乌司他丁对婴幼儿体外循环中sICAM-1及vWF的影响
胡明品 李兴旺 吴国伟 陈小玲 鹿文青 李宝青
1.温州医科大学附属第二医院育英儿童医院麻醉科,浙江温州325027;2.温州医科大学附属第二医院育英儿童医院小儿心胸外科,浙江温州325027;3.温州医科大学附属第二医院育英儿童医院检验科,浙江温州325027
乌司他丁对婴幼儿体外循环中sICAM-1及vWF的影响
胡明品1李兴旺1吴国伟2陈小玲1鹿文青1李宝青3
1.温州医科大学附属第二医院育英儿童医院麻醉科,浙江温州325027;2.温州医科大学附属第二医院育英儿童医院小儿心胸外科,浙江温州325027;3.温州医科大学附属第二医院育英儿童医院检验科,浙江温州325027
目的探讨婴幼儿体外循环(CPB)对可溶性细胞间黏附分子-1(sICAM-1)和血管性假性血友病因子(vWF)水平影响及乌司他丁干预性治疗效果。方法选择2014年5月~2015年4月择期婴幼儿心内直视手术48例,随机分为乌司他丁(U)组24例,对照组(C)组24例。前者在体外循环预充液中加入1万U/kg乌司他丁。分别于麻醉诱导后切皮前(T0)、CPB开始后30 min(T1)、CPB结束时(T2)、CPB结束后3 h(T3)、CPB结束后24 h(T4)等5个时间点于桡动脉抽取动脉血,测定血浆中sICAM-1、vWF的浓度。结果与T0比较,C组患儿在T1~T4时的sICAM-1和vWF血浆浓度明显升高,差异有统计学意义(P<0.01),U组患儿在T1~T2时的sICAM-1和vWF血浆浓度较T0时明显升高,差异有统计学意义(P<0.01);与C组比较,U组在T1~T4各时间点的血浆sICAM-1和vWF的浓度明显下降,差异有统计学意义(P<0.01)。结论给予ULI 10000 U/kg干预后,血浆sICAM-1和vWF水平明显降低,对CPB中婴幼儿血管内皮细胞具有保护作用。
乌司他丁;婴幼儿;体外循环;细胞黏附分子;von Willebrand因子
小儿体外循环(cardiopulmonary bypass,CPB)是一个非生理学过程,能够诱发机体产生全身炎症反应,导致重要器官如心肺的不同程度损伤,延迟患儿的术后康复,严重者可导致患儿多器官衰竭,甚至死亡[1,2]。近年来人们对乌司他丁(ulinastatin,ULI)减轻CPB所致炎症反应及肺损伤进行了大量研究[3-6],但对婴幼儿CPB时可溶性细胞间黏附分子-1(cell adhesion molecules,sICAM-1)和血管性假性血友病因子(von Willebrand factor,vWF)水平影响的报道尚少,本研究旨在探讨ULI是否能减轻婴幼儿CPB时血管内皮细胞的激活和破坏。现将结果报道如下。
1 对象与方法
1.1 研究对象
选择2014年5月~2015年4月在我院小儿心胸外科接受择期先天性心脏病手术的患儿48例,男31例,女17例,年龄6~36个月,体重7~17 kg。均经超声心动图确诊为房室水平左向右分流型先心病,其中房间隔缺损7例,室间隔缺损31例,房间隔缺损合并室间隔缺损10例,无其他合并症。按随机数字表法随机分为对照组(C组)24例,乌司他丁组(广东天普生化医药股份有限公司,10万U/支,产品批号:031308123)(U组)24例。本研究经医院伦理委员会批准,患儿父母或监护人均签署知情同意书,两组一般情况比较,差异均无统计学意义(P>0.05),具有可比性。见表1。
表1 两组患儿一般情况比较(±s,n=24)
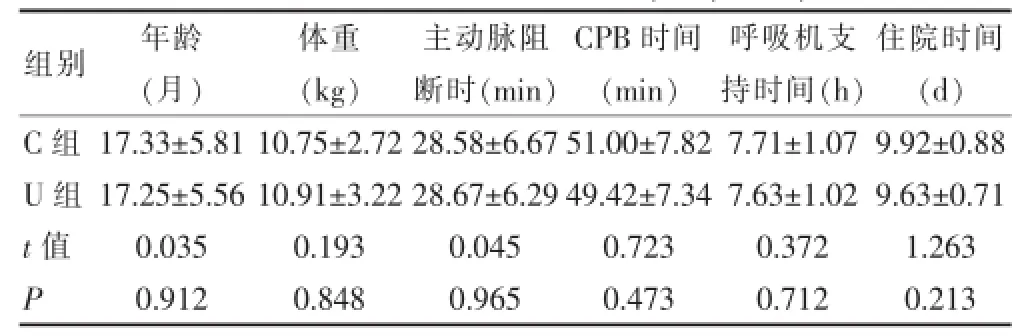
表1 两组患儿一般情况比较(±s,n=24)
