结缔组织病相关肺间质病变的血清生物标志物研究进展
殷 培,石桂秀
(1.四川大学华西临床医学院2011级硕士研究生,四川 成都 610041;2.厦门大学附属第一医院风湿免疫科,福建 厦门 361003)
肺间质病变(interstitial lung disease,ILD)是由多种原因引起的肺间质炎症性疾病,是炎症、组织损伤和试图修复等综合因素作用的结果。表现为呼吸困难,限制性通气障碍,一氧化碳弥散量降低等,严重影响患者生活质量。ILD目前尚缺乏良好的治疗手段,其中位生存期只有2~3年[1]。根据病因明确或不明确,一般将ILD分为非特发性ILD和特发性ILD[2]。在明确病因的ILD中,结缔组织病(connective tissue disease,CTD)是引起 ILD 的重要原因[3]。CTD是一组以自身免疫异常造成多器官系统损伤为特征的疾病,而这其中就包括呼吸系统。多种CTD均可以引起ILD,包括:类风湿关节炎、系统性红斑狼疮、硬皮病、原发性干燥综合症、多发性肌炎/皮肌炎、抗合成酶综合征、混合性CTD和未分化的CTD等。这些疾病在呼吸道的不同组成部分都有着不同的发病率和患病率的风险[4,5](表1)。在病理组织学检查中,CTD-ILD有着各种不同的分型,包括普通型间质性肺炎(usual interstitial pneumonia,UIP)、非特异性间质性肺炎(nonspecific interstitial pneumonia,NSIP)、弥漫性肺泡损伤(diffuse alveolar damage,DAD)、闭塞性细支气管炎机化性肺炎(bronchiolitis obliterans organizing pneumonia,BOOP)、淋巴细胞性间质性肺炎(lymphocytic interstitial pneumonia,LIP)等[5]。不同的病理亚型,其病程、治疗效果、预后皆不尽相同。
CTD-ILD是结缔组织疾病的重要并发症,也是肺间质病变的重要病因。CTD-ILD呈现了很高的发病率。一项回顾性研究中,经2.1年的随访后,114例ILD患者中近1/3可确诊为CTD-ILD[6]。而西班牙的一项研究则显示176例患者中有41.4%患者确诊CTD-ILD[7]。CTD-ILD的发病率可能远高于目前观点,有流行病学研究认为“亚临床”ILD更为常见,约发生在40%的CTD中,而这一阶段可能是CTD-ILD的可治疗阶段[8]。CTD-ILD是一组发病率高、预后差的疾病[9],早期干预可以及时控制炎症,修复已经受损的肺泡间质。若炎症反应持续,组织损伤及结构改变持续发展,则会使受损肺泡壁广泛破坏、功能性肺泡毛细血管单元丧失,胶原性疤痕组织聚集,造成不可逆转的组织破坏[3,10]。因此早期发现和治疗CTD-ILD对改善患者预后有重要影响[4,11,12]。
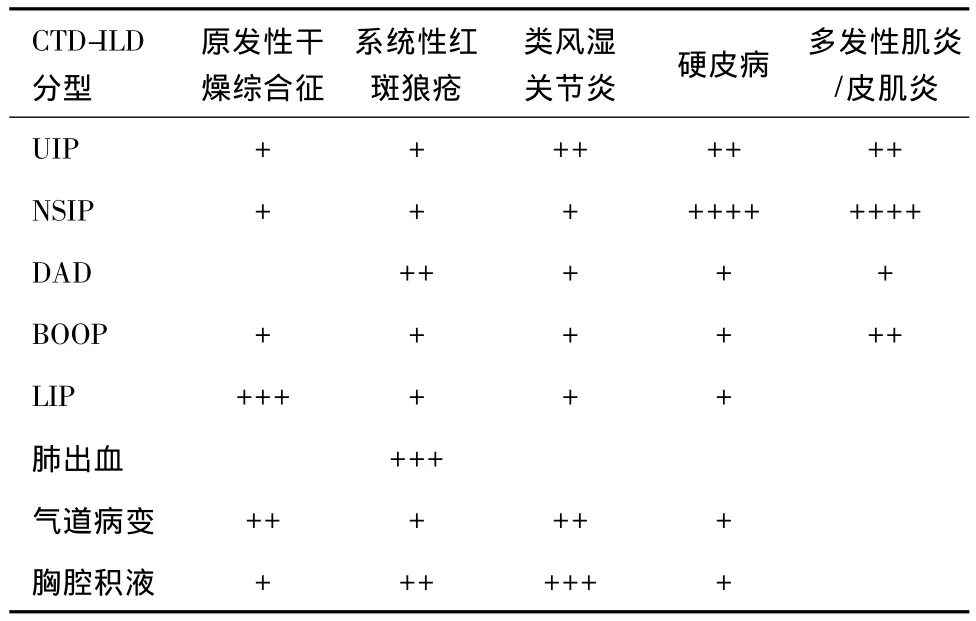
表1 常见CTD伴发ILD的频率
然而,CTD-ILD的诊断很复杂,怀疑肺部受损的患者需全面评估职业史、肺部症状、肺外表现有关的环境因素等才能做出相关判断。目前诊断CTD-ILD主要依靠高分辨率CT(high-resolution computed tomography,HRCT)和肺活检。HRCT检测昂贵,肺活检为有创检查。因判断手法的单一,和患者对放射性检查及有创检查的接受度低等原因,常因漏诊、误诊而耽误治疗时机。甚至有时进行了多种检查后仍不能确诊CTD-ILD[13],因此寻找简便、易行、可靠的早期诊断方法成为目前该领域研究的热点。检测相关血清生物标志物简单易行,本文对目前相关研究进行综述。
1 基质金属蛋白酶(matrix metalloproteinases,MMPs)及基质金属蛋白酶组织抑制因子(tissue inhibitor of metalloproteinasas,TIMPs)
