint principle component analysis and hierarchical clustering analysis,Bull.Korean Chem.Soc.30(2009) 2287–2293.
☆Peer review under responsibility of Xi'an Jiaotong University.
*Corresponding author at∶Sophisticated Analytical Instrument Facility(SAIF), CSIR-Central Drug Research Institute,Lucknow 226031,India.
Tel.∶+91 9455551934,+91 0522 2738433;fax∶+91 0522 2623405.
E-mail addresses:brijesh_kumar@cdri.res.in,gbrikum@yahoo.com(B.Kumar).
http∶//dx.doi.org/10.1016/j.jpha.2015.05.002
2095-1779/©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license (http∶//creativecommons.org/licenses/by-nc-nd/4.0/).
 Journal of Pharmaceutical Analysis2015年5期
Journal of Pharmaceutical Analysis2015年5期
- Journal of Pharmaceutical Analysis的其它文章
- Application of analytical instruments in pharmaceutical analysis
- JPA Prize in 2014
- High-sensitivity simultaneous liquid chromatography–tandem mass spectrometry assay of ethinyl estradiol and levonorgestrel in human plasma☆
- Determination of atractylon in rat plasma by a GC–MS method and its application to a pharmacokinetic study☆
- Application of RP–HPLC method in dissolution testing and statistical evaluation by NASSAM for simultaneous estimation of tertiary combined dosages forms☆
- Multiple responses optimization in the development of a headspace gas chromatography method for the determination of residual solvents in pharmaceuticals☆
