Synthesis and application of an environmentallyfriendly antiscalant in industrial cooling systems
Huang Jingyi Liu Guangqing, Xue Mengwei Zhou Yuming,3 Yao Qingzhao,3
(1School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)(2School of Biochemical and Environmental Engineering, Nanjing Xiaozhuang University, Nanjing 211171, China)(3Jiangsu Optoelectronic Functional Materials and Engineering Laboratory, Nanjing 211189, China)
Synthesis and application of an environmentallyfriendly antiscalant in industrial cooling systems
Huang Jingyi1Liu Guangqing1,2Xue Mengwei2Zhou Yuming1,3Yao Qingzhao1,3
(1School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)(2School of Biochemical and Environmental Engineering, Nanjing Xiaozhuang University, Nanjing 211171, China)(3Jiangsu Optoelectronic Functional Materials and Engineering Laboratory, Nanjing 211189, China)
Allylpolyethoxy carboxylate macromonomers, possessing polyethylene oxygen long chains, were synthesized by advanced technology of the polyether cap. A novel double-hydrophilic block copolymer was prepared through free radical polymeric reactions in aqueous solution and its performance on CaCO3inhibition and dispersancy activity towards Fe2O3was evaluated in recirculating cooling water systems. The study shows that acrylic acid-allylpolyethoxy carboxylate has a significant ability to inhibit the precipitation of calcium carbonate and an excellent dispersing capability to stabilize iron (Ⅲ) in industrial cooling systems. X-ray diffraction shows that there is a number of vaterite crystals in the presence of the phosphorous free and non-nitrogen copolymer. The change in crystal forms is also confirmed by the Fourier-transform infrared spectra, the scanning electron microscopy and the transmission electron microscopy. The inhibition mechanism is proposed and it shows that the interactions between calcium and polyethylene glycol (PEG) are the fundamental impetus for restraining the formation of the scale in cooling water systems.
phosphorous free antiscalant; calcium carbonate; disperse iron (Ⅲ); industrial cooling systems
Open recirculating cooling water systems are frequently used because they provide economical heat removal and the recirculation of water conservation is accomplished with substantial cost reductions. Dissolved and suspended matter contained in the water is concentrated in cooling water recirculation. The precipitation of calcium carbonate scale on heat transfer surfaces widely occurs, which involves the deposition of an insulating layer onto the internal walls owing to its inverse temperature-solubility characteristics[1]. Deposits formation may cause severe corrosion and deterioration in the heat exchange. The most common and effective method of controlling scale formation is the use of chemical additives as scale inhibitors that retard or prevent it, even at very small concentrations[2]. Several studies about calcium carbonate scale formation in the absence and presence of inhibitors have been carried out[3-6].
Regarding accelerated aquatic eutrophication, the popularity of inhibitors containing high phosphorus is diminishing. As a result, the current trend for inhibitor usage is toward more environmentally friendly “green” chemicals. On the other hand, the design or optimization of the recycling-water process on an industrial scale demands a thorough understanding of all the fundamental parameters that govern the various operations involved. Therefore, the inhibition varying with the solution temperature, pH, Ca2+and Fe2+concentrations should be tested.
Ferrous ion, which is soluble in an aqueous medium, is oxidized to the ferric ion. At a pH of approximately 5 and above, it precipitates out in the form of iron hydroxide Fe(OH)3, or iron oxide Fe2O3, and other iron compounds where the iron has an oxidation state of three, hereinafter referred to as iron (Ⅲ). It is known that trace amounts of iron (Ⅲ) on the order of 1-5 mg/L, when present in a circulation system, can adversely affect the performance of scale-control agents such as copolymers of acrylic acid. Therefore, optimum precipitation inhibitors are not only effective against scale but also effective at stabilizing iron in the solution.
The present work attempts to discover and explore the effectiveness of a structurally well-defined calcium-carbonate and iron (Ⅲ) inhibitor which is phosphor free and has a superior calcium tolerance. The inhibitor employed in this paper is double-hydrophilic block copolymer of acrylic acid (AA)-allylpolyethoxy carboxylate (APEL).
1 Methodology
1.1 Materials
APEL was synthesized from allyloxy polyethoxy ether (APEG) in our laboratory according to Ref.[7]. AA is an analytically pure grade and was supplied by Zhongdong Chemical Reagent Co. (Nanjing, Jiangsu, China). Distilled water was used in all the studies.
1.2 Synthesis of AA-APEL
A 5-neck round bottom flask, equipped with a thermometer and a magnetic stirrer, was charged with 90 mL distilled water and 0.1 mol APEL and heated to 70 ℃ with stirring under a nitrogen atmosphere. Then, 1.5 mol AA in 18 mL distilled water (the mole ratio of APEL and AA was 1∶15) and the initiator solution (3.0 g ammonium persulfate in 18 mL distilled water) were added separately at constant flow rates over a period of 1.0 h. The reaction was then heated to 80 ℃ and maintained at this temperature for an additional 2.0 h, ultimately producing an aqueous polymer solution containing approximately 35% solids. The synthesis of AA-APEL is given as
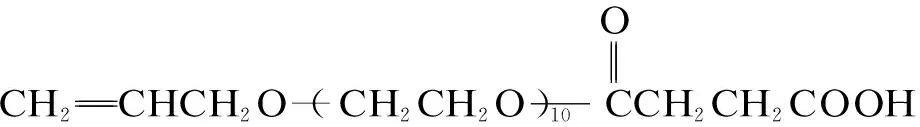
(1)
1.3 Characterization
The Fourier-transform infrared (FT-IR) spectra was taken on a Bruker FT-IR analyzer (VECTOR-22, Bruker Co., Germany) by using the KBr-pellet method (compressed powder). The X-ray diffraction (XRD) patterns of the CaCO3crystals were recorded on a Rigaku D/max 2400 X-ray powder diffractometer with Cu Kα (λ=1.540 6) radiation (40 kV, 120 mA). Powder samples were mounted onto a sample holder and scanned at a scanning speed of 2(°)/min between 2θ=20°-60°. The shape of calcium carbonate scale was observed with a scanning electron microscope (S-3400N, HITECH, Japan). The light transmittance of ferrous solutions was measured by spectrophotometric measurements on a UV3100-PC ultraviolet and visible spectrometer (Mapada, China) at 420 nm. The formation of the flowers structure was confirmed by a transmission electron microscope (JEM-2100SX, Japan).