1.1 MMPsMMPs是降解细胞外基质(extracellular matrix,ECM)组成部分的关键酶,与结缔组织重塑有关[14]。MMP-1是一种间质胶原酶,主要定位于肺泡上皮细胞,可以裂解堆积的Ⅰ型和Ⅲ型纤维胶原分子,有抗纤维化的作用。最近有研究证明,MMP-1通过结合人角质形成细胞和单核细胞的细胞膜上β1整合素调节迁徙活动[15]。在类风湿关节炎(Rheumatoid arthritis,RA)合并急性起病弥漫性ILD(acute-onsetdiffuse interstitiallung disease,AoDILD)患者中,MMP1血清表达水平显著增加[16]。在RA患者中,MMP-3血清水平被认为与关节损害密切相关,是系统性炎症的标志物,也是引起细胞外基质降解及最终导致软骨、韧带及骨破坏的最重要的蛋白酶[17]。在RA-AoDILD患者中,MMP-3血清水平明显降低,MMP-3/MMP-1的比值降低[16]。MMP-7又称溶素(matrilysin),是针对广泛的细胞外基质蛋白的金属蛋白酶,被认为在ILD中高表达。在硬皮病(scleroderma,SSc)合并肺纤维化的患者中,血清MMP-7表达较高,并与肺弥散水平低相关[18,19]。MMP-9 又称明胶酶 B(gelatinase B),与慢性炎症性自身免疫性疾病相关,与健康对照组相比较,系统性硬化症合并肺间质病变(SSc-ILD)患者的支气管肺泡灌洗液中MMP-9含量明显增加[20],在血清中的表达水平有待进一步证实。MMP-12又称巨噬细胞弹性蛋白酶(macrophage elastase),主要由巨噬细胞以酶原方式分泌,可降解多种细胞外基质,弹性蛋白是其主要底物。在SSc-ILD的患者,MMP-12血循环水平增加,并与肺纤维化严重程度相关[21]。
1.2 TIMPsTIMPs是MMPs抑制剂,两者之间的平衡可以改建细胞外基质和维护基底膜完整。TIMP-1水平已被证明与SSc-ILD的存在相关,与弥散水平微弱负相关[22]。在 RA-AoDILD时,血清TIMP-2降低,TIMP-1、TIMP-3升高[16]。在 RA-ILD,血清MMP-9/TIMP-1比值降低可以反映肺组织病理变化的严重程度[23],在SSc-ILD患者肺泡灌洗液中也发现了相似的结论[20]。
2 趋化因子CCL家族和CXCL家族
白细胞炎症趋化因子根据其开端两个半胱氨酸残基的位置不同分为CCL[Chemokine(C-C motif)ligand]和 CXCL[Chemokine(C-X-C motif)ligand],两者都可结合于白细胞表面表达的受体。
2.1 趋化因子CCL家族 ①趋化因子2(CCL2):在一项随访期3年的研究中,血清CCL2变化与肺活量(vital capacity,VC)的变化密切相关,但是此项研究中肺功能下降的患者人数并不多,可能对结果造成一定影响[24]。在另一项研究中,支气管肺泡灌洗液CCL2浓度与SSc患者是否合并ILD、肺功能参数、CT 评分相关[25]。②趋化因子 18(CCL18):CCL18又称为肺活化调节的趋化因子(pulmonary and activation regulated chemokine,PARC),主要由Th2型细胞因子激活,由肺泡巨噬细胞产生,作为多种单核细胞的趋化因子在肺中高水平表达。有研究表明 SSc-ILD患者血清 CCL18水平增加[26]。在Kodera等人的研究中,SSc-ILD患者血清CCL18水平与ILD活动度相关,并可能比KL-6(Krebs vonden lungen-6)或 SP-D(Surfactant protein D)与 SSc-ILD的关联更为紧密[27]。Tiev等人的超过4年的前瞻性队列研究中,血清CCL18水平可以独立预测SSc-ILD恶化[28]。③趋化因子24(CCL24):CCL24即嗜酸粒细胞趋化因子2(Eotaxin-2)或髓系造血细胞抑制因子2(myeloid progenitor inhibitory factor 2,MPIF-2)。CCL24是静止T淋巴细胞、嗜酸性粒细胞活化的趋化因子。它具有较低的中性粒细胞的趋化活性,但在单核细胞和活化的淋巴细胞没有趋化活性。在RA-AoDILD患者中,CCL24血清水平明显降低[16]。
