Effects of fertilization on population density and productivity of herbaceous plants in desert steppe
JieQiong Su , XinRong Li , HaoTian Yang ,2
1. Shapotou Desert Experimental Research Station, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
2. University of Chinese Academy of Sciences, Beijing 100049, China
1 Introduction
Water and nutrients can co-limit plant growth and reproduction in desert ecosystems (Harpoleet al.,2007). Usually, water is believed to be the first factor limiting plant growth in desert ecosystem. However,nitrogen (N) as well as phosphorus (P) has been shown to impact plant growth in consistent ways, due to the increasingly anthropogenic inputs on nutrients to the Earth’s ecosystems (Austinet al., 2004; Elseret al., 2007). Herbaceous plants, as an important component and primary producer of the desert ecosystem,is of importance to prevent desertification and protect biodiversity (Hallet al., 2011; Waseemet al., 2011).
Recently, community composition and productivity of herbaceous vegetation are experiencing impacts from altering soil nutrients, and the plant response is more sensitive for herbaceous vegetation relative to woody plants (Boyer and Zedler, 1998, 1999). N and P fertilizers can promote plant growth and increase community productivity in desert ecosystems through either individual (Chenet al., 2004; Qiuet al., 2004;Maet al., 2007; Zhenget al., 2007) or combined ways(Jameset al., 2005; Harpoleet al., 2007). However,the plant response to fertilizer additions is known to differ both between and within plant communities(Wescheet al., 2007; Xia and Wan, 2008).
It is well known that plant productivity is largely determined by soil fertility, which can be meliorated by surface fertilization (Liet al., 1999). However, far fewer studies have been reported on desert steppe,which is controlled by soil moisture and is covered by fewer plants, in contrast to the grassland and alpine meadow. In this study, we estimate the effects of fertilization on herbaceous plants on desert steppe at a regional scale. The specific objectives of the present study are to (1) estimate the effect of applications of fertilizer to desert steppe on population density and biomass; (2) compare fertilization response on total community of the dominate species; (3) suggest guidelines for fertilization frequency to manage and restore the desert ecosystem.
2 Methods
2.1 Study site
The experimental site, Cuiliugou (37°32′N,105°02′E), is located 40 km west of the Shapotou Desert Research and Experiment Station of the Chinese Academy of Sciences. This region is located in the southeastern fringe of the Tengger Desert, northwestern China. It is a typical transitional zone between steppe desert and desert steppe, with a mean altitude of 1,250 m. The mean annual air temperature is 10.4 °C, and the minimum and maximum temperatures are –25.1 °C in January and 38.1 °C in July, respectively. The mean annual precipitation (MAP) is 180.2 mm; and more than 80 % of annual precipitation occurs during May to September. Average annual wind velocity is 2.8 m/s;an average of 59 dust-storm days occurs annually. The soil is a loamy sand soil. The groundwater is too deep to sustain a large area of vegetation cover. The experimental site is a species-poor system, typically dominated by several herbaceous species, such asStipa breviflora,Tragus mongolorumk,Cleistogenes songorica,Allium polyrhizum,Enneapogon brachystachyus,Salsola ruthenica,andArtemisia capillaris(Li, 2005).
2.2 Fieldwork
Three experimental treatments (N, P, and NP)were replicated in eight 2.5m×7.0m blocks in a randomized complete block design with no replication within blocks. Each treatment included four fertilization levels (0, 5, 10, and 20 g/m2). Each block was split into ten 1m×1m subplots separated by a 0.5-m buffer zone. Every ten plots of the same block were assigned randomly to receive fertilization treatment.N and P fertilizers were added in the solution as NH4NO3and NaH2PO4all at once in mid-May, respectively, and NP fertilizer was applied at an N/P ratio of 3/1. Fertilizers were dissolved in distilled water equivalent to 0.75 L/m2, and the same amount of water was added to the control plots at the same time as the fertilizer additions were made.
The experiment was carried out in 2009, in which annual precipitation was less than the MAP (Figure 1).The tested soil showed relatively high levels of potassium (K), but otherwise low levels of N and P (Table 1). The species, number of individuals, height, and coverage of the herbaceous plants in each plot were recorded monthly from June to September. The aboveground biomass was collected randomly in a 0.5m×0.5m area in each plot during 10–15 September,at the time when aboveground biomass of the herbaceous vegetation attained its peak value in this region.We distinguished between the main groups ofA.capillaris,A.polyrhizum,E.brachystachyusand the rest. The underground biomass was collected by mixing three root cores (5 cm diameter, 10 cm and 20 cm deep, respectively) in each plot. Plant samples were oven-dried at 75 °C for 48 h to a constant weight, and then weighed.