1.4 Static inhibition test
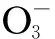
The solution was analyzed after every set of experiments with respect to soluble calcium ions using a standard solution of EDTA according to the Water Treatment Reagent Unit of Standardization Research Institute of Chemical Industry of China, the Application Guidebook on Water Quality of Circulation Cooling Water and Standards of Water Treatment Reagents (in Chinese). The inhibition efficiencyφis defined as
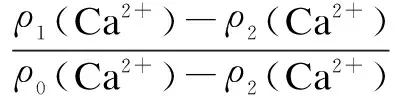
whereρ0(Ca2+) is the total concentrations of Ca2+, mg/L;ρ1(Ca2+) is the concentrations of Ca2+, mg/L, in the presence of the phosphorous free and non-nitrogen copolymer;ρ2(Ca2+) is the concentrations of Ca2+, mg/L, in the absence of the phosphorous free and non-nitrogen copolymer.
1.5 Iron dispersing ability
Ferrous compounds for precipitation experiments were prepared by adding a known volume of calcium stock solution to a beaker (1 000 mL) at room temperature under violent stirring, with a known volume of water. After temperature equilibration, the inhibitors were added before the iron(Ⅱ) (normally 5 to 10 mL) stock solution was added in such an amount that the final iron(Ⅱ) concentration would be 10 mg/L or the required values. Precipitation in these solutions was monitored by analyzing solutions for the light transmittance by using a UV spectrophotometer after these solutions were heated 5 h at a temperature of 50 ℃. The pH 9.0 of ferrous solutions was adjusted by using dilute solutions of borax.
Polymer efficacy as a iron (Ⅲ) inhibitor was evaluated by using the light transmittance of ferrous solutions, which was 100% after being heated for 5 h at a temperature of 50 ℃ in the absence of the inhibitor. The lower the light transmittance is, the better the polymer efficacy is as an iron (Ⅲ) inhibitor.
2 Results and Discussion
2.1 Characterization of inhibitor

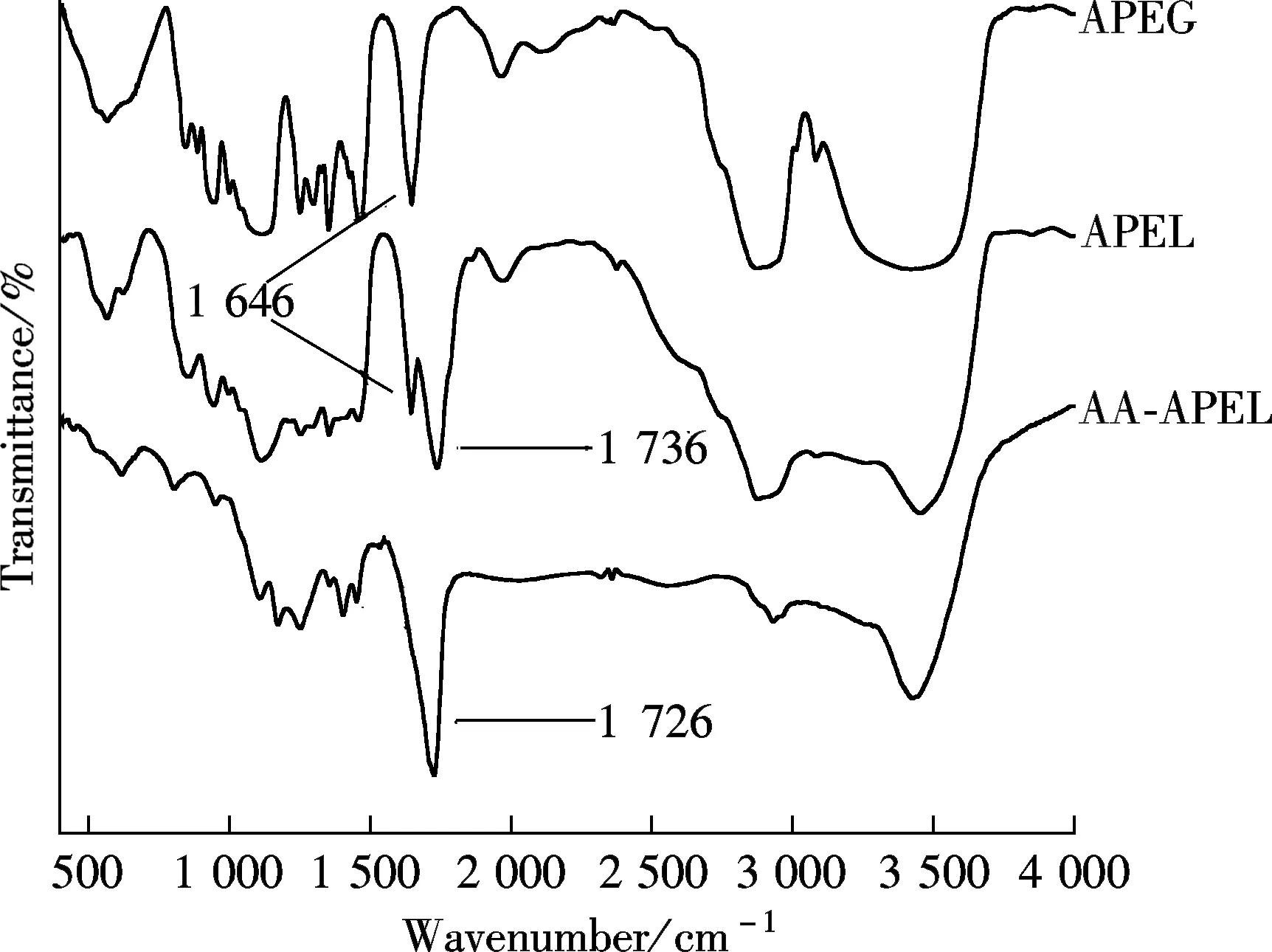
Fig.1 The FT-IR spectra
2.2 Effect of inhibitor dosage
The dosage of the inhibitor has a deep effect on the formation of iron (Ⅲ) precipitation. The light transmittance of ferrous solutions in the presence of AA-APEL in Tab.1 shows that AA-APEL has excellent dispersancy activity toward iron (Ⅲ). It is apparent that the copolymer dosage strongly affects the ability of the inhibitor to control the precipitation of iron (Ⅲ). When the dosage is 4 mg/L, the transmittance of the ferrous solution is 14.2% in the presence of AA-APEL. It should be noted that the similar tendency of the dosage on the performance behavior has been reported in earlier studies by polymeric dispersants inhibitors[8].
