60Co-γ射线辐照对长紫菜的诱变效果及优良品系分离与特性分析
李淑平,严兴洪,2*
(1. 上海海洋大学 水产与生命学院,上海 201306;2. 上海海洋大学 省部共建水产种质资源发掘与利用教育部重点实验室,上海 201306)
60Co-γ射线辐照对长紫菜的诱变效果及优良品系分离与特性分析
李淑平1,严兴洪1,2*
(1. 上海海洋大学 水产与生命学院,上海 201306;2. 上海海洋大学 省部共建水产种质资源发掘与利用教育部重点实验室,上海 201306)
长紫菜野生型品系(PD-WT)的叶状体经60Co-γ射线辐照处理后再培养4周,出现了枣红、浅桔红、黄绿、浅桔黄等颜色的色素变异细胞块,其百分率随着辐照剂量的增加而增加;同时,从基部到梢部,其变异率也呈现上升趋势。利用酶解法获得的单个色素变异细胞经离体培养后再生成叶状体,从再生体中筛选出具有明显生长优势的新品系(PD-5)。培养30~70 d,PD-5品系的叶状体最大绝对生长率和平均绝对生长率分别为3.76 cm/d和2.71 cm/d,分别是PD-WT品系的3.60倍和4.22倍。日龄70 d时,PD-5品系的叶状体平均体长为117.42 cm,是PD-WT品系的4.32倍。日龄45 d的叶状体,PD-5品系的叶绿素a和总藻胆蛋白含量分别为8.41 mg/g和97.07 mg/g,分别比PD-WT品系增加了25.71%和104.44%;PD-5品系的叶状体平均厚度为26.79 μm,比PD-WT品系降低了35.04%。PD-5品系的壳孢子放散总量高达421.16 万个/贝壳,是PD-WT品系的2.19倍。综上所述,与PD-WT品系相比,PD-5品系在叶状体的生长、3种主要光合色素和色素蛋白含量以及壳孢子放散量等方面,均表现出很明显的优势,有望被培养成可大规模栽培的新品种。
长紫菜;叶状体;60Co-γ射线;色素变异;体细胞再生体;优良品系
1 引言
紫菜属(Pyropia)[1]在系统分类学上隶属于红藻门(Rhodophycophyta)、红藻纲(Rhodophyceae)、红毛菜亚纲(Bangiophycidea)、红毛菜目(Bangiales)、红毛菜科(Bangiaceae),是具有重要经济和药用价值的大型藻类,广泛分布于世界各地,部分物种被广泛栽培[2]。在我国被大规模栽培的紫菜有长江以南的坛紫菜和长江以北的条斑紫菜,国内外对这两种紫菜的基础生物学、生活史、人工采苗、养殖技术及遗传育种等的研究均有大量报道[2]。
在自然界,长紫菜(Pyropiadentata)多分布于坛紫菜生长的潮位之上[3—4],现已被小规模试栽的长紫菜为生长慢、成熟早、产量低的野生型品系[5—7],因此,培育优质高产的长紫菜新品种对进一步扩大该紫菜的栽培具有一定的意义。
近十多年来,我国的坛紫菜和条斑紫菜遗传育种工作取得了长足的进步,已培育出了数个分别具有耐高温、耐低盐、耐低氮磷、生长快等不同特性的优良品系(种)[8—15],所采用的育种技术主要有诱变育种和杂交育种。诱变育种是指利用物理和化学等诱变因子,人工诱发生物体产生基因突变,从突变体中筛选出具有生长等优势的生物个体的过程[16],其中物理诱变具有变异率高、处理简单、周期短等优点,在紫菜遗传育种中也被广泛应用[17]。我国藻类学者已利用60Co-γ射线分别对野生型的坛紫菜和条斑紫菜进行人工诱变处理,筛选出了数个被国家认定的坛紫菜和条斑紫菜新品种,并在生产中得到了较大规模的推广[13—14,18]。
本文将首次利用60Co-γ射线对野生型长紫菜进行人工诱变处理,以期获得优质高产的长紫菜优良品系。
2 材料与方法
2.1 实验材料
本实验所用材料为长紫菜野生型品系(PD-WT),于2012年2月,采自广东省汕头市南澳岛,由果孢子萌发长成的自由丝状体被长期保存在实验室内,本文所用的培养液为MES培养基[19]。
2.2 野生型长紫菜叶状体的培养
取少量的长紫菜自由丝状体,用消毒刀片将其切碎,随后被配成丝状体切断的悬浮液,后者被均匀地铺洒于培养皿(Ф=9 cm)内,培养条件:温度(19±1)℃,光照密度20 μmol/(m2·s),光周期10L∶14D,每隔5 d更换一半的新鲜培养液。培养2周后,将光照强度增加至40 μmol/(m2·s),其他培养条件不变。待丝状体铺满培养皿后,将培养条件调整为:温度(23±1)℃,光照密度10 μmol/(m2·s),光周期8L∶16D。数周后,挑选已发育成熟的膨大藻丝,放入培养瓶内(250 mL)进行充气培养,并放入4~6根尼龙单丝供壳孢子附着。待尼龙丝上附着一定量的壳孢子后,将它们转移到新的培养瓶(250 mL)内充气培养,培养条件:温度(19±1)℃,光照密度50 μmol/(m2·s),光周期10L∶14D。当壳孢子萌发体的长度长至1 cm左右,将它们从尼龙单丝上刮下,继续充气培养。培养30 d后,随机取20棵叶状体进行培养,每隔5 d测量一次它们的长度和鲜质量,同时进行拍照记录,并更换一半的培养液。