组别年龄(月)C组U组体重(k g)主动脉阻断时(m i n)C P B时间(m i n)呼吸机支持时间(h)住院时间(d)t值P 1 7.3 3 ± 5.8 1 1 7.2 5 ± 5.5 6 0.0 3 5 0.9 1 2 1 0.7 5 ± 2.7 2 1 0.9 1 ± 3.2 2 0.1 9 3 0.8 4 8 2 8.5 8 ± 6.6 7 2 8.6 7 ± 6.2 9 0.0 4 5 0.9 6 5 5 1.0 0 ± 7.8 2 4 9.4 2 ± 7.3 4 0.7 2 3 0.4 7 3 7.7 1 ± 1.0 7 7.6 3 ± 1.0 2 0.3 7 2 0.7 1 2 9.9 2 ± 0.8 8 9.6 3 ± 0.7 1 1.2 6 3 0.2 1 3
1.2 纳入及排除标准
CPB中血管内皮细胞的释放受温度、血氧分压、CPB时间等因素的影响,为了减少各种因素对测定指标的影响,规定了以下病例纳入标准:①年龄在6~36个月之间先心病患儿;②经心脏多普勒测得肺动脉压力均≤30 mmHg;③肝肾功能无异常,无上呼吸道感染,未使用激素及免疫调节剂和抗凝治疗等;④体温在36.5℃~37.5℃(耳温)之间。排除标准:①经心脏多普勒测得肺动脉压力≥30 mmHg;②患儿有明显的血流动力学障碍;③体温低于36.5℃或高于37.5℃;④有严重的上呼吸道感染,或使用激素及免疫调节剂治疗。
1.3 方法
1.3.1 麻醉与CPB所有患儿均经口气管插管采用以芬太尼为主的静吸复合麻醉。麻醉诱导:咪达唑仑0.1 mg/kg、维库溴铵0.1 mg/kg、芬太尼(5~10)μg/kg静注;麻醉维持:全过程泵注丙泊酚(2~3)mg/(kg·h),并分别于切皮、CPB开始前静注咪达唑仑(0.1~0.3)mg/kg+芬太尼(5~10)μg/kg+维库溴铵0.05 mg/kg,同时根据麻醉深度和血流动力学变化适当复合吸入七氟醚。采用美国Sarns 8000型CPB机、百特OXIM-Plus膜式氧合器、1/4管道及无血预充技术,预充液用乳酸林格氏液作为基础液,按(0.5~1.5)g/kg量加入20%甘露醇,按2 mg/100 mL剂量加入肝素。按3 mg/kg剂量行全身肝素化后,常规插管建立CPB,术中检测激活全血凝固时间(ACT)>480 s,停机后按1.5∶1比例以鱼精蛋白中和体内的肝素。
1.3.2 用药方法U组使用ULI 1万U/kg,于CPB开始后直接加入预充液中,C组除用生理盐水替代ULI外,其他条件均相同。
1.4 观察指标
分别于麻醉诱导后切皮前(T0)、CPB开始后30 min(T1)、CPB结束时(T2)、CPB结束后3 h(T3)、CPB结束后24 h(T4)于桡动脉抽取动脉血1.8 mL置于含有枸橼酸的抗凝管中,立即以3000 r/min离心10 min,取上清液1 mL分别置于2个EP管中,放入-80℃冰箱中以备检测vWF、sICAM-1浓度(上海酶联生物科技有限公司)。由于CPB期间血液稀释程度不断发生变化,为排除其对测定值的影响,两组不同时段的测定结果均由HCT校正,公式为:校正值=(实测值×术前HCT)/实测HCT。
1.5 统计学处理
采用SPSS 13.0软件包进行分析,计量资料采用均数±标准差(±s)表示,采用重复测量方差分析和独立样本t检验进行统计分析,P<0.05为差异有统计学意义。
2 结果
与C组比较,U组在T1~T4各时间点的血浆sICAM-1和vWF的浓度明显下降,差异有统计学意义(P<0.01);与T0比较,C组患儿在T1~T4时的血浆sICAM-1和vWF浓度较基础值显著升高,差异有统计学意义(P<0.01);U组患儿在T1~T2时的血浆sICAM-1和vWF浓度较T0时明显升高,差异有统计学意义(P<0.01),在T3~T4时与T0时比较,差异无统计学意义(P>0.05)。C组患儿血浆sICAM-1和vWF浓度在T0~T4各时间点之间比较,差异均有统计学意义(P<0.01);U组患儿血浆sICAM-1和vWF浓度在T2~T3时间点与T1、T4时间点之间两两比较,差异均有统计学意义(P<0.01或P<0.05),在T0、T3、T4时间点之间比较,差异无统计学意义(P>0.05)。见表2、3。