2.2 CXCL家族 ①CXCL9及CXCL10:在肌炎相关的ILD患者血清中CXCL9及CXCL10水平升高[29]。CXCL10血清水平升高可见于各种自身免疫性疾病,并显示出较强的Th1型淋巴细胞趋化活性[29]。SSc合并ILD与那些不合并ILD或正常对照组相比,CXCL10血清水平显著增加[30]。然而这一结论尚有争议。最近一项回顾性的纵向研究发现,SSc患者的CXCL10血清水平随着时间的推移并没有与肺功能的变化相关[24]。②CXCL12又称基质细胞衍生因子-1(SDF-1)。CXCL12及其受体CXCR4的表达是在组织修复中招募内皮祖细胞的至关重要的一环。循环CXCR4+祖细胞已被观察到与皮肤和肺受累的相关性[31],研究表明在SSC-ILD患者肺组织中CXCL12及其受体CXCR4表达存在并升高[32]。
3 白细胞介素(interleukin,IL)家族
有研究认为血清白细胞介素-6(IL-6)是可以预测SSc-ILD早期肺功能下降和死亡率的指标[33]。在RA-AoDILD患者中,IL-2Rα、IL-1受体拮抗剂显著增加[16]。IL-22与 SSc-ILD的关系尚不十分明确,有待进一步确认[34]。
4 肺泡上皮蛋白
4.1 血清表面活性蛋白A(Surfactant protein A,SP-A)和D(SP-D)SP-A和SP-D由II型肺泡上皮细胞产生。研究表明SSc肺纤维化患者血清SPA 和 SP-D 明显高于无纤维化患者[35,36],并且 SP-D具有很高的敏感性[37]。一项前瞻性小规模研究显示,SSC-ILD患者SP-D血清水平会随着时间的推移而增加[36]。而另一项小样本研究中,SSC-ILD患者SP-D 水平下降或稳定[37]。
4.2 糖蛋白Krebs vonden lungen-6(KL-6)KL-6是一种高分子量的粘蛋白样糖蛋白,由Ⅱ型肺泡上皮细胞和支气管上皮细胞强烈表达,可以加重细胞的损伤或再生。此外,KL-6已被证明有对肺成纤维细胞纤维化和抗细胞凋亡的作用,可能在SSc-ILD发病中发挥作用[38]。相比单发SSc患者,SSc肺纤维化患者KL-6血清水平明显升高,并与VC和肺一氧化碳弥散量(DLco)水平呈负相关[37,39]。KL-6 血清水平在SSc-ILD更具特异性[37]。博内拉等人研究表明KL-6可作为CTD-ILD及反应其活动性的血清标志物[40]。在皮肌炎/多发性肌炎合并ILD患者,KL-6 血清水平也有升高[41,42]。
5 骨桥蛋白(osteopontin,OPN)
OPN(也被称为Eta-1)是由包括破骨细胞、T细胞、巨噬细胞、树突状细胞、成纤维细胞等多种细胞分泌的促炎性细胞因子,属于粘附分子整合素家族[43]。OPN在肺泡炎症时表达升高,在纤维化炎症浸润消退阶段达到顶峰[44],参与 RA、SSc 发病[45,46]。有研究表明在 RA-AoDILD 患者中,OPN血清水平显著增加[16]。
6 中性粒细胞弹性蛋白酶(neutrophil Elastase,NE)
多形核嗜中性白细胞(polymorphonuclear neutrophilic leukocyte,PMN)弹性蛋白酶是一种丝氨酸蛋白酶,可以调节细胞外基质的形成,对继发肺损伤进行重塑。Hara等研究指出,SSC-ILD患者血清PMN弹性蛋白酶水平显著增加,并与SP-D和KL-6相关[47]。
7 氧化应激标志物
8-异前列腺素作为脂质过氧化的标记,其血清水平在 SSC-ILD升高,和肺活量及 DLco呈负相关[48]。
8 抗Jo-1及抗CADM-140(Anti-clinically amyopathic dermatomyositis-140)