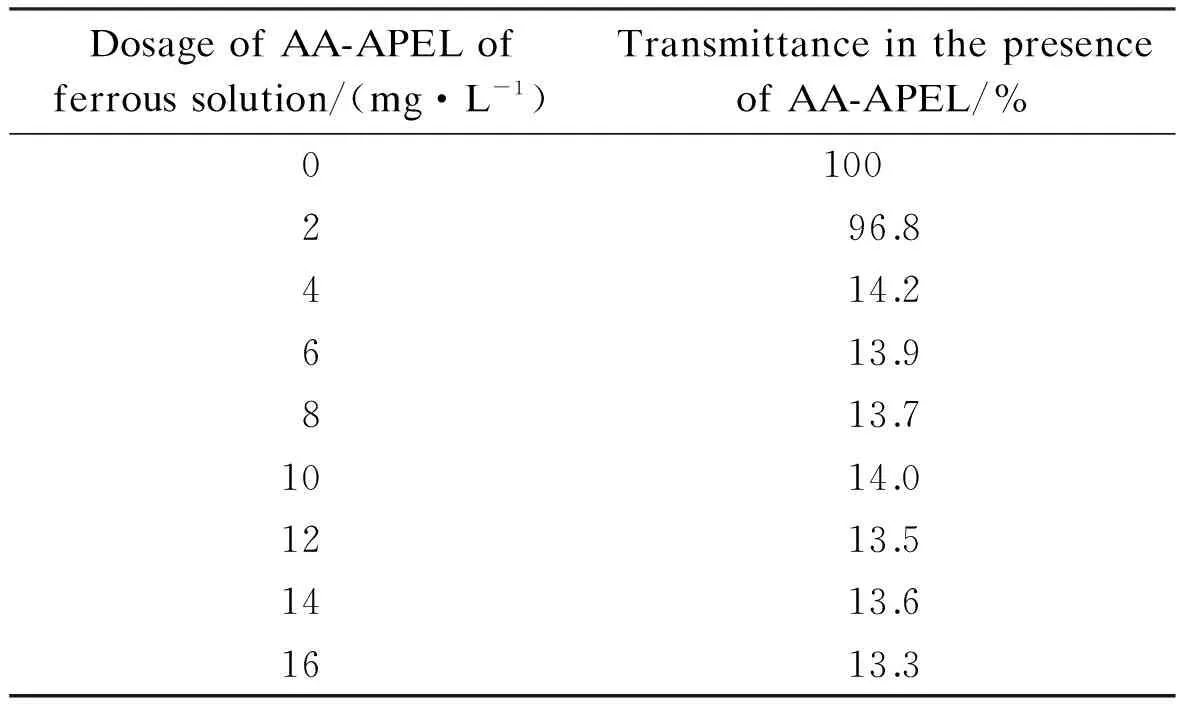
Tab.1 Iron (Ⅲ) inhibition of AA-APEL
2.3 Influence of solution property
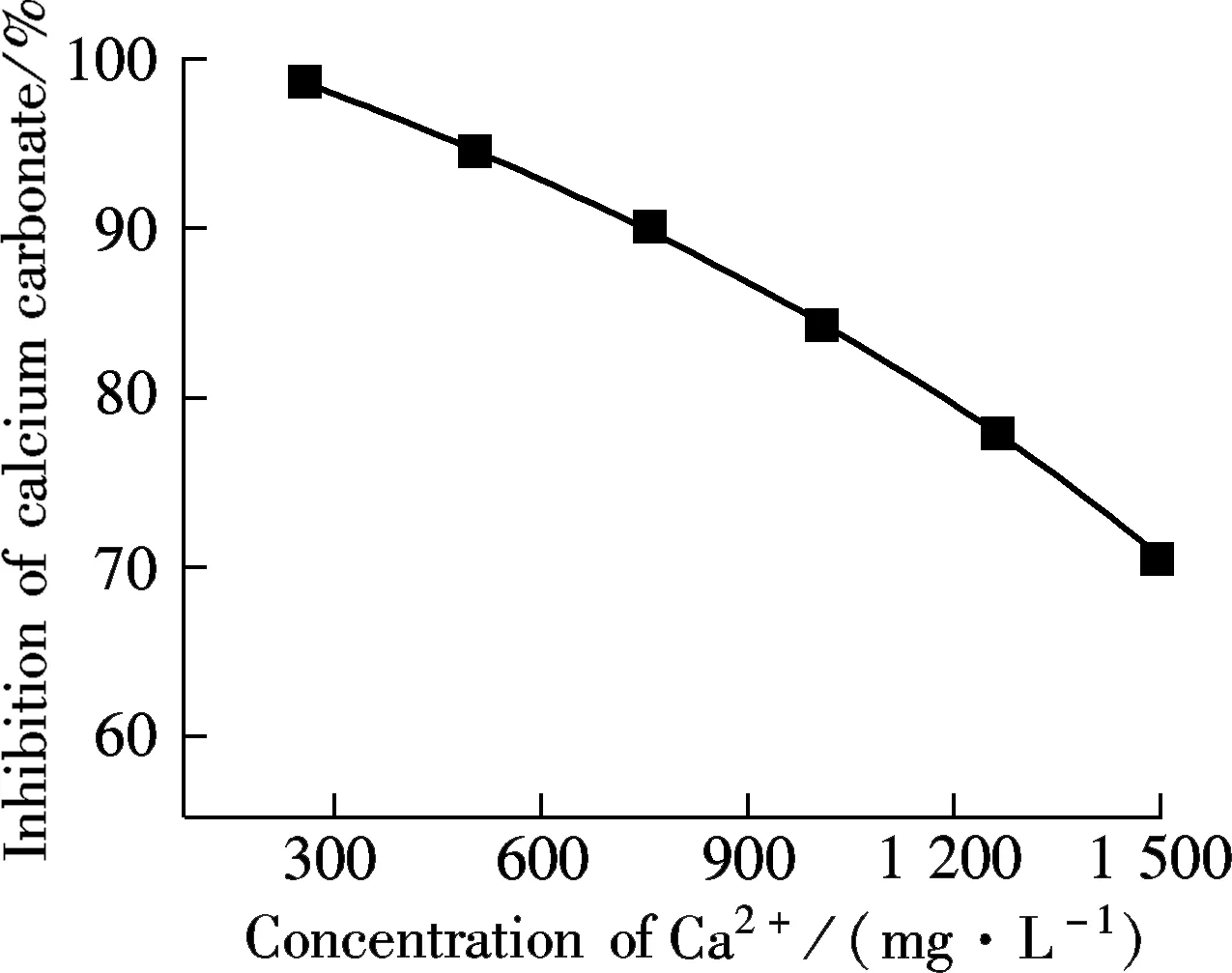
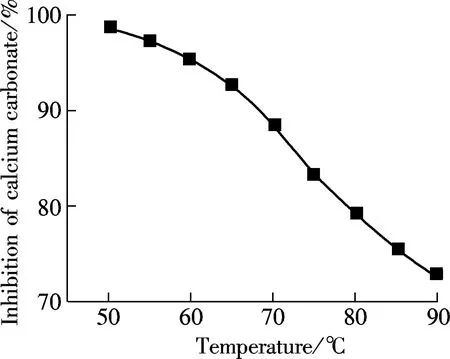
(a)
(b)
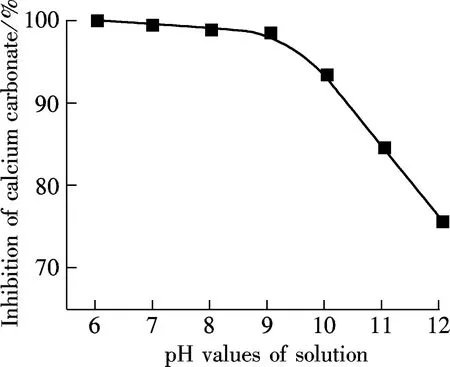
(c)
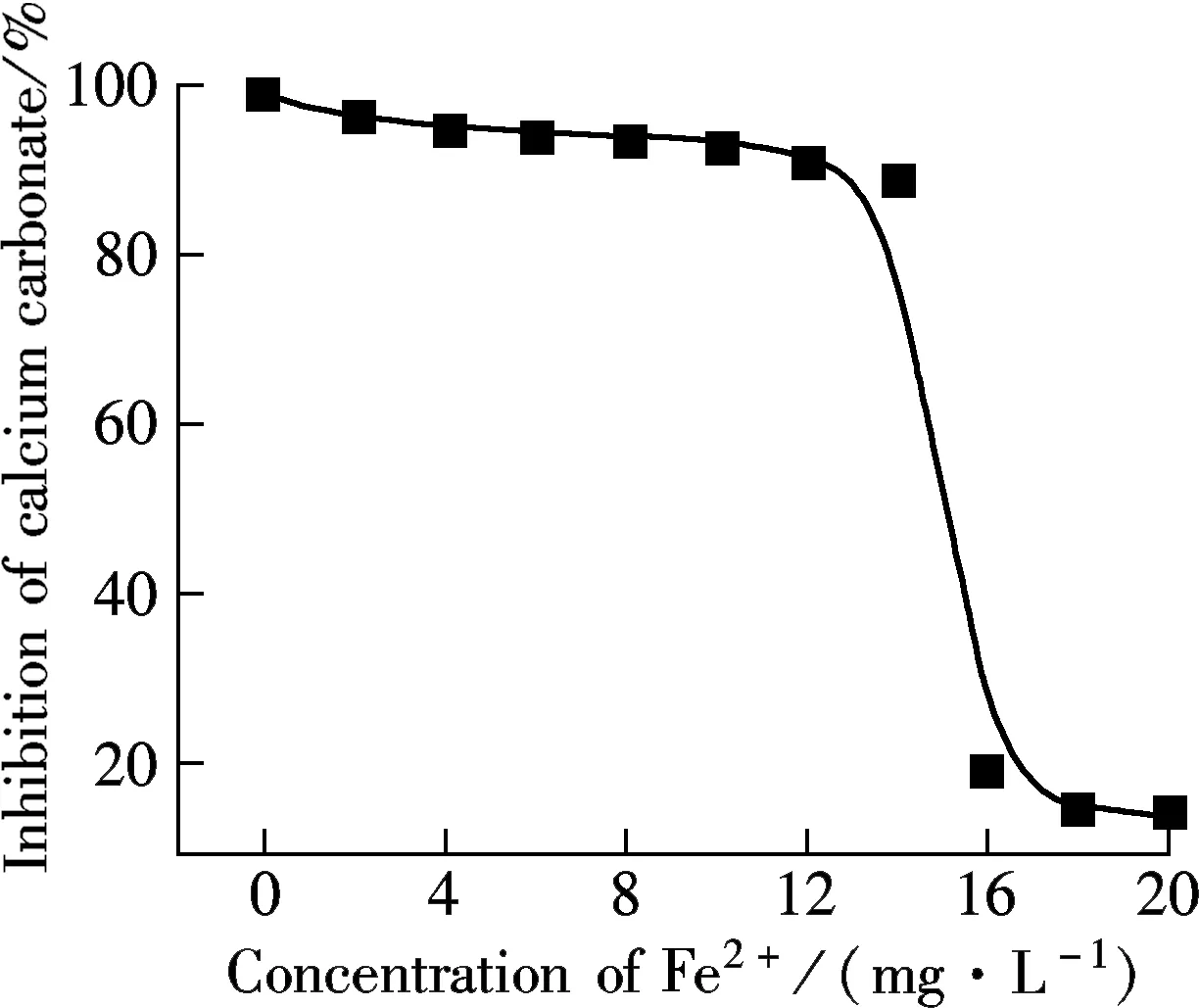
(d)
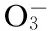
2.4 Characterization of scales
The CaCO3precipitated phases are identified by XRD, and the spectra are shown in Fig.3. In the absence of the AA-APEL copolymer, calcite is the main crystal form (see curve a). As shown in curve b, in the presence of the AA-APEL copolymer, there are a number of vaterite crystals interlarding. The change of crystal forms was also confirmed by the FTIR spectra, as shown in Fig.4. As shown by the curve in Fig.4, the peaks at 876 and 712 cm-1reflect the feature of calcite, and the peaks at 1 490 and 745 cm-1reflect the feature of vaterite[10].