2.3 叶状体的诱变处理与色素变异体分离
选择健康的长紫菜壳孢子萌发体(体长3~4 cm)作为诱变材料,以60Co-γ射线为诱变源,辐照剂量分设1 000、1 400和1 800 Gy 3组。辐照后的萌发体先在黑暗下培养24 h,再恢复光照进行培养,培养条件:温度(19±1)℃,光照密度50 μmol/(m2·s),光周期10L∶14D。培养3~4周后,从每个辐照组中随机取3棵叶状体,从基部到梢部每隔1 cm统计10个视野(×20)内的色素变异细胞块数;同时,用荧光倒置显微镜(Nikon Eclipse 90i,日本尼康公司)拍照并记录色素变异细胞块的类型。培养一段时间后,将含色素变异细胞块的叶状体酶解,分离出单个变异细胞,进行体外植株再生培养,方法同文献[19]。培养4周后,从中挑选具有明显生长优势的单颜色变异体,进行单株充气培养,培养条件同上。
进一步分析实验组和对照组酒样的质量差别,发现主要表现在放香的纯正度、入口柔和度、酒体协调度、醇厚度、后口干净程度5个方面,对这5个分项的评分进行了系统性分析,结果见图2。
2.4 优良品系的纯系分离与F1叶状体的生长特性分析
当单色变异体成熟时,利用它的单性生殖获得其丝状体[20],即为新品系的遗传纯合丝状体。当新品系的自由丝状体藻落长到一定大小时,将其用家用粉碎机粉碎后接种于文蛤壳表面上,培养成贝壳丝状体,方法同文献[21]。待贝壳表面的丝状体长满后,培养液更换为含氮磷比例为1∶10的灭菌海水,同时,培养条件调整为:温度(29±1)℃,光照密度50 μmol/(m2·s),光周期8L∶16D。当贝壳丝状体成熟后,再将成熟的单个贝壳丝状体置于(19±1)℃下进行充气刺激培养,并放入尼龙单丝供壳孢子附着,让壳孢子萌发成F1叶状体。当叶状体日龄达30 d时,随机取20棵叶状体,每隔5 d测量一次它们的长度和鲜质量,同时拍照记录藻体的形态和更换一半的培养液。叶状体的绝对生长率和相对生长率计算公式同文献[22],分别如下式(1)和(2)所示:
K1=(L-L0)/t,
(1)
K2=(lnN-lnN0)/t,
(2)
式中,L、N代表某一次叶状体长度的测量值,N0、L0则为上一次叶状体长度的测量值,单位是cm,t代表前后两次测量的时间间隔,单位为d。
2.5 F1叶状体的活体吸收光谱和主要色素蛋白含量测定
取培养45 d的F1叶状体,用分光光度计(UV-2600,日本岛津公司)分别测定叶状体在350~750 nm波长下的活体吸收光谱曲线和主要光合色素含量。叶状体在不同波长下的吸光值测定方法同文献[23],根据吸光值用Origin8.5软件再绘制出吸收光谱曲线;叶绿素a含量的测定方法同文献[24],藻红蛋白和藻蓝蛋白含量的测定方法同文献[25]。
2.6 叶状体的厚度测量
2.7 壳孢子放散量统计
将各品系成熟的贝壳丝状体,单个放入含50 mL培养液的一次性塑料杯子中进行充气培养,培养条件:温度(19±1)℃,光照密度50 μmol/(m2·s),光周期10L∶14D。每个品系设置3个平行组。每天中午12点后将杯中的孢子水搅拌均匀后倒入培养皿(Φ=9 cm)中,静置培养24 h待壳孢子附着后,在光学显微镜下统计20个视野(10倍)内的壳孢子数量。当壳孢子开始放散后连续计数20 d以获得单个贝壳的壳孢子放散总量。壳孢子的日放散量计算公式:
壳孢子日放散量=
每个视野内壳孢子平均数,
(3)
式中,π为圆周率,取值为3.14;10×10倍显微镜的视野直径为22 mm。
3 结果
3.1 叶状体诱变效果与色素突变体分离
长紫菜野生型品系的叶状体经不同剂量的60Co-γ射线辐照处理后,再培养2周,在显微镜下可观察到叶状体上呈点状分布的各类色素变异细胞,它们呈不规则状镶嵌于正常体细胞之间。培养2~3周后,色素变异细胞逐渐分裂成大小不一的色素变异细胞块(图版Ⅰ)。
如图版Ⅰ所示,野生型细胞与色素变异细胞块间有明显的界限,变异细胞块多为枣红、砖红、浅红紫、紫红、红褐、浅桔红、浅桔黄等颜色,少数为草绿、黄绿和灰褐色。如表1所示,对照组的叶状体上未观察到色素变异细胞块,而辐照组的色素变异细胞块数随着辐照剂量的增加而逐渐增加。如表2所示,同一个叶状体中不同部位的色素变异细胞块出现的频率是不同的,从基部到梢部,其变异频率逐渐增加。
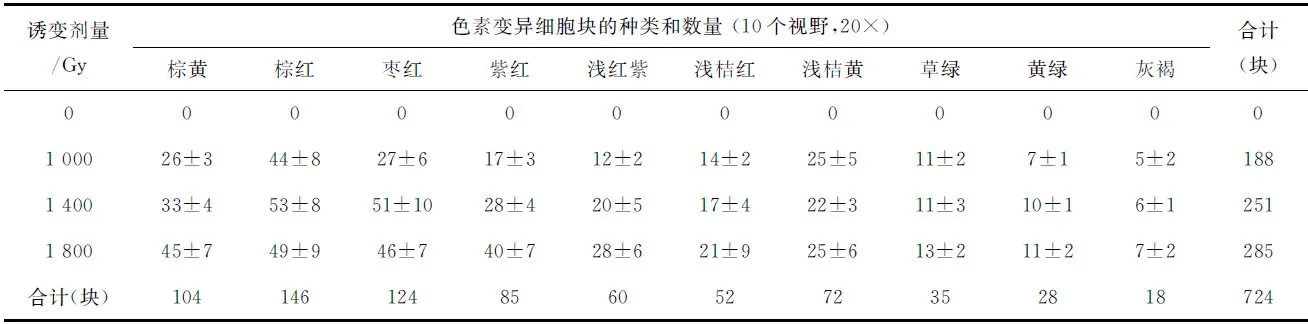
表1 长紫菜野生型品系(PD-WT)的叶状体经不同剂量的60Co-γ射线辐照后再培养25 d时出现的色素变异细胞块的种类和数量Tab.1 The types and numbers of the color-mutated cell-clusters appeared in the blades of the wild-type strain (PD-WT) in Pyropia dentata after being irradiated by 60Co-γ ray in different doses and cultured for 25 days
