Water sources of plants and groundwater in typical ecosystems in the lower reaches of the Heihe River Basin
YunFeng Ruan , LiangJu Zhao , HongLang Xiao , GuoDong Cheng ,MaoXian Zhou , Fang Wang
1. Key Laboratory of Ecohydrology and Integrated River Basin Science of CAS, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
2. Key Laboratory of Heihe Ecohydrology and Basin Science of Gansu Province, Lanzhou, Gansu 730000, China
3. University of Chinese Academy of Sciences, Beijing 100049, China
1 Introduction
In terrestrial ecosystems, the potential water sources of plants include precipitation (snow in winter), soil water (both shallow and deep), river water,and groundwater. These different water bodies have different hydrogen and oxygen isotopic compositions(δD, δ18O). Previous studies proved that plant roots do not discriminate against specific water isotopes during water uptake, and there is no fractionation during wa-ter transfer in xylem (Dawsonet al., 1998; Alessioet al., 2004). Therefore, the isotopic composition of root water can determine the potential plant water sources.By comparing δD and δ18O of plant root water and their potential water sources, we can understand the potential water sources (such as precipitation, river,groundwater, and shallow and deep soil water) of plants (Gat, 1996) and their respective contributions to plants (Ehleringeret al., 2000; Hall-Asplandet al.,2005; Phillipset al., 2005). Also, at annual and seasonal scales, the δD and δ18O of root and stem water can reflect the variations of potential plant water sources (White, 1989), and plant water sources vary according to variations of plant species, water environments, and growing seasons (Linet al., 1996; Liet al., 2006).
There are many applications of plant water sources and water utilization in different ecosystems such as deserts, forests, riparian and coast forests(Ehleringer and Dawson, 1992; Brunelet al., 1995;Chimner and Cooper, 2004). In desert regions between Utah and Arizona in America, for example,variations of the xylem water δD in desert dominant plants have indicated that annual and succulent plants only use summer precipitation; perennial plants use summer precipitation and deep soil water; and deep-rooted perennial plants use groundwater and winter soil water (Ehleringeret al., 1991). Linet al.(1996) simulated increased 25- and 50-mm summer rain events by enriched deuterium isotopes water,and found that the absorption of rain water by perennial shrubs differed greatly by species in a cold desert ecosystem. In riparian forests in Australia,Eucalyptusused not only shallow soil water and river water originating from precipitation, but also groundwater in riverbanks (Thorburn and Walker,1993; Dawson and Pate, 1996). The transpiration water ofAcer negundoin a riparian forest in Utah originated from groundwater (Dawson and Ehleringer, 1991), but from river water and precipitation in Arizona (Kolbet al., 1997).
Along the banks of perennial rivers in the California mountains, trees mainly use the upper soil water in the early growing season, and mainly use groundwater when the soil is dry (Smithet al., 1991). In addition,more groundwater is used when shallow soil water is decreased gradually (Thorburn and Walker, 1993).These results demonstrate that plant water sources vary by the plant species, water environment, and growing season. In China, comprehensive studies on plant water sources have used stable hydrogen and oxygen isotopic techniques (Caoet al., 2002), but there are few study cases about the plant water sources in arid-region ecosystems (Ohteet al., 2003;Zhaoet al., 2008).
By measuring the δ18O and δD of precipitation of the upper reaches of the Heihe River Basin, and soil water and shallow groundwater of a riparian forest, an artificial shrub forest, and Gobi in the lower reaches of the Heihe River Basin, we investigated the recharge sources of shallow groundwater and soil water of these ecosystems. In addition, variations of δ18O values for root water ofPopulous euphraticaand theTamarix ramosissimain the riparian forest ecosystem,ofHaloxylon ammodendronin the artificial forest ecosystem, and ofReaumuria soongoricain the Gobi ecosystem, as well as the δ18O values for soil water and groundwater, revealed the plant water sources and effective water uptake areas in these different ecosystems. This information on the mechanisms of plant water use and the strategies of adapting to extremely dry environments will be useful in restoring and rebuilding damaged desert ecosystems, and maintaining their stability and sustainable development.
2 Materials and methods
2.1 Study area
The study area is located near Ejina, an oasis that lies in western Inner Mongolia in the lower reaches of the Heihe River Basin, which is the second-largest inland river basin in China. This region is extremely arid, with a dry climate, low precipitation, strong evaporation, high winds, and long hours of sunshine.In Ejina, the mean annual precipitation is 42 mm/a;during 1957 to 2003 the maximum annual rainfall was 103 mm and the minimum was 7 mm, and 70%–80%of the rainfall occurs between June and September (Siet al., 2005). During our study period of June 21 to 22,23 to 24, and 26 to 27 in 2010, the annual rainfall was only 8 mm. Because the mean and the highest potential evapotranspiration are 3,755 mm/a and 4,035 mm/a, respectively, which is 89 times more than the rainfall, Ejina is considered one of the driest regions in China.