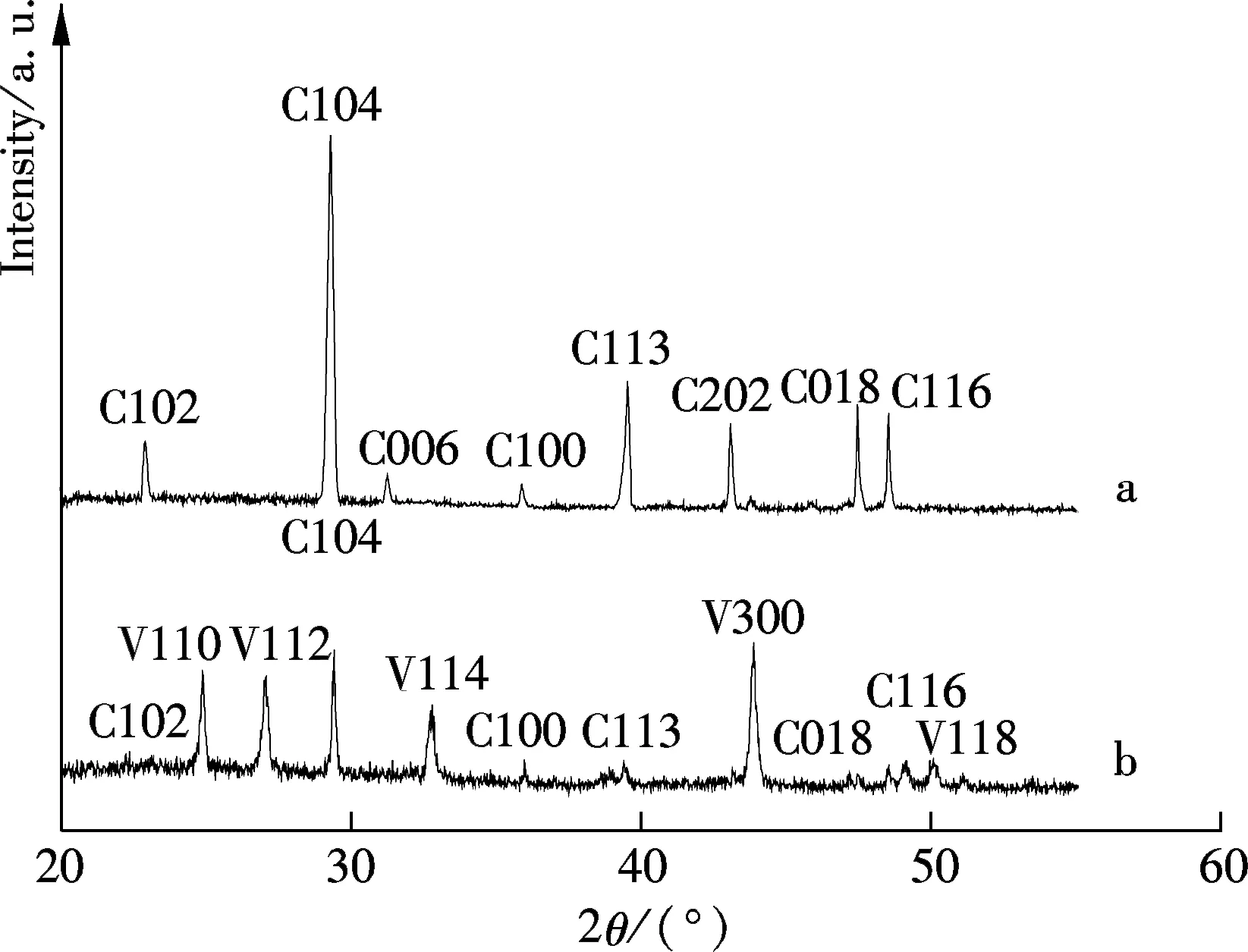
Fig.3 The XRD pattern of the CaCO3crystals without the copolymer (curve a) and with the copolymer (curve b) (C represents calcite and V represents vaterite)
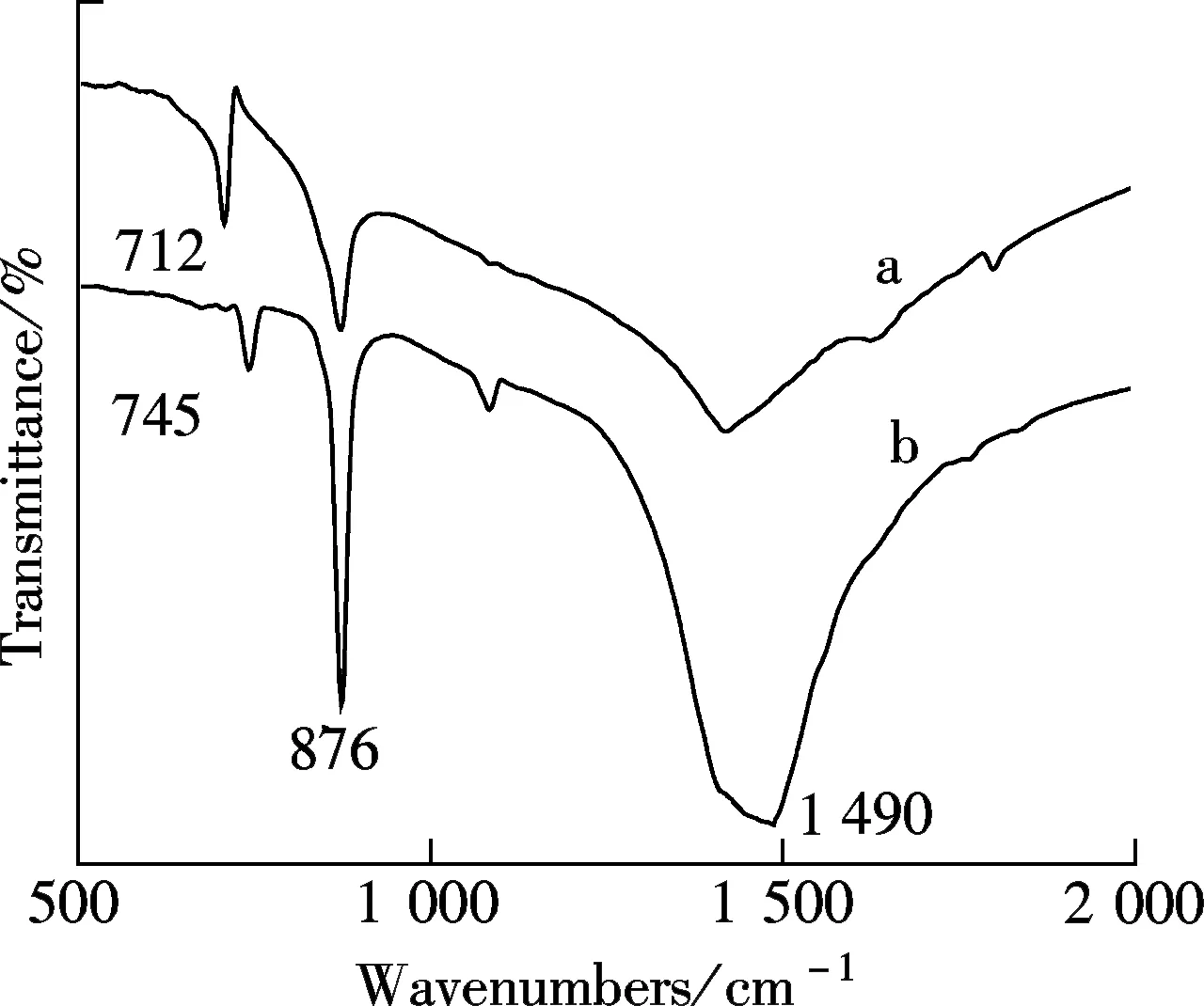
Fig.4 FTIR spectra of CaCO3precipitates in the absence of the copolymer (curve a) and in the presence of the copolymer (curve b)
It is well known that calcite is the most thermodynamically stable, and vaterite is the least stable form in the three polymorphic forms of CaCO3[10]. Vaterite is the initial phase formed when CaCO3supersaturated; calcite can be formed from the transformation of aragonite or vaterite in the absence of inhibitors[11]. The calcium carbonate precipitate obtained from the artificial cooling water without the AA-APEL copolymer has XRD patterns (see curve a in Fig.3) corresponding to calcite. Curve b in Fig.3 shows the X-ray diffraction spectrum for CaCO3precipitates in the presence of the AA-APEL copolymer, in which there are the (110), (112), (114), (300), and (118) weak peaks corresponding to vaterite. It is found that thermodynamically unstable vaterite can be stabilized kinetically in the presence of the AA-APEL copolymer.