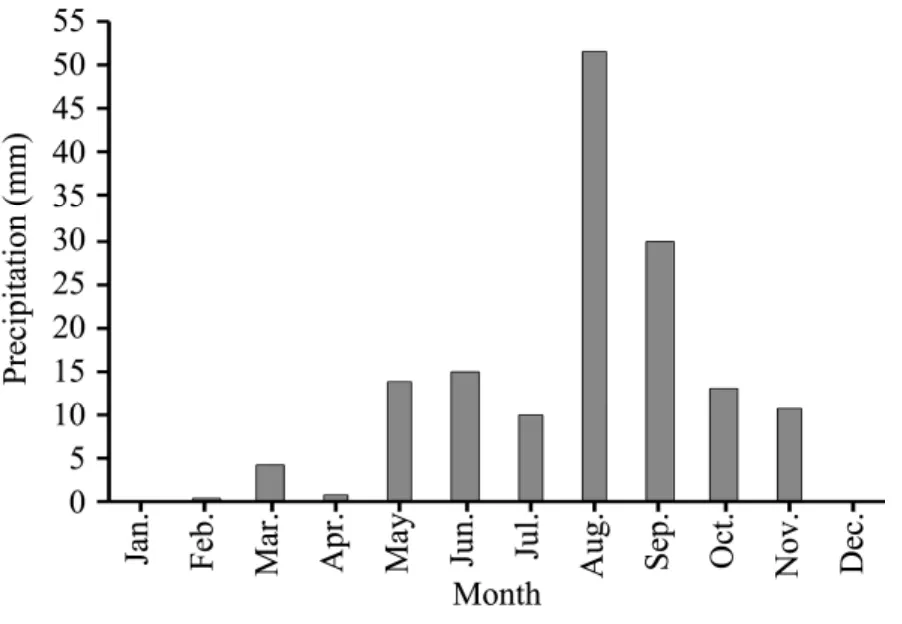
Figure 1 The amount of monthly precipitation of Shapotou area in 2009

Table 1 Physico-chemical properties of the experimental soil (mean±SE)
2.3 Chemical analysis
Measurement of soil physico-chemical properties included soil sampling at depths of 0–10 cm and 10–20 cm in each plot. Air-dried soil samples were sieved to pass through a 2-mm mesh prior to analysis.The soil pH was determined with a pH meter (PHS-4,Jiangsu Manufactory of Electrical Analysis Instruments, Jiangyin, China) in a soil/water solution (1:2.5,w:v). The soil organic carbon was determined using the K2Cr2O7oxidation method. The soil moisture in each plot was measured using a TDR probe (TDR300 Spectrum Technologies, Plainfield, IL) at 2-week intervals during May to September. The soil nutrient content (total and available N, P, and K) was determined using the routine methods of Bao (2000).
2.4 Data analysis
All the values expressed are mean±SE (standard error) of the eight replicates. When data deviated markedly from assumptions of normality and homogeneity of variances, data were transformed using lgX.To test whether the impacts of fertilizer addition on community density and biomass differed between fertilization levels, one-way analysis of variance(ANOVA) followed by multiple comparisons using Duncan’s multiple-range test was performed to separate means. Linear and exponential regression models were used to determine the general relationship between fertilization levels and biomasses for the dominant species and the total population. Statistical differences were considered significant atp<0.05. All statistical analyses were performed using SPSS 16.0;all the figures were drawn by Origin 8.0.
3 Results
3.1 Effects of fertilizer addition on plant density
The changes on population density in response to additions of N, P, and NP fertilizers are given in table 2. In general, plant density on both total community and dominant species (A.capillaris,A.polyrhizum,andE.brachystachyus) decreased as the fertilization levels increased, no matter whether the fertilizer application was single or combined. More specifically,N fertilizer significantly decreased not only total community density but also density of the three dominant species in 20 g/m2treated plots (p<0.05), as compared with the unfertilized control plots. However,in the case of P and NP fertilizers, only the density ofA.polyrhizumsignificantly decreased in 20 g/m2treated plots (p<0.05).