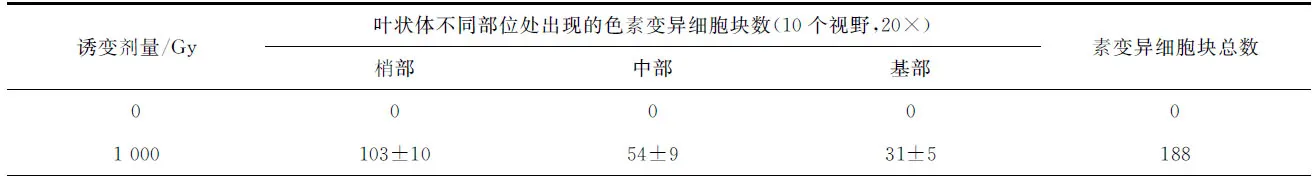
表2 经不同剂量的60Co-γ射线辐照处理的长紫菜野生型品系(PD-WT)叶状体再培养25 d后在不同部位处出现的色素变异细胞块数量Tab.2 Numbers of color-mutated cell-clusters appeared at different parts of Pyropia dentata blades of the wide-type strain(PD-WT) after being irradiated by 60Co-γ ray in different doses and cultured for 25 days
含色素变异细胞的叶状体被酶解后获得了大量的体细胞,后者经30多天的再生培养长成了叶状体,从中分离出单色的色素变异体,待它们长大后,再筛选出一颗生长优势最明显的变异体,它的颜色呈褐红色,基部偏绿,梢部偏红,生长很快。当它成熟时,发生单性生殖产生了纯合丝状体,被命名为PD-5品系。
3.2 选育品系F1叶状体的生长特性分析
PD-5品系的壳孢子萌发体的藻体颜色非常一致,藻体基部偏绿,而梢部偏红,与最初的母体一致,其性别全为雌性。PD-5品系的藻体为细长型,较PD-WT品系稍宽(图版Ⅱ)。
如图1所示,相同日龄的F1叶状体,PD-5品系的平均体长远大于PD-WT品系。日龄30 d的叶状体,PD-5品系的平均体长达8.95 cm,是PD-WT品系的5.93倍。日龄60 d后,PD-WT品系的叶状体就进入了缓慢生长期,而PD-5品系仍处于快速生长期。日龄70 d的叶状体,PD-5品系的平均体长已达117.42 cm,是PD-WT品系的4.32倍。由表3可知,PD-5品系的叶状体绝对生长率在日龄45~50 d时出现最大值,为3.76 cm/d,而PD-WT品系的最大绝对生长率却出现在35~40 d,仅为
1.045 cm/d。培养的第30~45 d期间,PD-5品系的叶状体特定生长率没有比PD-WT品系显示出优势,但是,培养45 d之后,PD-5品系的相对生长率就逐渐超过PD-WT品系,尤其是在60 d之后两者的差异就更加明显。
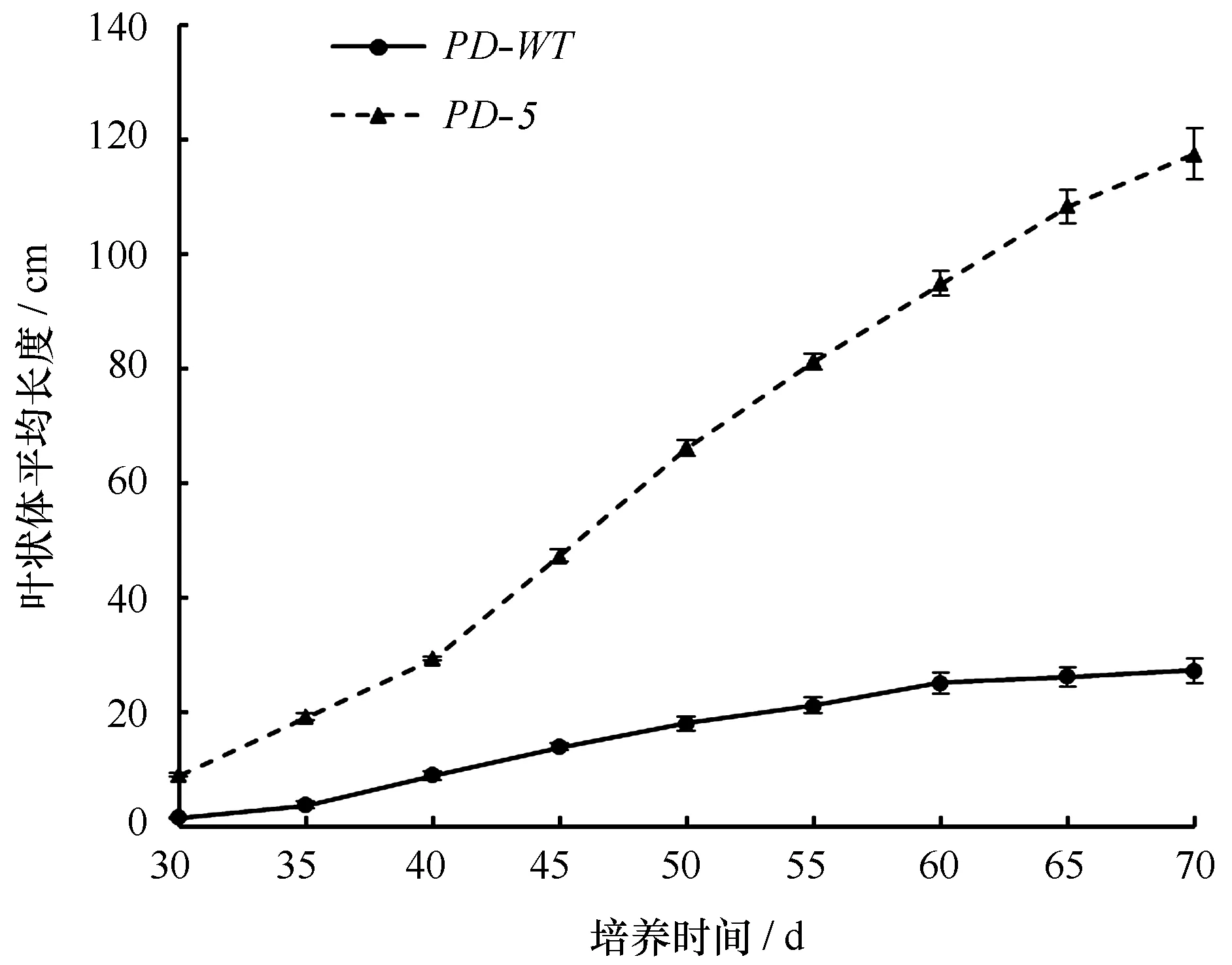
图1 长紫菜选育品系(PD-5)与野生型品系(PD-WT)F1叶状体的生长曲线Fig.1 The growth curves of F1 gametophytic blade of the improved strain (PD-5) and the wide-type strain (PD-WT) in Pyropia dentata
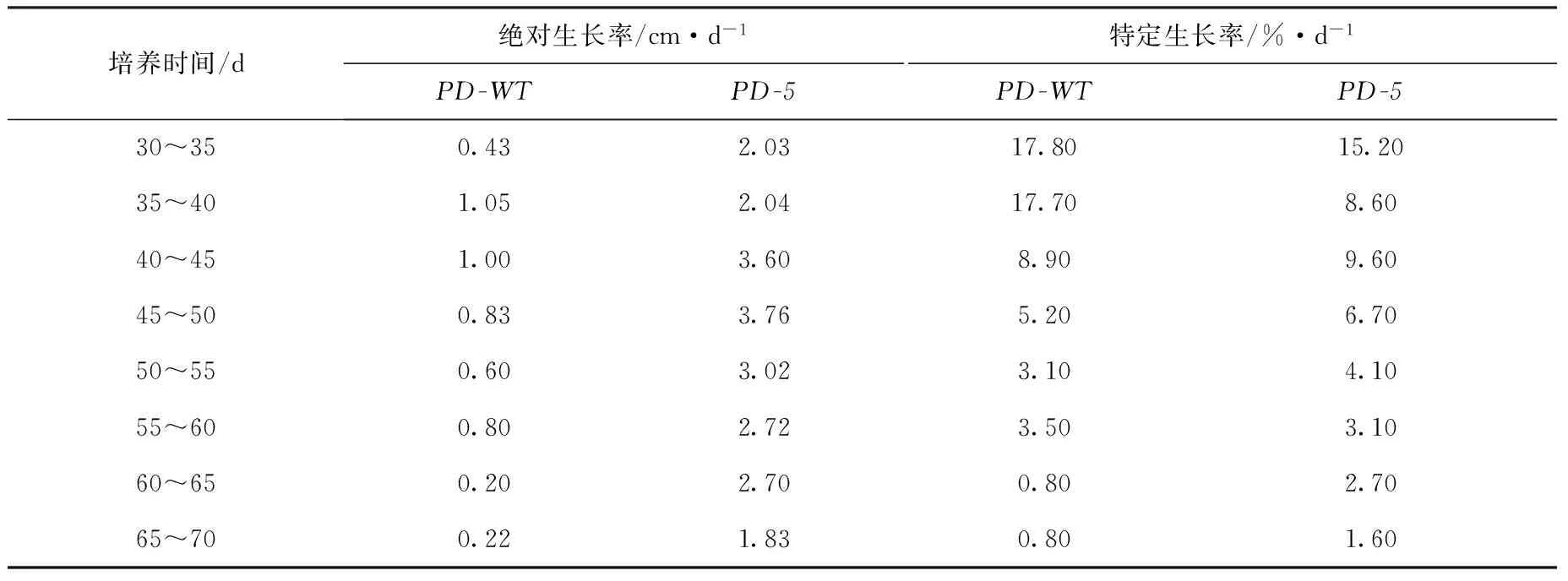
表3 长紫菜选育品系(PD-5)与野生型品系(PD-WT)F1叶状体的生长率Tab.3 Growth rates of the F1 gametophytic blades of the improved strain (PD-5) and the wide-type strain (PD-WT) in Pyropia dentata
由图2可知,在生长前期(30 d之前),PD-5品系的叶状体平均鲜质量并未表现出明显生长优势;35 d后,PD-5品系的叶状体平均鲜质量进入快速生长期,而PD-WT品系仍处于缓慢增长期。日龄70 d时,PD-5品系的单棵叶状体平均鲜质量为1.98 g,是PD-WT品系的9.81倍。
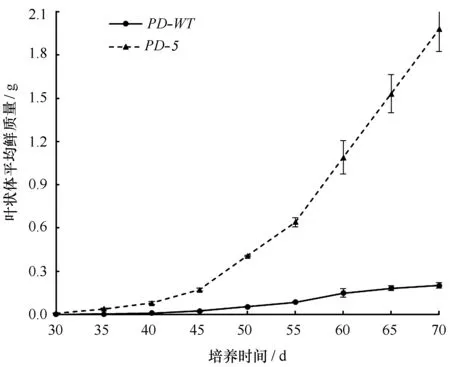
图2 长紫菜选育品系(PD-5)与野生型(PD-WT)F1叶状体的平均单棵鲜质量Fig.2 The fresh weight of per F1 gametophytic blades of the improved strain (PD-5) and the wide-type strain (PD-WT) in Pyropia dentata
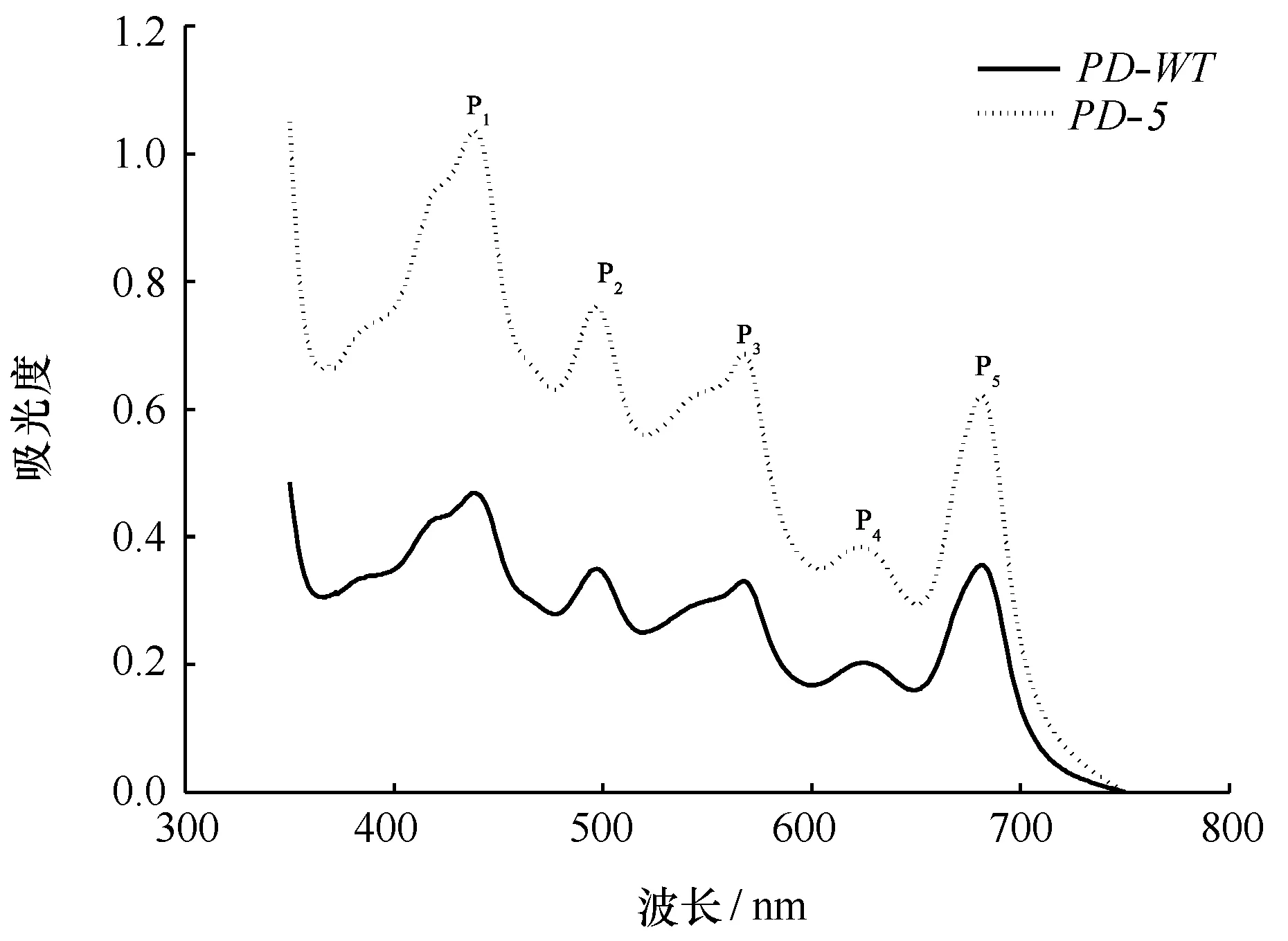
图3 日龄45 d的长紫菜选育品系(PD-5)和野生型品系(PD-WT)F1叶状体的活体吸收光谱曲线Fig.3 In vivo absorption spectra of F1 gametophytic blades of the improved strain (PD-5) and the wide-type strain (PD-WT) after being cultured for 45 days in Pyr-opia dentata