Our research was mainly conducted in three typical ecosystems of the lower reaches of the Heihe River Basin (Figure 1). The major vegetation of those ecosystems arePopulous euphraticaand shrubs ofTamarix ramosissimaandSophora alopecuroidein the riparian forest,Haloxylon ammodendronin the artificial shrub forest which was planted in the 1990s,and the naturally dominantReaumuria soongoricain the Gobi ecosystem.
The riparian forest is located in the protection zone of Qidaoqiao in the southeast part of the Ejina(42°01'N, 101°14'E); its area is 1,333 hm2. From 1960 to 2007 the mean annual temperature was 8.8 °C, with a mean January temperature of -11.3 °C and a mean July temperature of 26.9 °C. The annual average frost-free period is 146 days and there are 3,426.14 sunshine hours. The age of the riparian forest varied from 25 to 30 years, and the mean height and diameter ofPopulous euphraticawere about 10 m and 18.3±5.7 cm, respectively. The density ofPopulous euphraticavaried from 500 to 1,000 trees per hm2(Xiaoet al.,2010), with additional coverage ofTamarix ramosissima,Sophora alopecuroide, andTaraxacum officinale(dandelion). The soil texture was sandy loam at 40 cm and from 80 to 100 cm, and the remaining soil layer was silty sand. The organic matter content was 0.724%and 0.127% from 0 to 30 cm and from 30 to 200 cm,respectively. The groundwater level was near 2 m during the study period.
The artificial shrub forest is located at the south end of Ejina (41°57'N, 101°01'E). It was planted in a 3m×4m grid in 1994-1995 and the plant height ofHaloxylon ammodendronwas between 2.2 and 2.4 m.The age of these trees was between 16 and 17 years.This forest is irrigated once a year and the groundwater depth was 2.6 m during the study period.
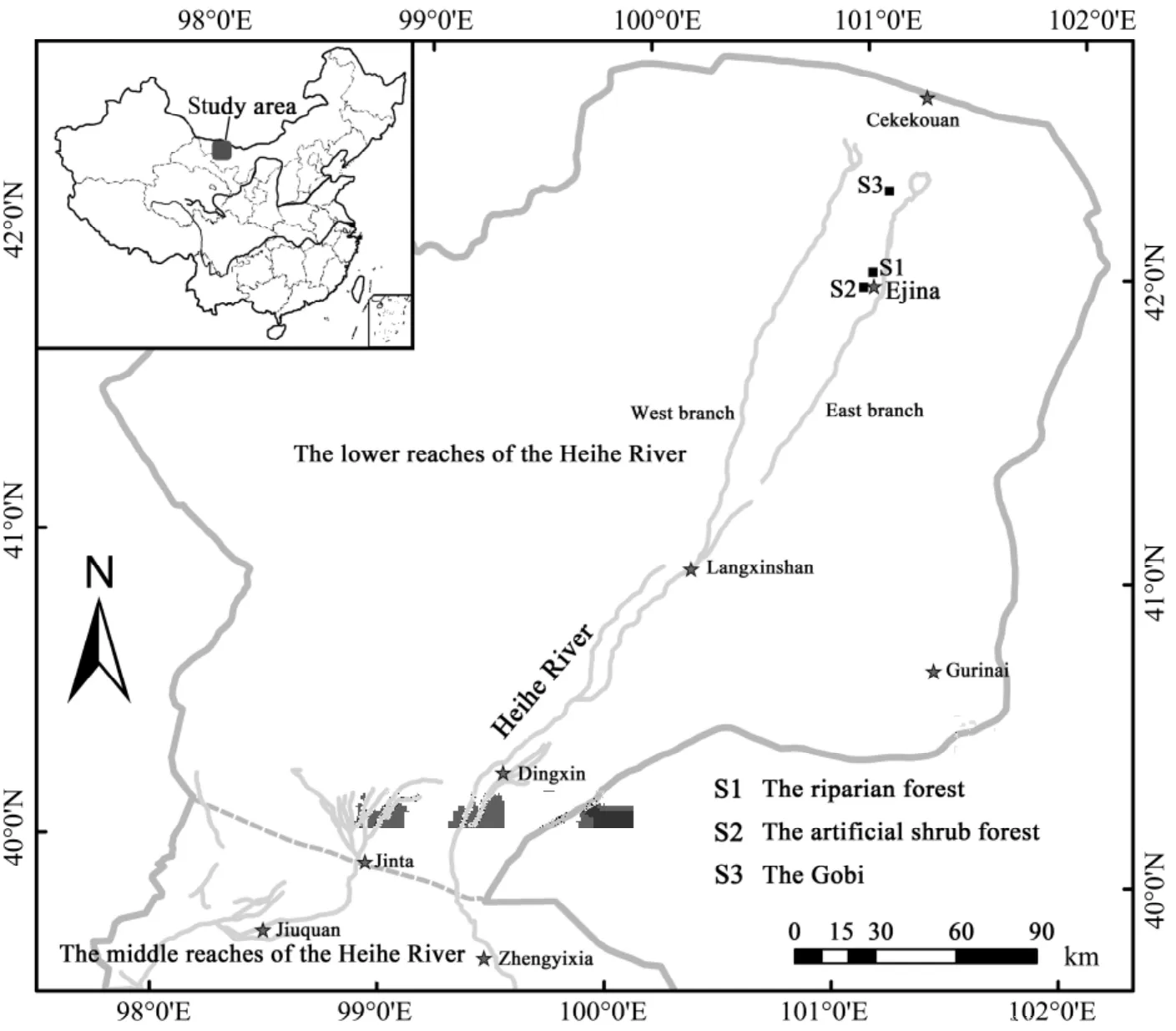
Figure 1 Study sites of plant water sources in the lower reaches of the Heihe River Basin
The Gobi ecosystem is located in the north part of Ejina (42°16'N, 101°07'E).Reaumuria soongoricawas the dominant species in that ecosystem, with coverage of less than 10%, mean plant height of 17.3 cm, and density of 57 trees per 100 m2. The soil texture was sandy soil from the surface to 1.85 m, and a clay layer below 1.85 m. The groundwater depth was greater than 3 m.