表2 两组患儿sICAM-1浓度变化(±s,μg/L)
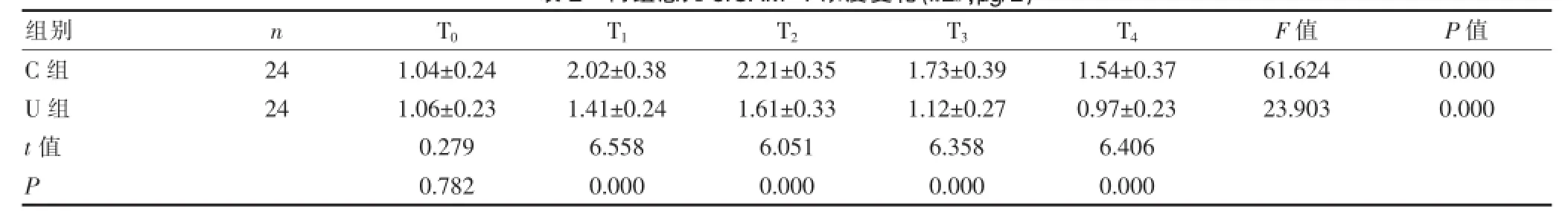
表2 两组患儿sICAM-1浓度变化(±s,μg/L)
组别n T0T1T2T3T4F值P值C组U组t值P 2 4 2 4 1 . 0 4 ± 0 . 2 4 1 . 0 6 ± 0 . 2 3 0 . 2 7 9 0 . 7 8 2 2 . 0 2 ± 0 . 3 8 1 . 4 1 ± 0 . 2 4 6 . 5 5 8 0 . 0 0 0 2 . 2 1 ± 0 . 3 5 1 . 6 1 ± 0 . 3 3 6 . 0 5 1 0 . 0 0 0 1 . 7 3 ± 0 . 3 9 1 . 1 2 ± 0 . 2 7 6 . 3 5 8 0 . 0 0 0 1 . 5 4 ± 0 . 3 7 0 . 9 7 ± 0 . 2 3 6 . 4 0 6 0 . 0 0 0 6 1 . 6 2 4 2 3 . 9 0 3 0 . 0 0 0 0 . 0 0 0
表3 两组患儿vWF浓度变化(±s,U/mL)
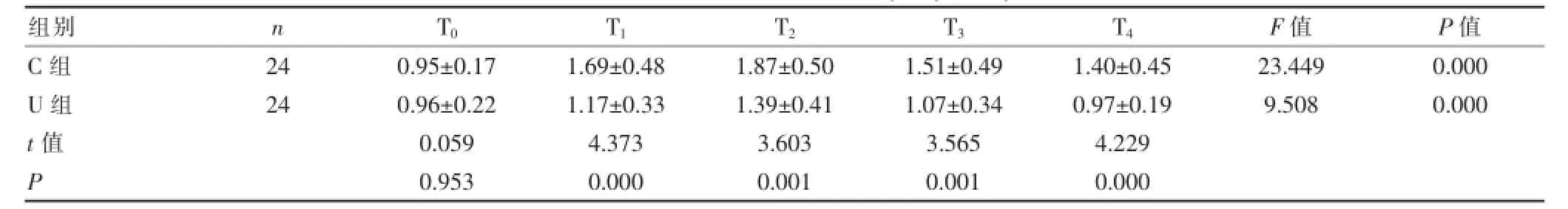
表3 两组患儿vWF浓度变化(±s,U/mL)
组别n T0T1T2T3T4F值P值C组U组t值P 2 4 2 4 0 . 9 5 ± 0 . 1 7 0 . 9 6 ± 0 . 2 2 0 . 0 5 9 0 . 9 5 3 1 . 6 9 ± 0 . 4 8 1 . 1 7 ± 0 . 3 3 4 . 3 7 3 0 . 0 0 0 1 . 8 7 ± 0 . 5 0 1 . 3 9 ± 0 . 4 1 3 . 6 0 3 0 . 0 0 1 1 . 5 1 ± 0 . 4 9 1 . 0 7 ± 0 . 3 4 3 . 5 6 5 0 . 0 0 1 1 . 4 0 ± 0 . 4 5 0 . 9 7 ± 0 . 1 9 4 . 2 2 9 0 . 0 0 0 2 3 . 4 4 9 9 . 5 0 8 0 . 0 0 0 0 . 0 0 0
3 讨论
CPB是常见的引起明显全身炎性反应的外科情况,小儿先天性心脏病心内直视手术大多在CPB下进行。在婴幼儿期,由于效应器官的功能发育尚不够成熟(如肺循环),更容易出现严重肺部并发症[7]。因此怎样能有效的避免和减少由此带来的不良反应,适时应用药物干预,对预防和减轻肺损伤具有重要的临床意义[8,9]。ULI是广谱、高效的多种蛋白酶抑制剂,有抑制细胞炎症介质释放、稳定细胞膜、保护器官功能及促进组织修复等作用[10-12]。最新的研究发现ULI能够抑制多型核粒细胞弹性蛋白酶的活性,对肺功能起保护作用[13,14],但目前关于ULI能否抑制婴幼儿CPB过程中血管内皮细胞的激活和破坏及肺功能有无保护作用尚需深入研究[15,16]。
血管内皮细胞是合成和分泌sICAM-1的主要场所,CPB后,血管内皮细胞受到激活/损伤,大量炎性介质及细胞因子可通过信息传导使血管内皮细胞合成sICAM-1增多,也可由于血管内皮细胞膜损伤,通透性增加,导致sICAM-1释放增加,故sICAM-1浓度的升高可以作为血管内皮细胞损伤或激活的标志[17,18]。在炎症反应过程中,抑制sICAM-1的过度表达可减轻肺损伤[19]。本研究结果表明CPB开始后两组血浆sICAM-1浓度呈进行性升高,在CPB结束时其量达到最大值;CPB结束后3 h和24 h两组均有所下降,但C组sIcAM-1浓度仍较CPB前显著升高,说明CPB可以引起术后细胞黏附分子表达增加,使其血浆浓度升高,导致全身炎症反应加重及术后并发症的发生。