Fig.5 shows the SEM micrographs of CaCO3precipitate formed in artificial cooling water. In the presence of the AA-APEL copolymer (see Fig.5(b)), obvious changes can be noted in distribution, size, and morphology of the CaCO3precipitate compared to Fig.5(a). The CaCO3precipitate lost its sharp edges, and the form was distorted. In addition, its size was decreased to 1 to 3 μm, smaller than those deposited in the uninhibited solution.

(a) (b)
Fig.5 SEM micrographs of CaCO3precipitates. (a) In the absence of the copolymer; (b) In the presence of the copolymer
A typical TEM and corresponding diffraction pattern of orthorhombic calcite particles are shown in Figs.6(a) and (c). The orthorhombic type morphology is displayed in the TEM image and the diffraction pattern appears to be a perfect single crystal without hint of the underlying powder rings.
The TEM micrograph (see Fig.6(b)) in the presence of the AA-APEL copolymer indicates the excellent inhibition results from the formation of the flowers structure. It is worth paying attention to the fact that the spherical vaterite and calcite induced by AA-APEL is porous (see Fig.6(b)). These spheres are aggregations of smaller microcrystals and both the aperture and particle diameter are all in nanoscale (1 to 100 nm). From the selected area electron-diffraction patterns (see Fig.6(d)), where more or less uniform and slightly diffused rings were found, it was concluded that the crystallites were randomly oriented in the particle.