2.2 Methods and sample analysis
2.2.1 Sampling methods
Soil water and plant root samples were collected in all three ecosystems on June 21 to 22, 23 to 24,and 26 to 27 in 2010. Root samples of four plants ofPopulous euphraticas,Tamarix ramosissimas,Ha-loxylon ammodendrons, andReaumuria soongoricas,were collected along with soil samples. The distribution of roots and variations of soil texture of the soil profiles are shown in figure 1, and soil samples were collected according to these distributions and variations. The saturated layers of the riparian forest and the artificial shrub forest were 180 and 250 cm, respectively, and soil samples of the saturated layers were also collected. In the Gobi, the groundwater depth was more than 3 m. The soil texture was sand and gravel material from the surface to 185 cm, and was clay below 185 cm. Typically, two samples were collected at each site: an 8-mL sample with two replicates for soil and plant water extraction, and a 15-mL sample with three replicates for soil water content measurement. All the samples were sealed and kept frozen until analysis.
2.2.2 Water extraction and sample analysis
Water was extracted from the plant and soil samples by a cryogenic vacuum distillation method(Dawson and Ehleringer, 1993) at the Isotopic Laboratory of Pretreatment of the Key Laboratory of Ecohydrology of the Inland River Basin, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences. The δ18O values for different water bodies were measured at the Key Laboratory of Ecohydrology of the Inland River Basin. An isoprime isotope ratio mass spectrometer was used to measure the δ18O values after the reaction of the water samples in high-temperature pyrolysis(reduction furnace), and the experimental error of the δ18O value was less than 0.5‰. The results were calibrated using the standard materials of V-SMOW(Nelson, 2000). The soil water content was measured by the weight method.
2.2.3 Data analysis
For a single water source, samples and sources had the same δD and δ18O values when there was no isotopic fractionation. For two water sources, the δD and δ18O values were between the values of the two sources. For two or more water sources, we could not determine the water sources without increasing the number of isotopic species measured.
Multiple possible water sources were mixed to produce a single isotope value; these potential water sources were considered the "initial" water sources which have been converted to soil water through precipitation, groundwater, and runoff. The soil water that was directly absorbed by plants was extracted from artificially divided soil profiles, either shallow soil water or deep soil water. The principle is that if there exist several water sources, the isotopic compositions of the plant water should be a linear combinatory value of the isotopic compositions of the various water sources; therefore, the endmember linear mixing model (Phillips, 2001) can be formulated from the following equations:

where δ18Oprepresents the δ18O value of plant water;δ18Oirepresents the δ18O value of the potential water sourcei; andfirepresents the proportional contributions of potential water sourceito plant water uptake.The isotopic compositions of soil water of different soil layers and groundwater were analyzed with IsoSource software (Phillips and Gregg, 2003) to evaluate the respective contributions of soil water and groundwater to root water. The fractional increment was set at 1%, and the uncertainty level was set at 0.2%.
3 Results and discussion
3.1 Variations of soil water content
There were significant differences in soil water content among the three typical ecosystems in the lower reaches of the Heihe River Basin (Figure 2).For example, in the riparian forest ecosystem, the groundwater level and the depth of the saturated-soil aquifer layer were 200 and 180 cm, respectively. Soil water content varied from 3.7% to 25.6%, and was close to or greater than 5.0% in all the profiles. From 80 to 135 cm, the soil texture was sand gravel, with the lowest soil water content (the mean soil water content of 20 cm and from 80 to 100 cm was 4.0%);from 35 cm to 70 cm the soil texture was sand with slight amounts of clay, and mean the soil water content was 8.1% from 40 to 60 cm. The depth of the saturated aquifer layer was 180 cm and the soil water content was 25.6%. The roots ofPopulous euphraticaandTamarix ramosissimawere mainly distributed from 35 to 70 cm and from 100 to 125 cm.