U组在CPB开始后30 min和CPB结束时血浆sICAM-1的上升规律虽然与C组相似,但上升幅度较缓慢且停CPB后下降明显,特别是在CPB开始后30 min至CPB结束后24 h各时间点均明显低于对照组,说明给予ULI 10000 U/kg干预后,血浆sICAM-1水平明显降低,显著减轻了中性粒细胞与血管内皮细胞的粘附及减轻血管内皮损伤,表明ULI能部分减少CPB中血管内皮细胞的激活和破坏,对CPB中婴幼儿血管内皮细胞具有保护作用。这与Tanaka R等[20]的研究结果相一致。
vWF是由血管内皮细胞、巨核细胞合成的,其中内皮细胞是血浆vWF的主要来源。研究认为血浆中vWF的增加是内皮损伤的重要标志,反应了内皮细胞损伤的严重程度[21]。本研究结果显示,CPB后,两组血浆vWF含量均呈显著增加,CPB结束时达到高峰,CPB结束后3 h和24 h两组均有所下降,但C组vWF浓度较CPB前显著升高,说明CPB能引起内皮细胞损伤,导致微血栓的形成。U组在CPB开始后30 min和CPB结束时血浆vWF含量的上升规律虽然与C组相似,但上升幅度较缓慢且停CPB后下降明显,自CPB开始后30 min后至CPB结束后24 h各时点血浆vWF含量均明显低于对照组,说明ULI可以部分抑制炎症介质的过度释放,减轻血管内皮细胞损伤,保护微循环功能,从而稳定了内皮细胞膜。
综上所述,ULI通过抑制血浆ICAM-1和vWF过度表达,减轻婴幼儿CPB中血管内皮细胞被激活和破坏,从而起到保护血管内皮细胞的作用。但单用ULI尚不能完全避免CPB中内皮细胞的损害,仍需要进一步联合其他措施加强对血管内皮细胞影响的研究。
[1]Makki M,Scheer I,Hagmann C,et al.Abnormal interhemispheric connectivity in neonates with D-transposition of the great arteries undergoing cardiopulmonary bypass surgery[J]. AJNR Am J Neuroradiol,2013,34(3):634-640.
[2]Davidson SJ,Tillyer ML,Keogh J,et al.Heparin concentra-tionsinneonatesduringcardiopulmonary bypass[J].J Thromb Haemost,2012,10(4):730-732.
[3]He S,Lin K,Ma R,et al.Effect of the urinary tryptin inhibitor ulinastatin on cardiopulmonary bypass-related inflammatory response and clinical outcomes:A meta-analysis of randomized controlled trials[J].Clin Ther,2015,37(3):643-653.
[4]Chen H,He MY,Li YM.Treatment of patients with sevre sepsis using ulinastatin and thymosin α1:A prospective,randomized,controlled pilot study[J].Chinese Medical Journal,2009,122(8):883-888.