在皮肌炎/多肌炎患者,最强的ILD预测因子是指向氨酰tRNA合成酶的自身抗体抗Jo-1[29,49]。抗CADM-140在皮肌炎,特别是无肌病皮肌炎出现,对合并 ILD 有着重要的预测意义[50,51]。Anti-CADM-140/MDA5(melanoma differentiation-associated gene 5,MDA5)抗体滴度可以预测迅速进展的皮肌炎合并 ILD 患者[52,53]。另外,有研究证实,转化生长因子 β1(Transforming growth factorβ1,TGF-β1)、结缔组织生长因子(Connective tissue growth factor,CTGF)、糖蛋白YKL-40、几丁质酶CHIT1、单核细胞趋化蛋白-1(monocyte chemoattractant protein-1,MCP-1)、热休克蛋白-90(heat-shock protein-90,Hsp90)、瓜氨酸 α和瓜氨酸β、胰岛素受体-1(insulin-like growth factor-1,IGF-1)、内皮素-1(endothelin-1,ET-1)、早期生长反应基因-1(Early growth response protein 1,Egr-1)等与CTD-ILD发病机制可能相关,是否可成为血清学标志还有待进一步研究[3]。
尽管目前已发现了很多CTD-ILD相关的标记物,但正如上文提到的,仍有许多与CTD-ILD发病机制相关的血清指标未纳入研究范畴。且目前研究均为小样本和单个实验室的研究,未能得到大规模研究及临床证实。目前对于CTD-ILD的研究集中在硬皮病、肌炎、类风湿关节炎合并肺间质病变中,对于系统性红斑狼疮、原发性干燥综合症等其他结缔组织病研究不足。相信随着新的生物标记物的发现,和进一步大样本多中心的研究,必将给CTD-ILD患者的治疗和预后提供有力的实验支持。
[1] Demedts M,Costabel U.ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias[J].The European respiratory journal,2002,19(5):794-796.
[2] Ryu JH,Daniels CE,Hartman TE,et al.Diagnosis of Interstitial Lung Diseases[J].Mayo Clinic Proceedings,2007,82(8):976-986.
[3] Castelino FV,Varga J.Interstitial lung disease in connective tissue diseases:evolving concepts of pathogenesis and management[J].Arthritis research & therapy,2010,12(4):213.
[4] Fischer A,du Bois R.Interstitial lung disease in connective tissue disorders[J].Lancet,2012,380(9842):689-698.
[5] Kim EA,Lee KS,Johkoh T,et al.Interstitial lung diseases associated with collagen vascular diseases:radiologic and histopathologic findings[J].Radiographics,2003,23(5):1340.
[6] Mittoo S,Gelber AC,Christopher-Stine L,et al.Ascertainment of col-lagen vascular disease in patients presenting with interstitial lung disease[J].Respiratory Medicine,2009,103(8):1152-1158.
[7] Martin-Mola E,Gomez-Carrera L.The impact of a jointly staffed clinic on the diagnosis of lung involvement and connective tissue diseases[J].Rheumatology,2011,50(3):434-436.