The groundwater level and the depth of the saturated-soil aquifer of the artificial shrub forest ecosystem were 260 and 250 cm, respectively (Figure 2).The soil water content was the greatest at the 250-cm depth (12.2%), followed by 10.4% at 100 cm and 4.7% from 40 to 80 cm. However, the soil water content was 1.4% between 160 and 200 cm due to the clay layer prevent infiltration of irrigation water. Below 230 cm, the soil water content increased gradually and became saturated at 250 cm due to recharge of the groundwater. The roots ofHaloxylon ammodendronwere mainly distributed from 40 to 60 cm vertically and about 100 cm horizontally.
The mean soil water content of the Gobi was as low as 1.4% above 160 cm due to its far distance from the river and its lack of recharge of irrigation water.From 175 to 180 cm, soil water content increased slightly to 2.4% due to the mixing of sand gravel and clay. The soil texture was clay below 185 cm, and the soil water content increased sharply to 15.8%, 18.0%,22.8%, and 28.0% at 185 cm, 200 cm, 220 cm, and 255 cm, respectively. The main roots ofReaumuria soongoricawere distributed vertically throughout the soil profile except at the sand and clay interface layer(Figure 2).
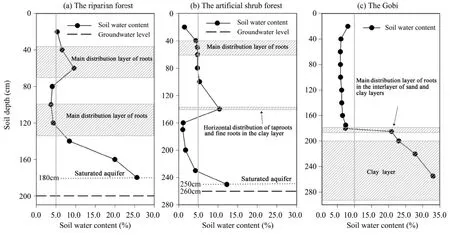
Figure 2 The soil water content and plant roots distribution of the typical ecosystems in the lower reaches of the Heihe River Basin
3.2 Correlations between precipitation in the upper reaches and soil water of the typical ecosystems in the lower reaches of the Heihe River Basin
The lower reaches of the Heihe River Basin is an extremely arid region, with a mean annual rainfall of 42 mm. Soil water recharge by precipitation can be ignored because the annual rainfall in 2010 was only 8 mm. In order to understand the water sources of soil water and shallow groundwater of the typical ecosystems in this area, we compared the δD–δ18O plots of soil water and shallow groundwater of the riparian forest, the artificial shrub forest, and the Gobi with the local meteoric water line (LMWL) of the upper reaches of the Heihe River Basin. Figure 3 shows that the δD–δ18O plots of soil water and shallow groundwater in the riparian forest ecosystem were all near the local meteoric water line (LMWL) of the upper reaches of the Heihe River Basin, except for the soil water above 40 cm. This indicates that soil water and shallow groundwater were recharged by river water which originates from precipitation in the upper reaches.
The δD–δ18O plots of deep soil water and shallow groundwater in the artificial shrub forest ecosystem were also close to the local meteoric water line(LMWL), showing that soil water of this area was also recharged by precipitation in the upper reaches of the Heihe River Basin. However, the δD–δ18O plots deviate from the local meteoric water line (LMWL),indicating that these samples had been affected by intense evaporation (Figure 3).
The Gobi is not recharged by the Heihe River due to its distant location from the river. Moreover, we did not collect soils deeper than 255 cm because of the thick clay layer. The δD–δ18O plots of soil water from a depth below 140 cm in the Gobi are far away from the local meteoric water line (LMWL), indicating that the soil water of this area does not receive the water supply of the middle and upper reaches of the Heihe River Basin (Figure 3). From this, we conclude that the soil water and shallow groundwater near the river in the lower reaches are mainly supplied by the water sources of the middle and upper reaches of the Heihe River Basin, and soil water far away from the river bank is derived from deep groundwater by capillarity.
3.3 Comparisons of the δ18O values in plant root water with its potential water sources
3.3.1 Variations of the δ18O values for soil water in the typical ecosystems
Previous studies have shown that shallow soil water is enriched with the heavier isotopes of both hydrogen and oxygen, and the D and18O values are significantly positive in dry seasons, primarily because of the fractionation in evaporation of hydrogen and oxygen isotopes. This leads to variations in isotopic compositions of soil water in different soil profiles(Zimmermannet al., 1967; Allison and Hughes, 1983).Figure 4 shows that in our study the δ18O values for soil water varied according to the different ecosystem types and varying climatic conditions, and the significant change layers for soil water in thePopulous euphraticaandTamarix ramosissimadistribution area were between 60–80 cm and 100–120 cm, respectively, in the riparian forest (Figure 4b). In the artificial shrub forest ecosystem, significant change occurred in the saturated soil layer between 230 and 250 cm (Figure 4c). In the Gobi, the soil texture was sandy from the surface to 175 cm, and was clay below 180 cm. Only the δ18O values of soil water below 140 cm were measured due to the low soil water content above 140 cm (Figure 4d). Except for the significantly negative δ18O values of the soil water in the interface layer of the sand and clay, the δ18O values of soil water gradually became more negative from 140 to 255 cm, suggesting that soil water evaporation was related to the ecosystem types and climatic conditions. For example, in the riparian forest ecosystem, the soil water evaporating layer was shallow (from 60 to 80 cm) in thePopulous euphraticadistribution area due to high canopy density and low soil water evaporation, whereas in theTamarix ramosissimadistribution area, the soil water evaporating layer was deeper (100–120 cm) due to lower canopy density and intense soil water evaporation,with less variation below 60 cm. The soil water evaporating layer of the artificial shrub forest was located near the saturated-soil aquifer (230 cm) due to low density and canopy and strong evaporation.The soil water evaporating layer of the Gobi was below 255 cm due to the extremely arid conditions in Ejina, the deep groundwater level, low precipitation recharge, and long-term strong evaporation.