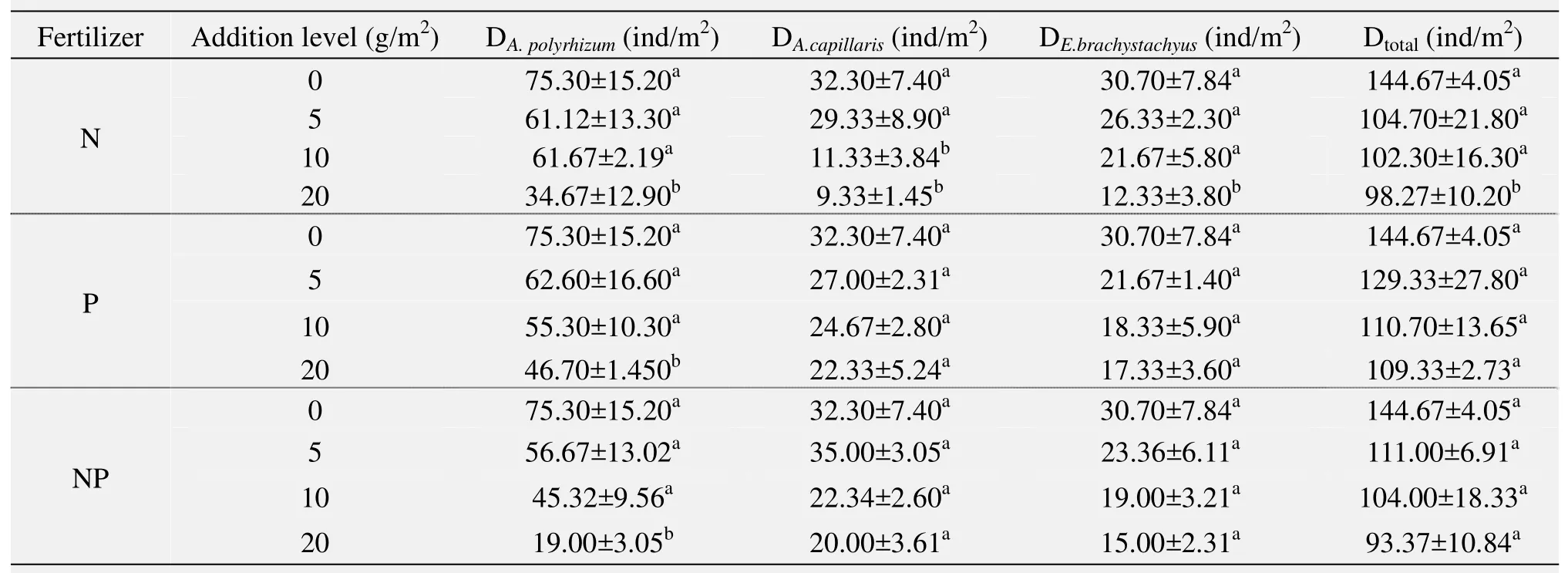
Table 2 Variations of population density under different fertilization levels (mean±SE, n=8)
3.2 Effects of fertilizer addition on plant aboveground biomass
As shown in figures 2 to 4, a general decreasing trend that is similar to the density (Table 2) was noted on aboveground biomasses of both the dominant species and the total population. Nonetheless, the regression models between fertilization levels and aboveground biomasses were different. In the case of total population, the aboveground biomass showed an exponential decrease with increasing fertilization levels regardless of the sort of fertilizer, with determination coefficients (R2) up to 0.898, 0.999, and 0.993 on plots treated with N, P, and NP fertilizers, respectively.
In contrast, significant species differences in degrees of biomass reduction were found for each of the three fertilizers. Specifically, an exponential reduction on plots treated with N fertilizer and a linear reduction on plots treated with P and NP fertilizers were observed forA.polyrhizum, whereas a completely opposite responding pattern was noted forA.capillaris; that is,linear reduction for N fertilizer and exponential reduction for P and NP fertilizers. As forE.brachystachyus,its aboveground biomass showed an exponential reduction on plots treated with single fertilizer of N and P,whereas plots treated with the combined NP fertilizer showed a linear reduction. Furthermore, aboveground biomasses of the three dominant species on fertilized plots decreased to less than a half of that produced on the unfertilized control plots (Figures 2 to 4).