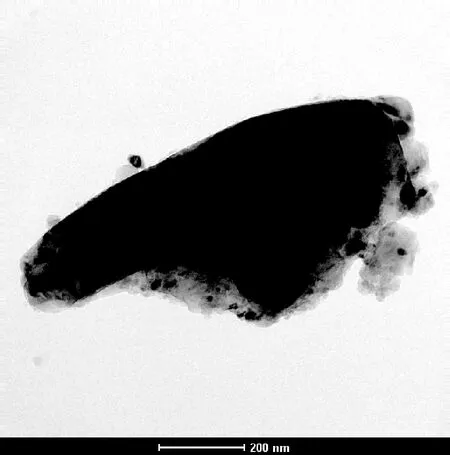
(a)
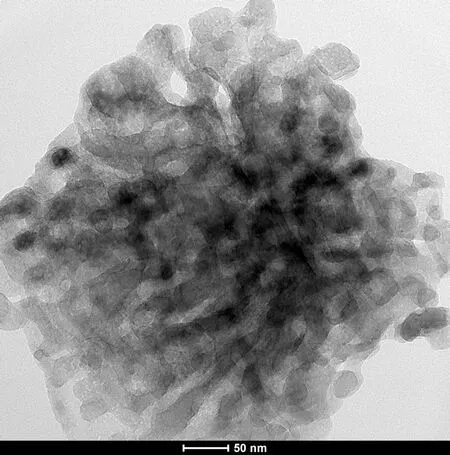
(b)
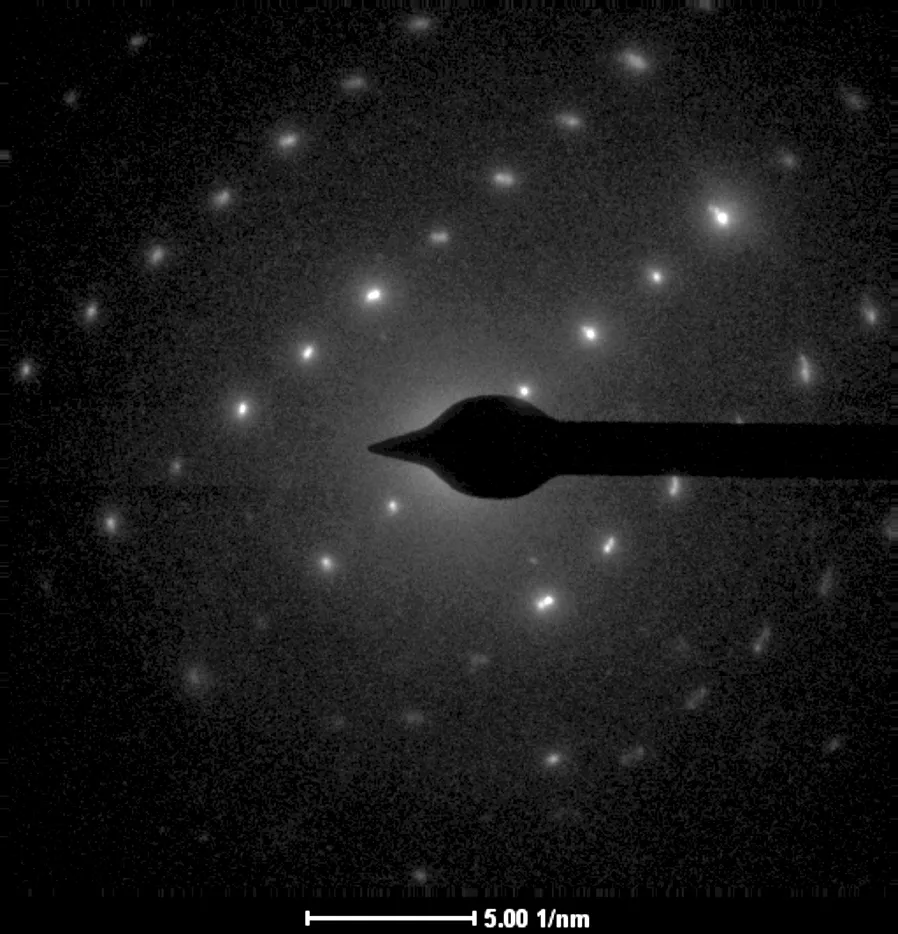
(c)

(d)
Fig.6 TEM micrograph and diffraction pattern of CaCO3precipitates. (a) TEM micrograph in the absence of the copolymer; (b) TEM micrograph in the presence of the copolymer; (c) Diffraction pattern in the absence of the copolymer; (d) Diffraction pattern in the presence of the copolymer
2.5 The mechanism of calcium carbonate and iron (Ⅲ) inhibition
Carboxyl segments and PEG are important parts in the matrices of AA-APEL. The functional groups of antiscalants exhibit a significant impact on their inhibitory power in terms of controlling scale precipitation[12]. PEG can recognize and encapsulate or react with positively charged calcium or iron ions on the surface of inorganic minerals, such as calcium carbonate, calcium sulphate and the like. Encapsulation or interaction, between the calcium ions and PEG, leads to the spontaneous formation of complex micelles and a core of PEG—Ca or PEG—Fe. At the same time, a stable corona of water-compatible carboxyl segments, surrounding the core, is formed towards the aqueous phase at the interface of the core. The core of PEG—Ca or PEG—Fe and the shell of carboxyl corona form the core-shell superstructure, where the aggregation of PEG—Ca or PEG—Fe is blocked through the steric and electrostatic repulsion of the —COO-corona[13]. The determinant during the formation process of core-shell superstructures is the phase separation between the —COO-corona and the PEG—Ca or PEG—Fe core domain, requiring a regular array of the molecular junctions between the PEG and the —COO-segments. It should be mentioned that the carboxyl segments surrounding the core can also interact with calcium ions.
3 Conclusion
AA-APEL possesses excellent calcium carbonate inhibition, approximately 98% at a level of 8 mg/L. AA-APEL has superior ability when stabilizing iron (Ⅲ) in solution. The light transmittance of ferrous solutions is about 14% in the presence of AA-APEL when the dosage is 4 mg/L.The AA-APEL copolymer maintains most of the activity under the conditions of the solution with a pH of 6 to 12, a temperature of 80 to 95 ℃, a calcium hardness of 300 to 1 500 mg/L, and at levels of 0 to 14 mg/L iron ions in aqueous solutions.
To the best of our knowledge, no reference to the phosphorous free and non-nitrogen copolymer of acrylic acid and allylpolyethoxy carboxylate used as a calcium carbonate scale inhibitor in cooling water has been found in the literature; and it is believed to represent a potentially new environmentally safe water treatment agent suitable for cooling water systems.