3.3 选育品系及野生型品系F1叶状体的活体吸收光谱特性和主要色素蛋白含量
在350~750 nm之间,D-5与PD-WT品系的叶状体活体吸收光谱曲线均出现5个明显的吸收峰,分别被标为P1、P2、P3、P4和P5,但PD-5品系的各吸收峰的峰值均显著高于PD-WT品系(图3)。
由表4可知,日龄45 d的PD-5品系叶状体的叶绿素a(Chla)含量为8.41 mg/g,比PD-WT品系增加了25.71%;PD-5品系的藻红蛋白(PE)和藻蓝蛋白(PC)含量分别是PD-WT品系的2.87倍和1.29倍,其总藻胆蛋白(PE+PC)含量比PD-WT品系增加了104.44%。
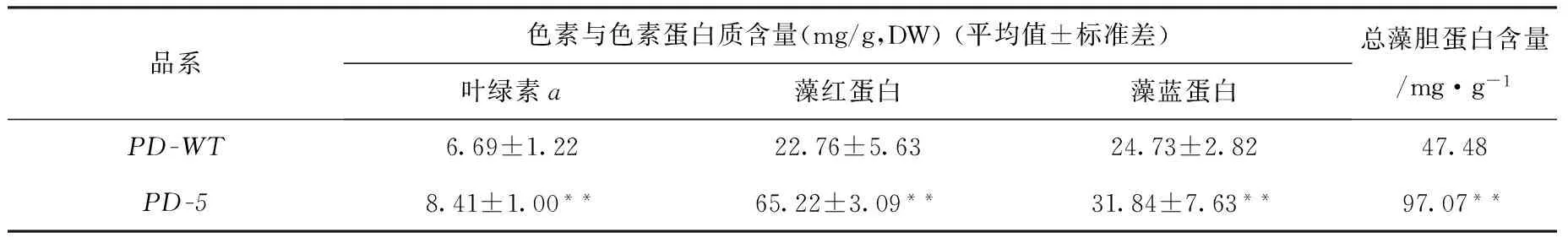
表4 日龄为45 d的长紫菜选育品系(PD-5)和野生型品系(PD-WT)F1叶状体的Chl a、PE、PC以及总藻胆蛋白(PE+PC)的含量(n=3)Tab.4 Contents of Chl a, PE, PC, Phycobiliprotein in F1 gametophytic blades of the improved strain (PD-5) and the wide-type strain(PD-WT) after being cultured for 45 days in Pyropia dentata (n=3)
注:**表示差异极其显著,(P<0.01,t-test),* 表示差异显著(P<0.05,t-test),下同。
3.4 F1叶状体的厚度
日龄45 d的叶状体得横切面观察发现,从叶状体的梢部到基部,其藻体厚度逐渐增加。PD-5品系叶状体的平均厚度为26.79 μm,比野生型品系薄了35.04%,独立样本T检验显示其差异极显著(见表5)。
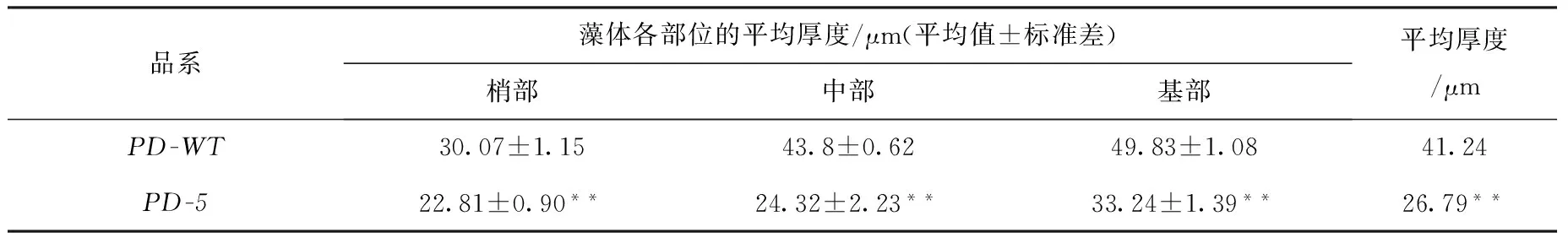
表5 日龄为45 d的长紫菜选育品系(PD-5)和野生型品系(PD-WT)的F1叶状体厚度(n=3)Fig.5 Thickness of different parts of F1 gametophytic blades of the improved strain (PD-5) and the wide-type strain (PD-WT) after being cultured for 45 days in Pyropia dentata (n=3)
3.5 选育品系的壳孢子放散量
自壳孢子放散开始连续统计20 d的壳孢子放散量后发现,在20 d 中,PD-5品系出现了3个大的放散高峰,放散量分别为86.70万、46.28万、65.16 万个/贝壳,均出现在前7天内;而PD-WT品系在前7天仅出现了一个大的放散高峰,放散量仅为56.69 万个/贝壳(图4)。由表6知,PD-5品系连续20 d的壳孢子放散总量为421.16 万个/贝壳,是野生型品系的2.19倍。

图4 长紫菜选育品系(PD-5)和野生型品系(PD-WT)在19℃下连续放散20 d的壳孢子日放散量Fig.4 Numbers of conchospores daily resealed from the improved strains (PD-5) and the wide-type strain (PD-WT) at 19oC during 20 days in Pyropia dentata
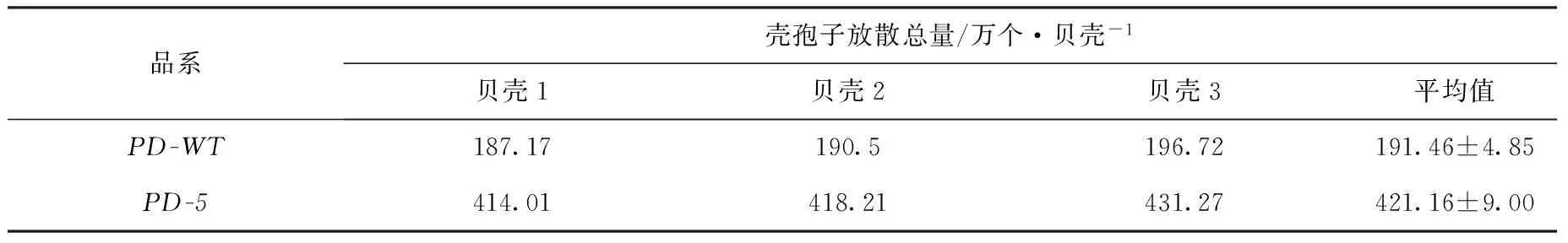
表6 长紫菜选育品系(PD-5)和野生型品系(PD-WT)在19℃下连续放散20 d的壳孢子放散总量Tab.6 Total numbers of conchospores released from the improved strains (PD-5) and the wide-type strain (PD-WT) at 19℃ during 20 days of conchospore-releasing in Pyropia dentata
4 讨论
目前,国内外在紫菜人工诱变育种的研究中,使用较多的诱变剂是化学诱变剂MNNG[26—30]和物理诱变剂γ-射线[24,31—35]。γ-射线的作用机理是破坏细胞内DNA或mRNA分子碱基对间的化学键,使碱基发生转换或置换,从而达到变异效果[31],它以使用方便,干净,穿透力强,诱发突变率高,周期短等优点而广泛使用[31—35]。我国藻类学者利用60Co-γ射线通过辐照处理坛紫菜和条斑紫菜的不同生长阶段(丝状体、叶状体、原生质体),均获得了良好的变异效果,已分离出了具有生长优势的色素突变体[31—33,35]。