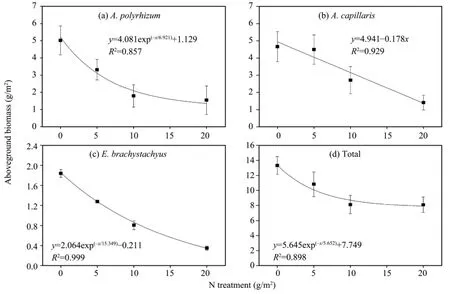
Figure 2 Changes of aboveground biomass under different N fertilization levels
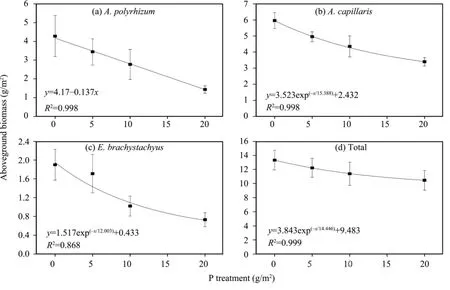
Figure 3 Changes of aboveground biomass under different P fertilization levels

Figure 4 Changes of aboveground biomass under different NP fertilization levels
3.3 Effects of fertilizer addition on plant underground biomass
The underground biomass production of the total population with different fertilizer addition levels is presented in figure 5. The underground biomass decreased sharply as the fertilization level increased,with the highest reduction noted on plots treated with the highest fertilization level at 20 g/m2, no matter whether the fertilizer application was single or combined. In addition, shallow roots showed more reduction than deep roots.
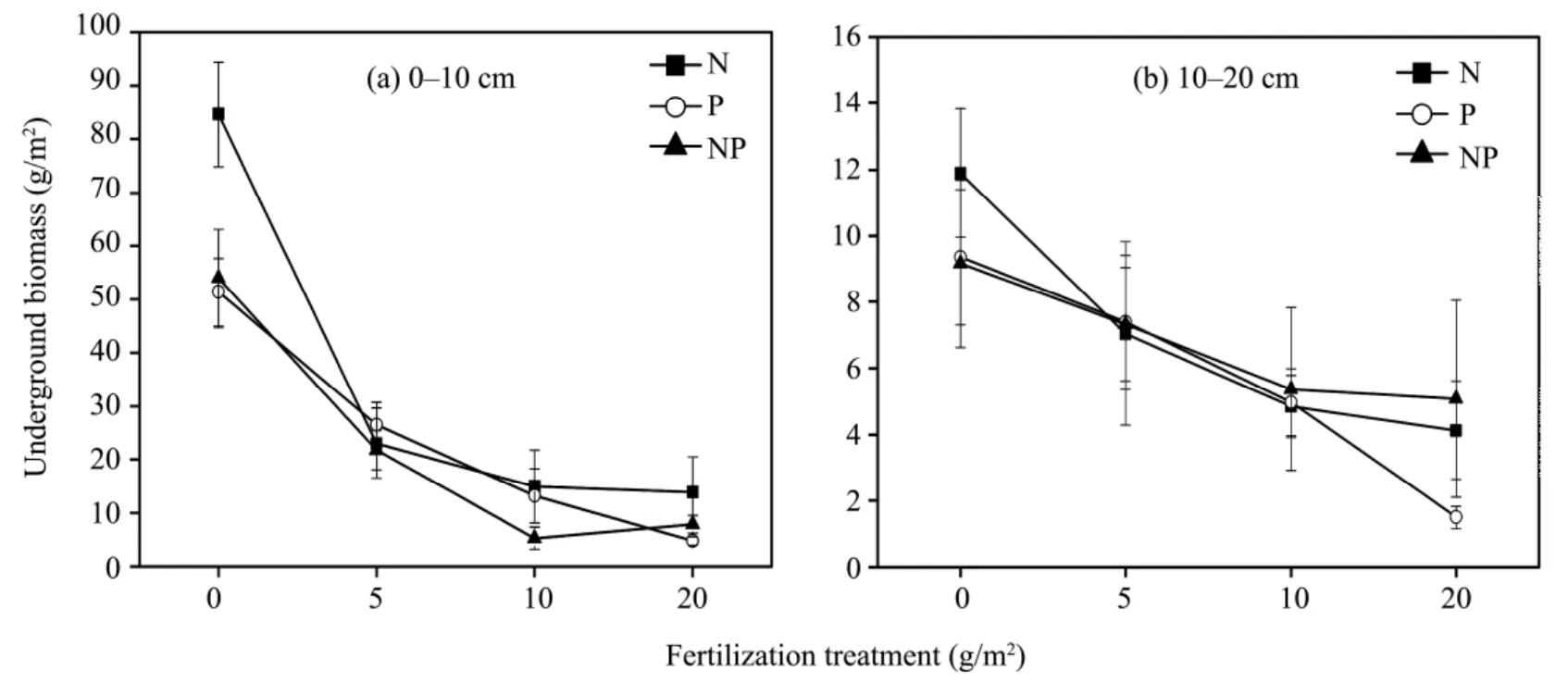
Figure 5 Changes of underground biomass under different fertilization levels
4 Discussion
In dryland ecosystems, the dominate species,which is robust with high density and large biomass,has an advantage over the rare and dwarf species because resources such as water and nutrients are limited. In this study, nonetheless, we found that not only total plant density but also density of the dominant species decreased in response to fertilizer additions,no matter if fertilizer applications were single or combined. The results reflected that fertilization to desert steppe could inhibit reproduction of herbaceous plants, even though the dominant species. This is largely because plant density is co-limited by numbers of the limiting factors (Clark and Tilman, 2008); that is, greater numbers of limiting factors can allow greater numbers of species to coexist through species’tradeoffs for different limiting resources. Thus, on the one hand, fertilization increased concentrations of the soil available nutrient, which enlarged the plant body because of enhanced tillering and reproduction, causing extreme competition among species and even the death of rare and dwarf species (Chenet al., 2004).On the other hand, soil moisture became the main limiting factor when nutrient limitation was eliminated, causing reduction in plant density as a result of lower nutrient uptake induced by water limitation(Gutiérrez, 1992).
The stimulation of aboveground biomass as a result of nutrient enrichment has been demonstrated in many terrestrial biomes (Chenet al., 2004; Qiuet al.,2004; Jameset al., 2005; Maet al., 2007; Zhenget al.,2007). However, we observed an opposite result that fertilizer additions decreased aboveground biomasses of both the total community and dominant species.This is consistent with the finding of St. Clairet al.