[1]Xyla A G, Mikroyannidis J, Koutsoukos P G. The inhibition of calcium carbonate precipitation in aqueous media by organophosphorus compounds[J].JournalofColloidandInterfaceScience, 1992, 153(2): 537-551.
[2]Kjellin P. X-ray diffraction and scanning electron microscopy studies of calcium carbonate electrodeposited on a steel surface[J].ColloidsandSurfacesA:PhysicochemicalandEngineeringAspects, 2003, 212(1): 19-26.
[3]Kumar T, Vishwanatham S S, Du K. A laboratory study on pteroyl-L-glutamic acid as a scale prevention inhibitor of calcium carbonate in aqueous solution of synthetic produced water[J].JournalofPetroleumScienceandEngineering, 2010, 71(1/2): 1-7.
[4]Zhou X H, Sun Y H, Wang Y Z. Inhibition and dispersion of polyepoxysuccinate as a scale inhibitor[J].JournalofEnvironmentalSciences, 2011, 23(Sup): 159-161.
[5]Suharso, Buhani, Bahri S, et al. Gambier extracts as an inhibitor of calcium carbonate (CaCO3) scale formation[J].Desalination, 2011, 265(1/2/3): 102-106.
[7]Du K, Zhou Y M, Wang Y Y. Fluorescent-tagged no phosphate and nitrogen free calcium phosphate scale inhibitor for cooling water systems[J].JournalofAppliedPolymerScience, 2009, 113(3): 1966-1974.
[8]Pecheva E, Pramatarova L, Altankov G. Hydroxyapatite grown on a native extracellular matrix: initialinteractions with human fibroblasts[J].Langmuir, 2007, 23(18): 9386-9392.
[9]Amjad Z. Constant composition study of crystallite growth of calcium fluoride. Influence of poly (carboxylic acids), polyphosphates, phosphonates, and phytate[J].Langmuir, 1991, 7(3): 600-603.
[10]Ueyama N, Hosoi T, Yamada Y, et al. Calcium complexes of carboxylate-containing polyamide with sterically disposed NHO hydrogen bond: detection of the polyamide in calcium carbonate by 13C cross-polarization/magic angle spinning spectra[J].Macromolecules, 1998, 31(21): 7119-7126.
[11]Chakraborty D, Agarwal V K, Bhatia S K, et al. Steady-state transitions and polymorph transformations in continuous precipitation of calcium carbonate[J].Industrial&EngineeringChemistryResearch, 1994, 33(9): 2187-2197.
[12]Senthilmurugan B, Ghosh B, Sanker S. High performance maleic acid based oil well scale inhibitors—development and comparative evaluation[J].JournalofIndustrialandEngineeringChemistry, 2011, 17(3): 415-420.
[13]Harada A, Kataoka K. Novel polyion complex micelles entrapping enzyme molecules in the core: preparation of narrowly-distributed micelles from lysozyme and poly(ethylene glycol)-poly-(aspartic acid) block copolymer in aqueous medium[J].Macromolecules,1998, 31(2): 288-294.
环境友好型阻垢剂的合成及其在工业冷却水中的应用
黄镜怡1刘广卿1,2薛蒙伟2周钰明1,3姚清照1,3
(1东南大学化学化工学院, 南京 211189)(2南京晓庄学院生物化工与环境工程学院, 南京 211171)(3江苏省光电功能材料工程实验室, 南京 211189)
采用绿色聚醚封端技术合成了含聚氧乙烯基重复单元的大分子单体烯丙基聚氧乙烯基羧酸,然后与丙烯酸在水相中通过自由基聚合反应合成了一种新型的双亲水嵌段共聚物阻垢剂,并深入研究了其在循环冷却水系统中阻碳酸钙和分散氧化铁的性能.实验结果表明该共聚物具有优异的阻碳酸钙和分散氧化铁性能,XRD表征表明加入无磷非氮的共聚物阻垢剂得到的碳酸钙晶体在方解石结构中混有了部分球文石结构,通过红外光谱、扫描电子显微镜和透射电子显微镜同样可以证实碳酸钙晶体结构的变化.提出了聚乙二醇链段与钙离子通过相互作用来抑制水垢形成的基本动力机理.
无磷阻垢剂; 碳酸钙;分散铁;工业冷却水循环系统
TQ085
Received 2014-04-28.
Biographies:Huang Jingyi(1973—), female, doctor, lecturer; Liu Guangqing (corresponding author), male, doctor, lecturer, 0539liuguangqing@163.com.
s:The National Natural Science Foundation of China (No.51077013), China Postdoctoral Science Foundation (No.2014M560381), Jiangsu Planned Projects for Postdoctoral Research Funds(No.1401033B), Transformation Program of Science and Technology Achievements of Jiangsu Province (No.BA2011086), the 333 High-Level Talents Training Project of Jiangsu Province (No.BRA2010033), the Project of Young Scientist Foundation of Nanjing Xiaozhuang University (No.2013NXY89).
:Huang Jingyi, Liu Guangqing, Xue Mengwei, et al. Synthesis and application of an environmentally friendly antiscalant in industrial cooling systems[J].Journal of Southeast University (English Edition),2014,30(4):514-519.
10.3969/j.issn.1003-7985.2014.04.019
10.3969/j.issn.1003-7985.2014.04.019
 Journal of Southeast University(English Edition)2014年4期
Journal of Southeast University(English Edition)2014年4期
- Journal of Southeast University(English Edition)的其它文章
- Improved design of reconfigurable frequency response masking filters based on second-order cone programming
- Experimental study on thermal characteristicsof a double skin façade building
- Gene expression profile in H4IIE rat hepatoma cells exposed to an antifouling booster biocide Irgarol-1051 degradation product
- L(1,2)-edge-labeling for necklaces
- Design and implementation of multi-hop video transmission experiment system in VANET
- Measurement of spatiotemporal characteristics of femtosecond laser pulses by a modified single-shot autocorrelation