Figure 3 Comparisons of δD and δ18O plots of soil water and shallow groundwater in the lower reaches to local meteoric water line(LMWL) of the upper reaches of the Heihe River Basin. The LMWL of the upper reaches of the Heihe River Basin was cited from He (2011) and the precipitation samples were collected from September 2007 to August 2010. PE, TR, and RS indicate Populous euphratica, Tamarix ramosissima, and Reaumuria soongorica, respectively, in the lower reaches of the Heihe River Basin
In summary, the most intense soil evaporation occurred from 60 to 80 cm and from 100 to120 cm (Figures 4a,4b), from 230 to 250 cm (Figure 4c), and below 255 cm (Figure 4d) in the riparian forest, the artificial shrub forest, and the Gobi of the lower reaches of the Heihe River Basin, respectively. This compares well with a study by Asbjornsenet al. (2007) in which the significant change layers of the δ18O values for a soil profile occurred from 80 to 100 cm in a midwestern U.S. savanna, and from 60 to 80 cm in a forest region with approximately 840 mm rainfall in the same vicinity. Those researchers concluded that the main soil water movement layers were related to precipitation and the ecosystem type (Asbjornsenet al., 2007).
3.3.2 The δ18O values for plant xylem water of the typical ecosystems as well as relationships with the potential water sources
Figures 4a and 4b compare the δ18O values of root water ofPopulous euphratica(-6.1‰) andTamarix ramosissima(-4.6‰) with the soil water and groundwater, respectively, in the riparian forest ecosystem. The δ18O value ofPopulous euphraticaroot water was similar to that of soil water at 40 cm(-5.6‰) and 60 cm (-6.9‰) and groundwater(-6.4‰), and theTamarix ramosissimaroot water was similar to that of soil water at 40 cm (-3.2‰), 60 cm (-5.7‰), and 80 cm (-5.9‰), indicating that the potential water sources ofPopulous euphraticaare groundwater and 40- and 60-cm soil water. That ofTamarix ramosissimawas soil water from 40-, 60-,and 80-cm soil. The similar δ18O values of root water ofPopulous euphraticaandTamarix ramosissimaand soil water of 40 cm and 60 cm were related to their root distributions in the soil profile (Figure 2).

Figure 4 Comparisons of the δ18O values in plant root water and the potential water sources in the typical ecosystems of the lower reaches of the Heihe River Basin. (a) and (b) represent the Populous euphratica and Tamarix ramosissima distribution areas of the riparian forest ecosystem, respectively; (c) and (d) represent the artificial shrub forest and the Gobi ecosystems, respectively
In the artificial shrub forest ecosystem, the δ18O values ofHaloxylon ammodendronroot water (-7.3‰)and soil water of 200 cm (-5.9‰), 230 cm (-7.4‰),the saturated soil layer (250 cm) (-7.6‰), and groundwater (-7.8‰) were similar (Figure 4c), indicating that the potential water sources ofHaloxylon ammodendronwere the soil water at 200 cm and 230 cm, the saturated soil layer, and groundwater.
In the Gobi ecosystem, the δ18O value ofReaumuria soongoricaroot water was -1.0‰, similar to the values of soil water at 175 cm (1.2‰), 180 cm(-1.0‰), 185 cm (-0.6‰), and 200 cm (-0.9‰),showing that the potential water sources ofReaumuria soongoricawere the soil water between 175 and 200 cm; these results also match with the layers where theReaumuria soongoricaroots were distributed(Figure 2).
3.4 Dependence of dominant plants on different water sources
IsoSource software was applied to the above results to calculate the relationships of plant water δ18O values and those of their potential water sources, and to reveal the contribution ratios of different potential water sources to the plant water sources in different ecosystems in the lower reaches of the Heihe River Basin (Table 1).
The results show that the contribution ratios of the 40- and 60-cm soil water and groundwater to the water sources ofPopulous euphraticawere 44.1%, 21.0%,and 34.9%, respectively, and of soil water from 40 cm,60 cm, and 80 cm toTamarix ramosissimawere 46.8%, 32.0% and 21.2%, respectively, in the riparian forest ecosystem. In the artificial shrub forest ecosystem, the contribution ratios of the soil water from 200 cm, 230 cm, the saturated soil water, and groundwater to the total plant water uptake byHaloxylon ammodendronwere 17.7%, 30.8%, 27.1%, and 24.3%,respectively. In the Gobi ecosystem,Reaumuria soongoricamainly absorbed the soil water between 175 cm and 200 cm, and the contribution ratios of soil water from 175 cm, 180 cm, 185 cm, and 200 cm were 22.7%, 29.1%, 26.5%, and 31.7%, respectively.