(2009) that fertilization can inhibit the development of aboveground biomass for herbaceous plants grown on dryland ecosystems with annual precipitation below the MAP. Although there were positive studies in desert steppe, the improved biomass largely benefited from annual precipitation above the MAP (Suet al.,2010, 2013). Several causes are put forward to explain the observed decreases in aboveground biomass.Firstly, this effect commonly referred to the scarce and unevenly distributed precipitation. More specifically, precipitation is the only effective supply of soil moisture for the vegetation in this region (Wanget al.,2005), with more than 60% occurring during August to October (Figure 1), leading to inhibitions on germination of annuals and resprouting of perennials(Zhanget al., 2011). In addition, the uptake of P by roots and then its translocation from roots to shoots could probably be restrained when soil moisture content was less than 25% of the field water holding capacity, resulting in an imbalance of the N/P ratio (Ji,2002). Furthermore, relatively low precipitation might decrease leaf construction and photosynthetic rate, which is expected to increase with increasing availability of N in the soil (Wanget al., 2007). Secondly, accumulations of high levels of N and P in the leaves would likely lead to deficiency of trace elements such as calcium, magnesium, and aluminum(Drenovsky and Richards, 2004). Then, the imbalanced nutrient uptake might cause reductions in plant net photosynthetic efficiency and increases in plant dark respiration rate, and eventually plant death(McLaughlinet al., 1991; Mcnultyet al., 1996; Baueret al., 2004).
The change of underground biomass is always related to the content of soil nutrients, as the root contacts directly with the substrate. As far as we known,the dominant species, such asA. capillarisandA.polyrhizum, have strong capability to compete for limited resources in desert ecosystems, due to their high root/shoot ratio (Jungk, 2001; Liet al., 2003;Walkeret al., 2003; Zhanget al., 2006). Thus, the decreased root biomass in this study was largely because of soil nutrient enrichment, leading to less competition of roots on nutrients, due to the reduced quantities of rootlets and root tips (Schulze, 1989;Perssonet al., 1998).
5 Conclusions
The fertilizer addition experiment in Shapotou desert steppe showed that soil nutrient enrichment decreased herbaceous plants’ density and biomass(both aboveground and root biomasses) of both total population and dominant species, no matter what kind of fertilizer was applied. The results suggest that fertilization to dryland ecosystems is not an optimal approach to promote plant growth when annual precipitation is below the MAP.