本试验选用60Co-γ射线辐照处理长紫菜叶状体,也获得了良好的变异效果。在60Co-γ射线辐照剂量方面,条斑紫菜的最适剂量为500 Gy[34],坛紫菜的为1 100 Gy[35],但本实验的结果表明,1 800 Gy组出现的变异细胞块最多。在变异细胞块的种类上,60Co-γ射线对野生型坛紫菜、条斑紫菜及长紫菜的变异效果差异不大,均是以红色型的变异细胞块偏多。
在生产中,衡量一种紫菜栽培品系的优劣主要取决于其叶状体的生长、主要色素和色素蛋白含量以及壳孢子放散量等。与长紫菜野生型品系相比,PD-5品系具有生长快、成熟晚和藻体薄等优势。另外,3种主要光合色素和色素蛋白含量的高低对商品紫菜饼的质量好坏起决定作用[36],因此,主要光合色素较高的PD-5品系比PD-WT的品质更好。生产上,进行壳孢子附网采苗时,放散量的大小和是否集中放散将直接影响采苗效果的好坏。与PD-WT品系相比,PD-5品系不仅总放散量增加了二倍,而且放散量集中,3个主要放散高峰均出现在头7 d,如果在生产中应用此品系,它能更早更多地采足壳孢子,早日下海栽培。由此可见,本文分离出来的PD-5品系有望被培育成具有优质高产且适应大规模生产应用的长紫菜新品种。
[1] Sutherland J E, Lindstrom S C, Nelson W A, et al. A new look at an ancient order: generic revision of the Bangiales (Rhodophyta) [J]. Journal of Phycology, 2011, 47(5): 1131-1151.
[2] 张学成, 秦松, 马家海, 等. 海藻遗传学[M]. 北京: 中国农业出版社, 2005: 190-192.
Zhang Xuecheng, Qin Song, Ma Jiahai, et al. The Genetics of Marine Algae[M]. Beijing: China Agriculture Press, 2005: 190-192.
[3] 曾呈奎, 张峻甫. 我国的紫菜与紫菜养殖[J]. 生物学通报, 1956(3): 29-33.
Zeng Chengkui, Zhang Junfu. The ChinesePorphyraand seaweed cultivation[J]. Biological Bulletin, 1956(3): 29-33.
[4] 张德瑞, 郑宝福. 中国的紫菜及其地理分布[J]. 海洋与湖沼, 1962, 4(3/4): 183-188.
Zhang Derui, Zheng Baofu. The ChinesePorphyraand their geographical distribution[J]. Oceanologia et Limnologia Sinica, 1962, 4(3/4): 183-188.
[5] King N G. Culture studies ofPorphyradentataandP.pseudolinearis(Bangiales, Rhodophyta), two dioecious species from Korea[J]. Hydrobiologia, 1999(398/399): 127-135.
[6] Notoya M, Kikuchi N, Matsuo M, et al. Culture studies of four species ofPorphyra(Rhodophyta) from Japan[J]. Nippon Suisan Gakkaishi, 1993, 59(3): 431-436.
[7] 许俊斌, 陈伟洲, 宋志民, 等. 不同培养条件对长紫菜叶状体生长及生理响应的研究[J]. 水产学报, 2013, 37(9): 1319-1327.
Xu Junbin, Chen Weizhou, Song Zhimin, et al. Effects of different culture conditions on growth and physiological response ofPorphyradentatathallus[J]. Journal of Fisheris of China, 2013, 37(9): 1319-1327.
[8] 严兴洪, 马少玉. 坛紫菜抗高温品系的筛选[J]. 水产学报, 2007, 31(1): 112-119.
Yan Xinghong, Ma Shaoyu. Selection of a high-temperature resistant strain ofPorphyrahaitanensis[J]. Journal of Fisheris of China, 2007, 31(1): 112-119.