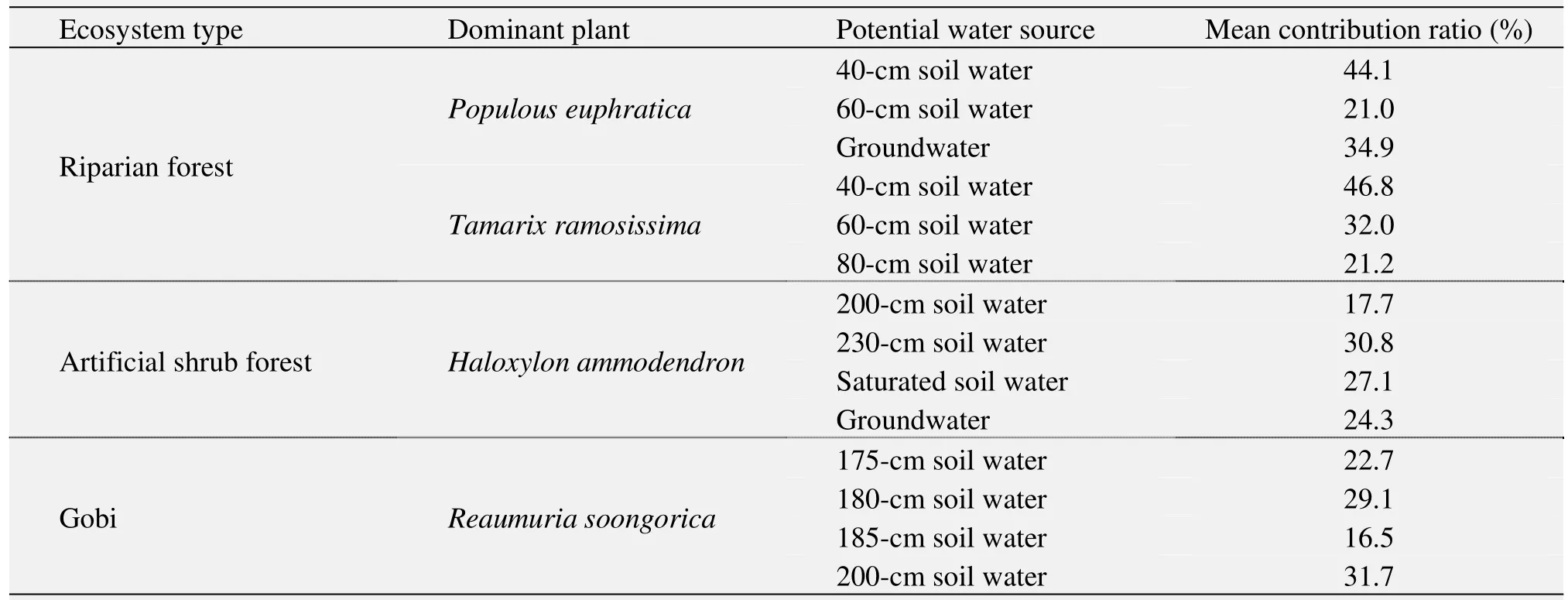
Table 1 The potential water sources and their contribution ratios to dominant plants in the typical ecosystems of the lower reaches of the Heihe River Basin
Our results suggest that soil water and shallow groundwater are the main plant water sources for desert plants to survive in arid desert regions, which is in agreement with Buschet al. (1992) and Synder and William (2000). The groundwater depth directly affects the soil water and nutrient dynamics, which are closely related to the vegetation growth, and it is the controlling factor in determining the distribution,growth, and species succession of desert vegetation as well as the development of desert oases, as found by Zhaoet al. (2003) and Fanet al. (2004). Xiaoet al.(2010) determined that the formation of tree rings ofPopulous euphraticais significantly related to the groundwater depth in the lower reaches of the Heihe River Basin, and water use efficiency ofPopulous euphraticaincreases with the increase of groundwater depth (Caoet al., 2012). Therefore, in the lower reaches of the Heihe River Basin adequate shallow groundwater and soil water levels are important to maintain the normal growth of desert plants.
4 Conclusions
In three typical ecosystems of the lower reaches of the Heihe River Basin, the recharge sources for soil water and groundwater vary according to their distance from the Heihe River channel. Soil water content, soil water evaporating layers, and plant water sources vary with the different ecosystem types.
1) The groundwater level, depth of the saturated soil aquifer, and the soil water content were the highest in the studied riparian forest ecosystem. Its recharge source is the Heihe River, which originates from precipitation in the upper reaches of the Heihe River Basin. In the artificial shrub forest ecosystem,the recharge source of soil water and groundwater is also the Heihe River, but the soil water content is relatively low due to this forest’s location far from the river, the small recharge amount, and strong local evaporation. The Gobi soil water contents were the lowest, due to its distance from the river and strong local evaporation.
2) The δ18O values of soil water in various profiles show that the intense evaporating layers of soil water become deeper with the decrease of vegetation coverage and groundwater level. The evaporating layers of the riparian forest, the artificial shrub forest, and the Gobi were from 60 to 120 cm, from 230 to 250 cm,and below 255 cm, respectively.