This research was supported by the Major State Basic Research Development Program of China (973 Program, Grant No. 2013CB429901-2), and the Foundation for Excellent Youth Scholars of Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences (Y451121001).
Austin AT, Yahdjian L, Stark JM,et al., 2004. Water pulses and biochemical cycle in arid and semiarid ecosystems. Oecologia,141: 221–235. DOI: 10.1007/s00442-004-1519-1.
Bao SD, 2000. Soil and Agricultural Chemistry Analysis. Agriculture Press, Beijing, China, pp. 138–162.
Bauer GA, Bazzaz FA, Minocha R,et al., 2004. Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (PinusresinosaAit.) stand in the NE United States. Forest Ecology and Management, 196: 173–186. DOI: 10.1016/j.foreco.2004.03.032.
Boyer KE, Zedler JB, 1998. Effects of nitrogen addition on the vertical structure of a constructed cord grass marsh. Ecological Applications, 8: 692–705. DOI: 10.1890/1051-0761(1998)008[0692:EONAOT]2.0.CO;2.
Boyer KE, Zedler JB, 1999. Nitrogen addition could shift plant community composition in a restored California salt marsh.Restoration Ecology, l7: 74–85. DOI: 10.1046/j.1526-100X.1999.07109.x.
Chen YM, Li ZZ, Du GZ, 2004. Effects of fertilization on plant diversity and economic herbage groups in alpine meadow. Acta Bot Boreal-Occident Sinica, 24(3): 424–429. DOI:10.3321/j.issn:1000-4025.2004.03.009.
Clark CM, Tilman D, 2008. Loss of plant species after chronic low-level nitrogen deposition to prairie grassland. Nature, 451:712–715. DOI: 10.1038/nature06503.
Drenovsky RE, Richards JH, 2004. Critical N:P values: Predicting nutrient deficiencies in desert shrublands. Plant and Soil, 259:59–69. DOI: 10.1023/B:PLSO.0000020945.09809.3d.
Elser JJ, Bracken MES, Cleland EE,et al., 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letter,10: 1135–1142. DOI: 10.1111/j.1461-0248.2007.01113.x.
Gutiérrez JR, 1992. Effects of low water supplementation and nutrient addition on the aboveground biomass production of annual plants in a Chilean coastal desert site. Oecologia, 90:556–559. DOI: 10.1007/BF01875450.
Hall SJ, Sponseller RA, Grimm NB,et al., 2011. Ecosystem response to nutrient enrichment across an urban airshed in the Sonoran Desert. Ecological Applications, 3: 640–660.
Harpole WS, Potts DL, Suding KN, 2007. Ecosystem responses to water and nitrogen amendment in a California grassland.Global Change Biology, 13: 2341–2348. DOI:10.1111/j.1365-2486.2007.01447.x.
James JJ, Tiller RL, Richards JH, 2005. Multiple resources limit plant growth and function in a saline-alkaline desert community. Journal of Ecology, 93: 113–126. DOI:10.1111/j.0022-0477.2004.00948.x.
Ji YJ, 2002. Primary study on fertilizer application to alpine rangeland in Qinghai, China. Pratacultural Science, 19(5):14–18. DOI: 10.3969/j.issn.1001-0629.2002.05.003.
Jungk A, 2001. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science, 164(2):121–129. DOI: 10.1002/1522-2624(200104)164:2<121:AID-JPLN121>3.0.CO;2-6.
Li DJ, Mo JM, Fang YT,et al., 2003. Impact of nitrogen deposition on forest plants. Acta Ecologica Sinica, 23(9): 1893–1900.
Li KJ, Zhang SF, Jia WZ,et al., 1999. Effect of long-term fertilization on crop yield and soil fertility in semi-arid area. Plant Nutrition and Fertilizer Science, 5(1): 21–25. DOI:10.3321/j.issn:1008-505X.1999.01.004.
Li XR, 2005. Influence of variation of soil spatial heterogeneity on vegetation restoration. Science in China (Series D: Earth Sciences), 48(11): 2020–2031. DOI: 10.1360/04yd0139.
Ma T, Wu GL, He YL,et al., 2007. The effect of simulated mowing of the fertilizing level on community production and compensatory responses on the Qinghai-Tibetan. Acta Ecologica Sinica, 27(6): 2288–2293.