[8] Bongartz T,Nannini C,Medina-Velasquez YF,et al.Incidence and mortality of interstitial lung disease in rheumatoid arthritis:a populationbased study[J].Arthritis and rheumatism,2010,62(6):1583-1591.
[9] Kocheril SV,Appleton BE,Somers EC,et al.Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia[J].Arthritis Care & Research,2005,53(4):549-557.
[10] Saketkoo LA,Matteson EL,Brown KK,et al.Developing disease activity and response criteria in connective tissue disease-related interstitial lung disease[J].The Journal of rheumatology,2011,38(7):1514-1518.
[11] Yamasaki Y,Yamada H,Yamasaki M,et al.Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis[J].Rheumatology,2007,46(1):124-130.
[12] Mukae H,Ishimoto H,Sakamoto N,et al.Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis[J].Chest,2009,136(5):1341-1347.
[13] Fischer A,West SG,Swigris JJ,et al.Connective tissue disease-associated interstitial lung disease:a call for clarification[J].Chest,2010,138(2):251-256.
[14] Nagase H,Woessner JF.Matrix metalloproteinases[J].The Journal of biological chemistry,1999,274(31):21491-21494.
[15] Pardo A,Selman M,Kaminski N.Approaching the degradome in idiopathic pulmonary fibrosis[J].The international journal of biochemistry & cell biology,2008,40(6-7):1141-1155.
[16] Oka S,Furukawa H,Shimada K,et al.Serum biomarker analysis of collagen disease patients with acute-onset diffuse interstitial lung disease[J].BMC immunology,2013,14:9.
[17] Fiedorczyk M,Klimiuk PA,Sierakowski S,et al.Correlations between serum matrix metalloproteinase(MMP-1,MMP-3,MMP-9,MMP-13)concentrations and markers of disease activity in early rheumatoid arthritis[J].Przeglad lekarski,2005,62(12):1321-1324.
[18] Rosas IO,Richards TJ,Konishi K,et al.MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis[J].PLoS medicine,2008,5(4):e93.
[19] Lota HK,Renzoni EA.Circulating biomarkers of interstitial lung disease in systemic sclerosis[J].International journal of rheumatology,2012,2012:121439.
[20] Andersen GN,Nilsson K,Pourazar J,et al.Bronchoalveolar matrix metalloproteinase 9 relates to restrictive lung function impairment in systemic sclerosis[J].Respir Med,2007,101(10):2199-2206.
[21] Manetti M,Guiducci S,Romano E,et al.Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis:correlation with severity of skin and pulmonary fibrosis and vascular damage[J].Annals of the rheumatic diseases,2012,71(6):1064-1072.
[22] Kikuchi K,Kubo M,Sato S,et al.Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis[J].Journal of the A-merican Academy of Dermatology,1995,33(6):973-978.
[23] Ji YX,Huang JA,Zong JP,et al.The serum levels of cytokines in patients with rheumatoid arthritis associated interstitial lung disease and their clinical significance[J].Zhonghua jie he he hu xi za zhi,2008,31(4):264-267.
[24] Hasegawa M,Fujimoto M,Matsushita T,et al.Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis[J].Clinical rheumatology,2011,30(2):231-237.
[25] Schmidt K,Martinez-Gamboa L,Meier S,et al.Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients[J].Arthritis research & therapy,2009,11(4):R111.
[26] Prasse A,Pechkovsky DV,Toews GB,et al.CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis[J].Arthritis and rheumatism,2007,56(5):1685-1693.
[27] Kodera M,Hasegawa M,Komura K,et al.Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis:a sensitive indicator of active pulmonary fibrosis[J].Arthritis and rheumatism,2005,52(9):2889-2896.
[28] Tiev KP,Hua-Huy T,Kettaneh A,et al.Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis[J].The European respiratory journal,2011,38(6):1355-1360.
[29] Richards TJ,Eggebeen A,Gibson K,et al.Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease[J].Arthritis and rheumatism,2009,60(7):2183-2192.
[30] Antonelli A,Ferri C,Fallahi P,et al.CXCL10(alpha)and CCL2(beta)chemokines in systemic sclerosis--a longitudinal study[J].Rheumatology,2008,47(1):45-49.
[31] Campioni D,Lo Monaco A,Lanza F,et al.CXCR4 pos circulating progenitor cells coexpressing monocytic and endothelial markers correlating with fibrotic clinical features are present in the peripheral blood of patients affected by systemic sclerosis[J].Haematologica,2008,93(8):1233-1237.