3) Plant water sources and the contribution ratios of different potential water sources to plant water sources vary with the differences in vegetation coverage, groundwater level, and the structure of soil profiles in the extremely arid region of the lower reaches of the Heihe River Basin. In the riparian forest, the water sources ofPopulous euphraticamainly come from 40- and 60-cm soil water and from groundwater, and those ofTamarix ramosissimacome from 40- to 80-cm soil water. In the artificial shrub forest ecosystem,Haloxylon ammodendronmainly uses soil water from 200 cm to the saturated layer and groundwater, andin the GobiReaumuria soongoricamainly uses soil water from 175 to 200 cm.
This project is supported by the National Natural Science Foundation of China (Grant Nos. 91325102,91025016 and 91125025) and the National Science &Technology Support Project (No. 2011BAC07B05).
Alessio GA, De Lillis M, Brugnoli E,et al., 2004. Water sources and water-use efficiency in Mediterranean coastal dune vegetation. Plant Biology, 6: 350–357. DOI: 10.1055/s-2004-820882.
Allison GB, Hughes MW, 1983. The use of natural tracers as indicators of soil water movement in a temperate semi-arid region.Journal of Hydrology, 60: 157–173. DOI: 10.1016/0022-1694(83)90019-7.
Asbjornsen H, Mora G, Helmers MJ, 2007. Variation in water uptake dynamics among contrasting agricultural and native plant communities in midwestern U.S. Agriculture. Ecosystems and Environment, 121: 343–356. DOI: 10.1016/j.agee.2006.11.009.
Brunel JP, Walker GR, Kennett-Smith AK, 1995. Field validation of isotopic procedures for determining source water used by plants in a semi-arid environment. Journal of Hydrology, 167:351–368. DOI: 10.1016/0022-1694(94)02575-V.
Busch DE, Ingraham NL, Smith SD, 1992. Water uptake in woody riparian phreatophytes of the southwestern United States: A stable isotope study. Ecological Application, 2: 450–459. DOI:10.2307/1941880.
Cao SK, Feng Q, Si JH,et al., 2012. Variations of foliar stable carbon isotope composition and water use efficiency inPopulus euphraticafor different plots. Journal of Glaciology and Geocryology, 34(1): 155–160.
Cao YL, Lu Q, Lin GH, 2002. Review and perspective on hydrogen stable isotopes technique in tracing plant water sources researches. Acta Ecologica Sinica, 21(1): 111–117. DOI:10.3321/j.issn:1000-0933.2002.01.015.
Chimner RA, Cooper DJ, 2004. Using stable oxygen isotopes to quantify the water source used for transpiration by native shrubs in the San Luis Valley, Colorado U.S.A.. Plant and Soil,260: 225–236. DOI: 10.1023/B:PLSO.0000030190.70085.e9.
Dawson TE, Ehleringer JR, 1991. Streamside trees that do not use stream water. Nature, 350: 335–337. DOI: 10.1038/350335a0.
Dawson TE, Ehleringer JR, 1993. Isotopic enrichment of water in the "woody" tissues of plants: Implications for plant water source, water uptake, and other studies which use the stable isotopic composition of cellulose. Geochimica et Cosmochimica Acta, 57: 3487–3492. DOI: 10.1016/0016-7037(93)90554-A.
Dawson TE, Pate JS, 1996. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia, 107: 13–20. DOI: 10.1007/BF00582230.
Dawson TE, Pausch RC, Parker HM, 1998. The role of hydrogen and oxygen stable isotopes in understanding water movement along the soil-plant-atmospheric continuum. In: Griffiths H(ed.). Stable Isotopes: Integration of Biological, Ecological and Geochemical Processes. Bios Scientific Publisher Limited,Oxford, pp. 169–183.
Ehleringer JR, Dawson TE, 1992. Water uptake by plants: Perspectives from stable isotope composition. Plant Cell and Environment, 15: 1073–1082. DOI: 10.1111/j.1365-3040.1992.tb01657.x.
Ehleringer JR, Phillips SL, Schuster WSF,et al., 1991. Differential utilization of summer rains by desert plants. Oecologia, 88:430–434. DOI: 10.1007/BF00317589.
Ehleringer JR, Roden J, Dawson TE, 2000. Assessing ecosystem-level water relations through stable isotope ratio analyses.In: Osvaldo ES, Robert BJ, Harold AM,et al.(eds.). Methods in Ecosystem Science. Springer-Verlag, New York, pp.181–198. DOI: 10.1007/978-1-4612-1224-9_13.
Fan ZL, Ma YJ, Zhang H,et al., 2004. Research of eco-water table and rational depth of groundwater of Tarim River Drainage Basin. Arid Land Geography, 27(1): 8–13. DOI:10.3321/j.issn:1000-6060.2004.01.002.
Gat JR, 1996. Oxygen and hydrogen isotopes in the hydrologic cycle. Annual Review of Earth and Planetary Sciences, 24:225–262. DOI: 10.1146/annurev.earth.24.1.225.