[5]Inoue K,Takano H,Yanagisawa R,et al.Protective effects of urinary trypsin inhibitor on systemic inflammatory response induced by lipopolysaccharide[J].Journal of Clinical Biochemistry and Nutrition,2008,43(3):139-142.
[6]Fang Y,Xu P,Gu C,et al.Ulinastatin improves pulmonary function in severe burn-induced acute lung injury by attenuating inflammatory response[J].Journal of Trauma-Injury,Infection and Critical Care,2011,71(5):1297-1304.
[7]Apostolakis E,Filos K,Koletsis E,et al.Lung dysfunction following cardiopulmonary bypass[J].J Card Surg,2010,25(1):47-55.
[8]De Vroege R,Van Oeveren W,Van Klarenbosch J,et al. The impact of heparincoated cardiopulmonary bypass circuits on pulmonary function and the release of inflammatory mediators[J].Anesth Analg,2004,98(6):1586-1594.
[9]Chong EX,Zou CW,Zhang MY,et al.Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A dissection after cardiopul monary bypass under deep hypothermic circulatory arrest[J]. Journal of Cardiothoracic and Vascular Anesthesia,2013,27(3):479-484.
[10]Ito K,Mizutani A,Kira S,et al.Effect of ulinastatin,a human urinary trypsion inhibitor,on the oleic acid-induced acute lung injury in rats via the inhibition of activated leukocytes[J].Injury,2005,36(3):387-394.
[11]Huang N,Wang F,Wang Y,et al.Ulinastatin improves survival of septic mice by suppressing inflammatory re sponse and lymphocyte apoptosis[J].J Surg Res,2013,182(2):296-302.
[12]Jieun Song,Jungmin Park,Jee-Young Kim,et al.Effect of ulinastatin on perioperative organ function and systemic inflammatory reaction during cardiac surgery:A randomized double-blinded study[J].Korean J Anesthesiol,2013,64(4):334-340.