[32] Tourkina E,Bonner M,Oates J,et al.Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease:reversal by caveolin-1 scaffolding domain peptide[J].Fibrogenesis &tissue repair,2011,4(1):15.
[33] De Lauretis A,Sestini P,Pantelidis P,et al.Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis[J].The Journal of rheumatology,2013,40(4):435-446.
[34] Truchetet ME,Brembilla NC,Montanari E,et al.Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis:association with interstitial lung disease[J].Arthritis research & therapy,2011,13(5):R166.
[35] Takahashi H,Kuroki Y,Tanaka H,et al.Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis[J].American journal of respiratory and critical care medicine,2000,162(1):258-263.
[36] Asano Y,Ihn H,Yamane K,et al.Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis[J].Arthritis and rheumatism,2001,44(6):1363-1369.
[37] Yanaba K,Hasegawa M,Takehara K,et al.Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis[J].The Journal of rheumatology,2004,31(6):1112-1120.
[38] Ohshimo S,Yokoyama A,Hattori N,et al.KL-6,a human MUC1 mucin,promotes proliferation and survival of lung fibroblasts[J].Biochemical and biophysical research communications,2005,338(4):1845-1852.
[39] Yamane K,Ihn H,Kubo M,et al.Serum levels of KL-6 as a useful marker for evaluating pulmonary fibrosis in patients with systemic sclerosis[J].The Journal of rheumatology,2000,27(4):930-934.
[40] Doishita S,Inokuma S,Asashima H,et al.Serum KL-6 level as an indicator of active or inactive interstitial pneumonitis associated with connective tissue diseases[J].Internal medicine,2011,50(23):2889-2892.
[41] Kubo M,Ihn H,Yamane K,et al.Serum KL-6 in adult patients with polymyositis and dermatomyositis[J].Rheumatology,2000,39(6):632-636.
[42] Fathi M,Barbasso Helmers S,Lundberg IE.KL-6:a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis[J].Journal of internal medicine,2012,271(6):589-97.
[43] Anborgh PH,Mutrie JC,Tuck AB,et al.Pre-and post-translational regulation of osteopontin in cancer[J].Journal of cell communication and signaling,2011,5(2):111-122.
[44] O'Regan A.The role of osteopontin in lung disease[J].Cytokine &growth factor reviews,2003,14(6):479-488.
[45] Zheng W,Li R,Pan H,et al.Role of osteopontin in induction of monocyte chemoattractant protein 1 and macrophage inflammatory protein 1beta through the NF-kappaB and MAPK pathways in rheumatoid arthritis[J].Arthritis and rheumatism,2009,60(7):1957-1965.
[46] Wu M,Schneider DJ,Mayes MD,et al.Osteopontin in systemic sclerosis and its role in dermal fibrosis[J].The Journal of investigative dermatology,2012,132(6):1605-1614.
[47] Hara T,Ogawa F,Yanaba K,et al.Elevated serum concentrations of polymorphonuclear neutrophilic leukocyte elastase in systemic sclerosis:association with pulmonary fibrosis[J].The Journal of rheumatology,2009,36(1):99-105.
[48] Ogawa F,Shimizu K,Muroi E,et al.Serum levels of 8-isoprostane,a marker of oxidative stress,are elevated in patients with systemic sclerosis[J].Rheumatology,2006,45(7):815-818.
[49] Tillie-Leblond I,Wislez M,Valeyre D,et al.Interstitial lung disease and anti-Jo-1 antibodies:difference between acute and gradual onset[J].Thorax,2008,63(1):53-59.
[50] Muro Y,Sugiura K,Hoshino K,et al.Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission[J].Rheumatology,2012,51(5):800-804.
[51] Sato S,Hirakata M,Kuwana M,et al.Autoantibodies to a 140-kd polypeptide,CADM-140,in Japanese patients with clinically amyopathic dermatomyositis[J].Arthritis and rheumatism,2005,52(5):1571-1576.
[52] Sato S,Hoshino K,Satoh T,et al.RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis:Association with rapidly progressive interstitial lung disease[J].Arthritis and rheumatism,2009,60(7):2193-2200.
[53] Sato S,Kuwana M,Fujita T,et al.Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease[J].Modern rheumatology/the Japan Rheumatism Association,2013,23(3):496-502.