Hall-Aspland SA, Hall AP, Rogers TL, 2005. A new approach to the solution of the linear mixing model for a single isotope:application to the case of an opportunistic predator. Oecologia,143: 143–147. DOI: 10.1007/s00442-004-1783-0.
He JQ, 2011. Spatial and temporal variations of stable isotopes of precipitation and river water of the inland river basin in Hexi,China. Ph.D. dissertation, Graduate School of the Chinese Academy of Sciences, Gansu, China.
Kolb TE, Hart SC, Amundson R, 1997. Boxelder water source and physiology at perennial and ephemeral stream sites in Arizona.Tree Physiology, 17: 151–160. DOI: 10.1093/treephys/17.3.151.
Li SG, Tsujimura M, Sugimoto A,et al., 2006. Seasonal variation in oxygen isotope composition of waters for a montane larch forest in Mongolia. Trees—Structure and Function, 20:122–130. DOI: 10.1007/s00468-005-0019-1.
Lin GH, Phillips SL, Ehleringer JR, 1996. Monsoonal precipitation responses of shrubs in a cold desert community on the Colorado Plateau. Oecologia, 106: 8–17. DOI: 10.1007/BF00334402.
Nelson ST, 2000. A simple, practical methodology for routine VSMOW/SLAP normalization of water samples analyzed by continuous flow methods. Rapid Communications in Mass Spectrometry, 14: 1044–1046. DOI: 10.1002/1097-0231(20000630)14:12<1044::AID-RCM987>3.0.CO;2-3.
Ohte N, Koba K, Yoshikawa K,et al., 2003. Water utilization of natural and planted trees in the semiarid desert of Inner Mongolia, China. Ecological Applications, 13: 337–351. DOI:10.1890/1051-0761(2003)013[0337:WUONAP]2.0.CO;2.
Phillips DL, 2001. Mixing models in analysis of diet using multiple stable isotopes: A critique. Oecologia, 127: 166–170. DOI:10.1007/s004420000571.
Phillips DL, Gregg JW, 2003. Source partitioning using stable isotopes: Coping with too many sources. Oecologia, 136:261–269. DOI: 10.1007/s00442-003-1218-3.
Phillips DL, Newsome SD, Gregg JW, 2005. Combining sources in stable isotope mixing models: alternative methods. Oecologia, 144: 520–527. DOI: 10.1007/s00442-004-1816-8.
Si JH, Feng Q, Zhang XY,et al., 2005. Vegetation changes in the lower reaches of the Heihe River after its water import. Acta Botanica Boreali-Occidentalia Sinica, 25(4): 631–640. DOI:10.3321/j.issn:1000-4025.2005.04.001.
Smith SD, Wellington AB, Nachlinger JL,et al., 1991. Functional responses of riparian vegetation to streamflow diversions in eastern Sierra Nevada. Ecological Application, 1: 89–97. DOI:10.2307/1941850.
Synder KA, William DG, 2000. Water sources used by riparian trees varies among stream types on the Pedro River, Arizona.Agricultural and Forest Metrology, 105: 227–240. DOI:10.1016/S0168-1923(00)00193-3.
Thorburn PJ, Walker GR, 1993. The source of water transpired byEucalyptus camaldulensis: Soil, groundwater, or streams? In:Ehleringer JR, Hall AE, Farqubar GD (eds.). Stable Isotopes and Plant Carbon-Water Relations. Academic Press, San Diego,pp. 511–527.
White JWC, 1989. Stable hydrogen isotope ratios in plants: A review of current theory and some potential application. In:Rundel PW, Ehleringer JR, Nagy KA (eds.). Stable Isotopes in Ecological Research. Springer-Verlag, New York, pp.142–162.
Xiao SC, Xiao HL, Si JH,et al., 2010. Study on the sub-diurnal radial growth ofPopulus euphratica. Journal of Glaciology and Geocryology, 32(4): 816–822.
Zhao LJ, Xiao HL, Cheng GD,et al., 2008. A preliminary study of water sources of riparian plants in the lower reaches of the Heihe Basin. Acta Geoscientica Sinica, 29(6): 709–718.
Zhao WZ, Chang XL, He ZB, 2003. Responses of distribution pattern of desert riparian forests to hydrologic process in Ejina oasis. Science in China (Series D: Earth Sciences), 33(suppl.):21–30.
Zimmermann U, Enhalt D, Munnich KO, 1967. Soil-water movement and evapotranspiration: Changes in the isotopic composition of the water. Isotopes in Hydrology, Vienna, IAEA, pp.567–585.
 Sciences in Cold and Arid Regions2014年3期
Sciences in Cold and Arid Regions2014年3期
- Sciences in Cold and Arid Regions的其它文章
- Spatiotemporal variations of maximum seasonal freeze depth in 1950s–2007 over the Heihe River Basin,Northwest China
- Retrieval study of lake water depth by using multi-spectral remote sensing in Bangong Co Lake
- Climate transformation to warm-humid and its effect on river runoff in the source region of the Yellow River
- Modeling tropical river runoff: A time dependent approach
- Effects of fertilization on population density and productivity of herbaceous plants in desert steppe
- Effects of sand burial on dune plants: a review