[9] 陈昌生, 纪德华, 谢潮添, 等. 坛紫菜耐高温品系选育及经济性状的初步研究[J]. 海洋学报, 2008, 30(5): 100-106.
Chen Changsheng, Ji Dehua, Xie Chaotian, et al. Preliminary study on selecting the high temperature resistance strains and economic traits ofPorphyrahaitanensis[J]. Haiyang Xuebao, 2008, 30(5): 100-106.
[10] 吕峰, 严兴洪, 刘长军, 等. 坛紫菜耐高温品系的选育与海区中试[J]. 上海海洋大学学报, 2010, 19(4): 457-462.
Lv Feng, Yan Xinghong, Liu Changjun, et al. Selection of a high-temperature tolerant strain ofPorphyrahaitanensisand its cultivation in sea area[J]. Journal of Shanghai Ocean University, 2010, 19(4): 457-462.
[11] 严兴洪, 陈敏. 坛紫菜耐低盐优良品系的筛选[J]. 上海海洋大学学报, 2008, 17(3): 316-320.
Yan Xinghong, Chen Min. Selection of low-salinity resistant improved varieties inPorphyrahaitanensis(Bangiales, Rhodophyta)[J]. Journal of Shanghai Ocean University, 2008, 17(3): 316-320.
[12] 柳佩娟, 纪德华, 谢潮添, 等. 坛紫菜耐低氮磷品系选育的研究[J]. 集美大学学报(自然科学版), 2009, 14(2): 15-20.
Liu Peijuan, Ji Dehua, Xie Chaotian, et al. Study on selection of low N and P resistant strains inPorphyrahaitanensis[J]. Journal of Jimei University (Natural Science), 2009, 14(2): 15-20.
[13] 宋武林. 坛紫菜优良品系“申福1号”苗种培育技术研究[J]. 南方水产, 2006, 2(4): 19-23.
Song Wulin. A research on the technique of conchosporelings about the premium strain of “Shenfu No. 1”inPorphyrahaitanensis[J]. South China Fisheries Science, 2006, 2(4): 19-23.
[14] 王长青, 严兴洪, 黄林彬, 等. 坛紫菜优良品系“申福2号”的特性分析与海区中试[J]. 水产学报, 2011, 35(11): 1658-1667.
Wang Changqing, Yan Xinghong, Huang Linbin, et al. Characterization of an improved strain (SF-2) ofPorphyrahaitanensis(Bangiales, Rhodophyta) and its pilot cultivation in mariculture farm[J]. Journal of Fisheries of China, 2011, 35(11): 1658-1667.
[15] 吴宏肖, 严兴洪, 宋武林, 等. 坛紫菜与Pyropiaradi种间杂交重组优良品系的选育与特性分析[J]. 水产学报, 2014, 38(8): 1079-1088.
Wu Hongxiao, Yan Xinghong, Song Wulin, et al. Selection and characterization of an improved strain produced by genetic recombinant of interspecific hybridization betweenPyropiahaitanensisandPyropiaradi[J]. Journal of Fisheries of China, 2014, 38(8): 1079-1088.
[16] 何培民, 秦松, 严晓军, 等. 海藻生物技术及其应用[M]. 北京: 化学工业出版社, 2007: 86-98.
He Peimin, Qin Song, Yan Xiaojun, et al. Seaweed Biological Technology and its Application[M]. Beijing: Chemical Industry Press, 2007: 86-98.
[17] 梁红. 植物遗传与育种[M]. 广州: 广东高等教育出版社, 2002: 197-206.
Liang Hong. Plant Genetics and Breeding[M]. Guangzhou: Guangdong Higher Education Press, 2002: 197-206.
[18] 全国水产技术推广总站. 2014水产新品种推广指南[M]. 北京: 中国农业出版社, 2014: 146-164.
National Fisheries Extension Center. 2014 Promotion of New Varieties of Aquatic Guide [M]. Beijing: Chinese Agriculture Press, 2014: 165-181.
[19] 王素娟, 张小平, 徐志东, 等. 坛紫菜营养细胞和原生质体培养的研究Ⅰ[J]. 海洋与湖沼, 1986, 17(3): 217-221.
Wang Sujuan, Zhang Xiaoping, Xu Zhidong, et al. A study on the cultivation of the vegetative cells and protoplasts ofP.haitanensisⅠ[J]. Oceanologia et Limnologia Sinica, 1986, 17(3): 217-221.
[20] 严兴洪, 李琳, 陈俊华, 等. 坛紫菜的单性生殖与遗传纯系分离[J]. 高技术通讯, 2007, 17(2): 205-210.
Yan Xinghong, Li Lin, Chen Junhua, et al. Parthenogenesis and isolation of genetic pure strains inPorphyrahaitanensis(Bangiales, Rhodophyta)[J]. Chinese High Technology Letters, 2007, 17(2): 205-210.
[21] 严兴洪, 梁志强, 宋武林, 等. 坛紫菜人工色素突变体的诱变与分离[J]. 水产学报, 2005, 29(2): 166-172.
Yan Xinghong, Liang Zhiqiang, Song Wulin, et al. Induction and isolation of artificial pigmentation mutants inPorphyrahaitanensisChang et Zheng (Bangiales, Rhodophyta)[J]. Journal of Fisheries of China, 2005, 29(2): 166-172.
[22] Stein J R. Handbook of Phycological Methods: Culture Methods and Growth Measurements[M]. London: Cambridge University Press, 1973: 289-311.
[23] Aruga Y, Miura A.Invivoabsorption spectra and pigment contents of the types of color mutants ofPorphyrapurpurea(Rhodophyta)[J]. Japanese Journal of Phycology, 1984, 32(3): 243-250.
[24] Kato M, Aruga Y. Comparative studies on the growth and photosynthesis of the pigmentation mutants ofPorphyrayezoensisin laboratory culture[J]. Japanese Journal of Phycology, 1984, 32: 333-347.
[25] 高洪峰. 不同生长期坛紫菜中藻胆蛋白的含量变化[J]. 海洋与湖沼, 1993, 24(6): 645-648.
Gao Hongfeng. The variation in the contents of phycobiliproteins fromPorphyrahaitanensiscollected in different growing stages[J]. Oceanologia et Limnologia Sinica, 1993, 24(6): 645-648.
[26] Yan X H, Aruga Y. Induction of pigmentation mutants by treatment of monospore germlings with NNG inPorphyrayezoensisUeda (Bangiales, Rhodophyta)[J]. Algae, 1997, 12(1): 39-54.
[27] 严兴洪, 田中次郎, 有贺佑胜. 条斑紫菜色彩突变体的诱导, 分离和特性分析[J]. 水产学报, 2000, 21(3): 221-228.
Yan Xinghong, Tanaka Jiro, Aruga Yusho. Isolation and characterization of pigmentation mutants inPorphyrayezoensisUeda (Bangiales, Rhodophyta)[J]. Journal of Fisheries of China, 2000, 21(3): 221-228.
[28] Yan X H. Studies on color type variants from mutagenized protoplasts ofPorphyrahaitanensisChang et Zheng &P.yezoensisUeda (rhodophycease)[J]. Chinese Journal of Oceanology and Limnology, 1993, 11(3): 235-244.
[29] Yan X H, Aruga Y. Genetic analysis of artificial pigmentation mutants inPorphyrayezoensisUeda (Bangiales, Rhodophyta) [J]. Phycological Research, 2000, 48(3): 177-187.
[30] 李永斌, 左正宏, 李博文, 等. MNNG(N-甲基-N’-硝基-N-亚硝基胍)诱变坛紫菜原生质体的初步研究[J]. 厦门大学学报(自然科学版), 2006, 45(3): 400-403.