McLaughlin SB, Anderson CP, Hanson PJ,et al., 1991. Increased dark respiration and calcium deficiency of red spruce in relation to acidic deposition at high-elevation southern Appalachian Mountain sites. Canadian Journal of Forest Research, 21:1234–1244.
Mcnulty SG, Aber JD, Newman SD, 1996. Nitrogen saturation in a high elevation New England spruce-fir stand. Forest Ecology and Management, 84: 109–121.
Persson H, Ahlstr K, Clemensson LA, 1998. Nitrogen addition and removal at Gardsjn effects on fine-root growth and fine-root chemistry. Forest Ecology and Management, 101: 199–206.
Qiu B, Luo YJ, Du GZ, 2004. The effect of fertilizer gradients on vegetation characteristics in alpine meadow. Acta Prataculturae Sinica, 13(6): 65–68.
Schulze ED, 1989. Air pollution and forest decline in a spruce(Picea abies) forest. Science, 244: 776–783. DOI:10.1126/science.244.4906.776.
St. Clair SB, Sudderth EA, Castanha C,et al., 2009. Plant responsiveness to variation in precipitation and nitrogen is consistent across the compositional diversity of a California annual grassland. Journal of Vegetation Science, 20: 860–870. DOI:10.1111/j.1654-1103.2009.01089.x.
Su JQ, Li XR, Li XJ,et al., 2010. Response of vegetation herb layer to nitrogen fertilizer in steppe desert. Journal of Desert Research, 30(6): 1336–1340.
Su JQ, Li XR, Li XJ,et al., 2013. Effects of additional N on herbaceous species of desertified steppe in arid regions of China: a four-year field study. Ecological Research, 28: 21–28. DOI:10.1007/s11284-012-0994-9.
Walker TS, Bais HP, Grotewold E,et al., 2003. Root exudation and rhizosphere biology. Plant Physiology, 132: 44–51.http://dx.doi.org/10.1104/pp.102.019661.
Wang B, Liu WZ, Dang TH,et al., 2007. Distribution features of soil water content in the profile of rainfed cropland with long-term fertilization. Plant Nutrition and Fertilizer Science,13(3): 411–416.
Wang XP, Zhang ZS, Zhang JG,et al., 2005. Review to researches on desert vegetation influencing soil hydrological processes.Journal of Desert Research, 25(2): 196–201.
Waseem M, Ali A, Tahir M, 2011. Mechanism of drought tolerance in plant and its management through different methods.Continental Journal of Agricultural Science, 5: 10–25.
Wesche K, Nadrowski K, Retzer V, 2007. Habitat engineering under dry conditions: the impact of pikas (Ochotona pallasi)on southern Mongolian mountain steppes. Journal of Vegetation Science, 18: 665–674. DOI: 10.1111/j.1654-1103.2007.tb02580.x.
Xia JY, Wan SQ, 2008. Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist, 179:428–439. DOI: 10.1111/j.1469-8137.2008.02488.x.
Zhang XL, Zeng FJ, Liu B,et al., 2011. Effect of irrigation on root growth and distribution of the seedlings ofAlhagi sparsifoliaShap., in the Taklimakan Desert. Journal of Desert Research,31(6): 1459–1466.
Zhang Z, Fan HW, Zhao JL,et al., 2006. Root distribution and dynamics of re-vegetated communities in desert area. Journal of Desert Research, 26(4): 637–643.
Zheng HP, Chen ZX, Wang SR,et al., 2007. Effect of fertilizer on plant diversity and productivity of desertified alpine grassland at Maqu, Gansu. Acta Prataculturae Sinica, 16(5): 34–39. DOI:10.3321/j.issn:1004-5759.2007.05.004.
 Sciences in Cold and Arid Regions2014年3期
Sciences in Cold and Arid Regions2014年3期
- Sciences in Cold and Arid Regions的其它文章
- Spatiotemporal variations of maximum seasonal freeze depth in 1950s–2007 over the Heihe River Basin,Northwest China
- Retrieval study of lake water depth by using multi-spectral remote sensing in Bangong Co Lake
- Climate transformation to warm-humid and its effect on river runoff in the source region of the Yellow River
- Modeling tropical river runoff: A time dependent approach
- Water sources of plants and groundwater in typical ecosystems in the lower reaches of the Heihe River Basin
- Effects of sand burial on dune plants: a review