[13]Qiu-Lan He,Fei Zhong,Fang Ye,et al.Does intraoperative ulinastatin improve postoperative clinical outcomes in patients undergoing cardiac surgery:A meta-analysis of randomized controlled trials[J].Biomed Res Int,2014,2014:630835.
[14]Umeadi C,Kandeel F,Al-abdullah H.Ulinastatin is a novel protease inhibitor and neutral procease[J].Transplant proc,2008,40(2):387-389.
[15]Wang X,Xue Q,Yan F,et al.Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass[J].Paediatr Anaesth,2013,23(3):209-216.
[16]Shen J,Gan Z,Zhao J,et al.Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats[J]. Toxicol and Industrial Health,2014,30(9):785-793.
[17]芶大明,魏兵华,张红,等.乌司他丁对体外循环犬血浆可溶性细胞间粘附分子-1、血管性假性血友病因子及MDA水平的影响[J].中华麻醉学杂志,2007,27(1):47-50.
[18]张士兵,周汝元.体外循环炎症反应中粘附分子变化及干预性治疗的研究[J].中华胸心血管外科杂志,2003,19(5):287-289.
[19]Kira S,Daa T,Kashima K,et al.Mild hypothermia reducesexpression of intercellular adhesion molecule-1(ICAM-1)and the accumulation of neutrophils after acidinduced lung injury in the rat[J].Acta Anaesthesiol Scand,2005,49(3):351-359.
[20]Tanaka R,Fujita M,Tsuruta R,et al.Urinary trypsin inhibitor suppresses excessive generation of superoxide anion radical,systemicinflammation,oxidativestress,and endothelial injury in endotoxemic rats[J].Inflamm Res,2010,59(8):597-606.
[21]Meyer NJ,Christie JD.Von willebrand factor and angiopoietin-2:Toward an actue lung injury endothelial endophenotype[J].Crit Care Med,2012,40(6):1966-1967.
Effect of ulinastatin on plasma levels of soluble intercellular adhesion molecule-1,von Willebrand factor during cardiopulmonaey bypass in infants
HU Mingpin1LI Xingwang1WU Guowei2CHEN Xiaoling1LU Wenqing1LI Baoqing3
1.Department of Anesthesiology,the 2nd Affiliated Hospital&Yuying Children’s Hospital of Wenzhou Medical University,Wenzhou325027,China;2.Department of Children Cardiothoracic Surgery,the 2nd Affiliated Hospital& Yuying Children’s Hospital of Wenzhou Medical University,Wenzhou325027,China;3.Department of Clinical Laboratory,the 2nd Affiliated Hospital&Yuying Children’s Hospital of Wenzhou Medical University,Wenzhou325027, China
ObjectiveTo investigate the effect of ulinastatin on plasma levels of soluble intercellular adhesion molecule-1,von Willebrand factor during cardiopulmonaey bypass and the effect of ulinastatin on vascular endothelial cell in CPB.MethodsA total of 48 infants undergoing open-heart surgery under CPB form May 2014 to April 2015 were selected in our hospital.Infants were randomly divided into control group(group C,n=24)and ulinastatin group (group U,n=24)in which infants received ulinastatin 10000 U/kg.Plasma concentrations of sICAM-1 and vWF were measureed before CPB(T0),30 min after the start of CPB(T1),at the end of CPB(T2),3 h(T3)after termination of CPB and 24 h(T4)after termination of CPB.ResultsThe concentrations of sICAM-1 and vWF were significantly increased during CPB as compared with the baseline values at T0in group C,the difference was statically significant(P<0.01).However,the concentrations of sICAM-1 and vWF at T1and T2increased more significantly than that at T0in group U, the difference was statically significant(P<0.01).Compared with control group,these indexes were much lower in group U at T1to T4,the difference was statically significant(P<0.01).ConclusionGiven ULI 10000 U/kg after the intervention,the plasma sICAM-1 and vWF are obviously reduced,the CPB in endothelial cells plays a protective role in infants.
Ulinastatin;Infant;Cardiopulmonary bypass;Cell adhesion molecules;von Willebrand factor
R726.5
A
1673-9701(2015)27-0001-04
2015-06-03)
浙江省省级公益性技术应用研究计划项目(2012 C33113)