Li Yongbin, Zuo Zhenghong, Li Bowen, et al. Studies on protoplasts morphological change and growth ofPorphyrahaitanensistreated with mutagen MNNG[J]. Journal of Xiamen University (Natural Science), 2006, 45(3): 400-403.
[31] 匡梅, 许璞, 王素娟. γ-射线对条斑紫菜和坛紫菜诱变作用的初步研究[J]. 上海水产大学学报, 1997, 6(4): 241-245.
Kuang Mei, Xu Pu, Wang Sujuan. A preliminary study of the mutagenesis of γ-rays onPorphyrayezoensisandP.haitanensis[J]. Journal of Shanghai Fishries University, 1997, 6(4): 241-245.
[32] 王素娟, 马凌波, 许璞, 等.60Co-γ射线诱变条斑紫菜丝状体的研究[J]. 海洋科学, 1999(4): 43-46.
Wang Sujuan, Ma Lingbo, Xu Pu, et al. Study on using gamma-ray to induce mutation in conchocelis ofPorphyrayezoensis[J]. Marine Sciences, 1999(4): 43-46.
[33] 梁志强. 坛紫菜遗传育种的初步研究[D]. 上海: 上海水产大学, 2004: 10-22.
Liang Zhiqiang. Primary study on genetics and breeding ofPorphyrahaitanenis[D]. Shanghai: Shanghai Fisheries University, 2004: 10-22.
[34] 纪德华, 陈昌生, 郑伟刚, 等.60Co-γ射线辐照坛紫菜叶状体及单克隆培养的研究[J]. 台湾海峡, 2005, 24(2): 171-177.
Ji Dehua, Chen Changsheng, Zheng Weigang, et al. Study of60Co-γ irradiation and monoclone culture in thallus ofPorphyrahaitanensis[J]. Journal of Oceanography in Taiwan Strait, 2005, 24(2): 171-177.
[35] 严兴洪, 张淑娟, 黄林彬.60Co-γ射线对条斑紫菜 (Porphyrayezoensis) 的诱变效果与色素突变体分离[J]. 海洋与湖沼, 2009, 40(1): 56-61.
Yan Xinghong, Zhang Shujuan, Huang Linbin. Induction and isolation of pigmentation mutants ofPorphyrayezoensisUeda (Bangiales, Rhodophyta) by60Co-γ ray irradiation[J]. Oceanologia et Limnologia Sinica, 2009, 40(1): 56-61.
[36] 纪德华, 谢潮添, 周修史, 等. 福建沿海野生坛紫菜主要品质性状分析[J]. 集美大学学报(自然科学版), 2011, 16(6): 401-406.
Ji Dehua, Xie Chaotian, Zhou Xiushi, et al. Analysis of the major quality traits in the wildPorphyrahaitanensisof Fujian coast[J]. Journal of Jimei University (Natural Science), 2011, 16(6): 401-406.
Isolation and characterization of an improved strain of Pyropia dentata (Bangiales, Rhodophyta) after being irradiated by60Co-γ ray
Li Shuping1, Yan Xinghong1,2
(1.CollegeofFisheriesandLifeSciences,ShanghaiOceanUniversity,Shanghai201306,China; 2.KeyLaboratoryofExplorationandUtilizationofAquaticGeneticResources,ShanghaiOceanUniversity,MinistryofEducation,Shanghai201306,China)
The young gametophytic blades ofPyropiadentata, developed from conchospores of the wild-type strain (PD-WT), were treated with60Co-γ ray to induce mutation in the experiment. After being cultured for 4 weeks, there were many color-mutated cell clusters showing bright red, red brown, red orange and yellow green in the treated blades. The results showed that the percentage of the colored-mutated cell-clusters increased as increasing of the irradiation dose and from the base to the tip of the blades. Single color-mutated cells were isolated enzymatically from the color-mutated blades and were regenerated into blades. An improved strain named asPD-5 strain with the growth advantages was selected from the regenerated blades. In the growth of the blades, the maximum and average absolute growth rates of this improved strain were 3.76 cm/d and 2.71 cm/d, which were 3.60 and 4.22 times that of thePD-WTstrain, respectively, during culture from 30 to 70 days. The mean length of F1gametophytic blades ofPD-5 strain was 117.42 cm which was 4.32 times that of thePD-WTstrain after being cultured for 70 days. The contents of Chlaand phycobiliprotein of thePD-5 strain were 8.41 mg/g and 97.07 mg/g, respectively, increasing by 25.71% and 104.44% in contrast with that of thePD-WT,respectively. The mean thickness of the 45-day-old blades of thePD-5 was 26.79 μm,decreasing by 35.04% in contrast with that of thePD-WT. The total numbers of the conchospores released from thePD-5 strain was 421.16×104per shell,which was 2.19 times that of thePD-WTstrain. The aboved results confirmed that thePD-5 strain was characterized by faster growth,higher contents of photosynthetic pigments,larger amount of releasing conchospores than the wild-type strain. Therefore,the improved strainPD-5 had great potential to be applied in commercial cultivation as a new strain.
Pyropiadentata; blades;60Co-γray; pigmentation mutation; regenerated blades; improved strain
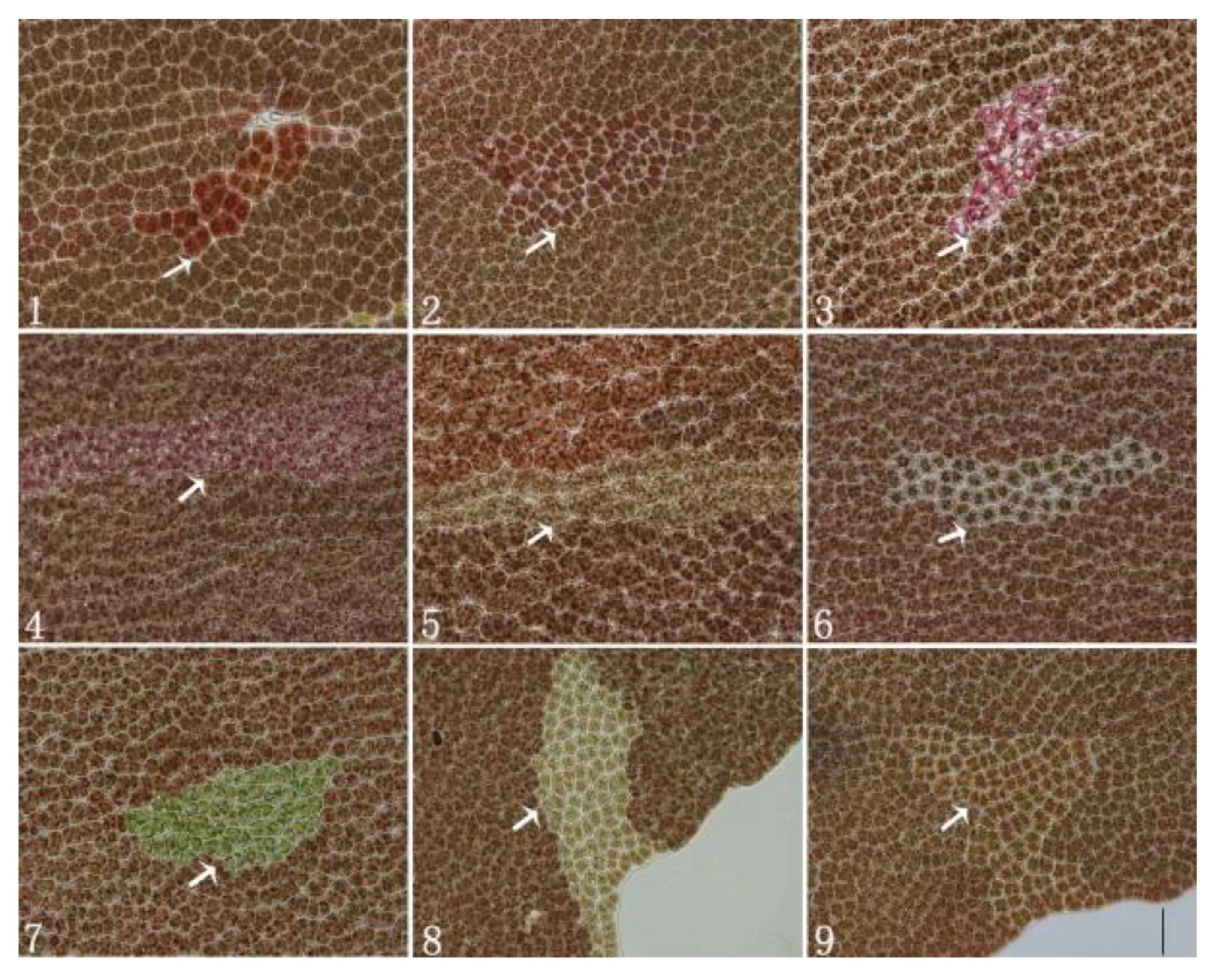
图版Ⅰ 经60Co-γ射线辐照后在长紫菜野生型品系(PD-WT)叶状体上形成的不同颜色变异色块显微照片PlateⅠ Micrographs of the color-mutated cell-clusters appeared in the gametophytic blades of the wild-type strain (PD-WT) in Pyropia dentata after being irradiated with 60Co-γ ray1~9分别为枣红色、砖红色、紫红色、暗红紫色、黄褐色、灰褐色、草绿色、浅桔黄色和浅桔红色的色素变异细胞块。箭头所指处为上述命名的色素变异细胞块 (图中标尺为50 μm)1-9. Reddish orange,bright red,red purple,light purple red,yellow brown,grey brown,bright green,yellow orange and light red orange color-mutated cell-clusters (arrowheads),respectively (Bar=50 μm)
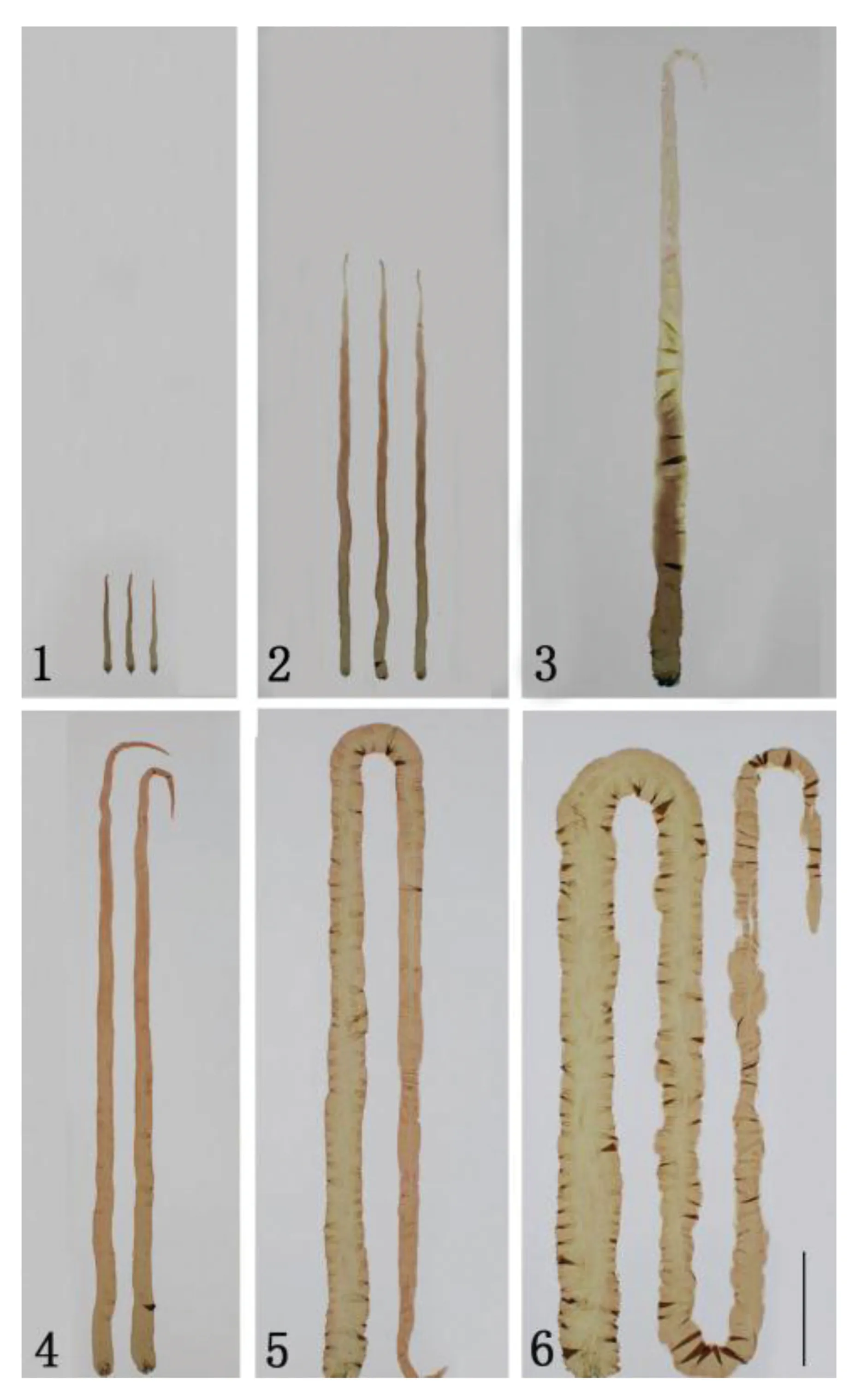
图版Ⅱ 长紫菜选育品系(PD-5)和野生型品系(PD-WT)的F1叶状体生长比较Plate Ⅱ Comparison of the growth of F1 gametophytic blades of the improved strain (PD-5) and the wide-type (PD-WT) strain in Pyropia dentate 1~3.分别培养35、45和55d的长紫菜野生型品系(PD-WT)的F1叶状体;4~6. 分别培养35、45、55d的长紫菜选育品系(PD-5)的F1叶状体(图中标尺为5 cm)1-3. The F1 gametophytic blades of the PD-WT strain,after being cultured for 35,45 and 55 days,respectively; 4-6. The F1 gametophytic blades of the PD-5 strain,after being cultured for 35,45 and 55 days,respectively(Bar=5 cm)
2015-04-29;
2015-06-23。
国家高科技研究发展计划(863计划)资助项目(2012AA10A411);国家自然科学基金资助项目(31072208);农业部公益性专项(200903030);国家农业科技成果转化资金项目(2013GB2C220537); 上海市科委重点科技攻关项目(10391901100);国家海洋局公益专项(201105008, 201105023);上海高校水产学一流学科建设项目资助;福建省省长专项基金(2014S1477-10)。
李淑平(1989—), 女, 山东省滨州市人,从事海藻遗传育种研究。E-mail:lishuping29@126.com
*通信作者:严兴洪, 教授, 博士生导师, 主要从事海藻遗传育种, 海藻生理生态与分子生物学。E-mail:xhyan@shou.edu.cn
10.3969/j.issn.0253-4193.2015.10.007
S917.3
A
0253-4193(2015)10-0069-11
李淑平,严兴洪.60Co-γ射线辐照对长紫菜的诱变效果及优良品系分离与特性分析[J].海洋学报,2015,37(10):69—79,
Li Shuping, Yan Xinghong. Isolation and characterization of an improved strain ofPyropiadentata(Bangiales, Rhodophyta) after being irradiated by60Co-γ ray[J]. Haiyang Xuebao,2015,37(10):69—79, doi:10.3969/j.issn.0253-4193.2015.10.007

