Synthesis of Phenyl Acetate from Phenol and Acetic Anhydride over Synthetic TS-1 Containing Template
Wu Guowen; Shi Chunfeng; Lin Min; Zhu Bin
(SINOPEC Research Institue of Petroleum Processing, Beijing 100083)
Synthesis of Phenyl Acetate from Phenol and Acetic Anhydride over Synthetic TS-1 Containing Template
Wu Guowen; Shi Chunfeng; Lin Min; Zhu Bin
(SINOPEC Research Institue of Petroleum Processing, Beijing 100083)
In this article, phenyl acetate was synthesized from phenol and acetic anhydride over titanium silicalite-1, in which the organic structure-directing agents were immobilized (TS-1-U). The reaction conditions were specified at a phenol to acetic anhydride molar ratio of 1:1.2, a catalyst dosage of 6 m%, and a reaction temperature of 70 ℃. A total of 96.5% of phenol was converted to phenyl acetate without producing any byproducts after 2.5 h of reaction. Besides, although the catalyst deactivation is inevitable, TS-1-U could be recycled for at least four times.
phenyl acetate; preparation; titanium silicalite-1; catalyst
1 Introduction
Phenyl acetate is an important medicine intermediate which is usually used as the solvent in the industry. It can be transformed into the compound with the adjacent hydroxyphenyl ketone[1]which can be used to synthesize antiarrhythmics andp-hydroxyphenyl ketone, which is the material for preparation of anti-inflammatory drugs[2]. Both of them are important materials in the pharmaceutical industry. In other words, phenyl acetate has a wide range of medical applications.
One of the major methods for synthesizing phenyl acetate applied in industry is to prepare the sodium phenate firstly. To a mixture containing a proper proportion of phenol and sodium hydroxide, acetic anhydride is added, and then phenyl acetate can be obtained by a series of operations such as stirring, neutralization, and so on. Another pathway can use phenol and acetyl chloride as the starting materials to obtain phenyl acetate. Disadvantages exist in both of these two pathways. The first pathway is simple but a phenyl acetate yield of 77%[3]is unsatisfactory and it is not environmentally friendly. The other pathway can reach a yield of about 95% but the raw materials are costly and the corrosion of equipment is severe[4-5]. In addition, some catalysts with poor stability used in the reaction of phenol on acetic anhydride are deactivated easily[6]. For example, one of the catalysts containing SO42-/TiO2is unstable and can be easily inactivated with a short service life.
Therefore, it is still necessary to develop efficient methods and more effective catalysts to prepare phenyl acetate. As it is known to all, zeolite is widely used as catalytic material over the past thirty years. The zeolite family is crystalline, hydrated alumino-silicates with micropores having regular structure. The TS-1 zeolite is one of zeolites containing Ti and possessing advantages of efficiency and environmental friendliness. Intensive research on TS-1, a kind of zeolite with the MFI structure, has resulted in a deep understanding of aspects including its synthesis, structure, chemical and physical properties, and catalytic preformance[7-9]. There are some familiar methods for preparing the TS-1 zeolite. One major and classical pathway is called the “mixed alkoxide method.” According to this pathway, a crystallized gel can be obtained by the controlled hydrolysis of tetraethyltitanate and tetraethylsilicate in the presence of tetrapropylammonium hydroxide. Crystallization under hydrothermal conditions can produce crystalline TS-1-U (TS-1 containing the template), calcination of which can yield TS-1. The more appropriate and effective way for preparing synthetic titanium silicalite-1 has been explored and improved by virtue of the following aspects: (1) Controlling the hydrolysis rate ofreactants; (2) Reducing the cost by using cheaper templating agent; (3) Selecting the appropriate post-processing methods; and (4) Adoption of other factors. Among them, reducing the cost of synthesis process is one of the key issues, since the expensive templating agent contributes to the high production cost. Therefore, it is important and meaningful to look for more cheap and effective templating agent or to make better use of the templating agent.
TS-1 zeolite exhibits special effect in selective oxidation reactions like the hydroxylation of phenol, benzene, and ammoximation of cyclohexanone in the presence of hydrogen peroxide in the liquid phase[10-17]. It has also been researched in other reactions to serve as the catalyst. But in the present work, the TS-1 zeolite is seldom used in the esteri fication reaction, in particular the phenol esterification reaction. Upon considering the waste templating agent obtained during calcining TS-1-U to TS-1, we can try to use TS-1-U as the catalyst to prepare phenyl acetate. And it is necessary to point out that the TS-1 zeolite can still been used in other catalytic oxidation reactions after recovery of TS-1-U followed by calcination process. However, there are few reports relating to the use of TS-1-U in esteri fication reaction. From that point, this paper describes an effective method for synthesis of phenyl acetate from phenol and acetic anhydride catalyzed by TS-1-U. The effects of different factors are discussed in detail including the reaction temperature, the reaction time, the different proportion of materials, and other factors. And the appropriate conditions will be studied in detail.
2 Experimental
2.1 Materials and reagents
Both of phenol and acetic anhydride were purchased from Beijing Yili Chemical Co, Ltd. The catalyst in this reaction was TS-1-U obtained from our own lab.
2.2 Preparation of phenyl acetate
The reaction of these reagents was carried out in a 250-mL flask. First of all, phenol and acetic anhydride were mixed with the catalyst TS-1-U at a proper mass ratio. And then the mixture was stirred at a specified temperature for a definite period of time. After the reaction, the catalyst in the resultant liquid was filtered out and the residues were analyzed by gas chromatography. The catalyst filtered out was washed repeatedly with water and then dried in a drying oven at 100 ℃ for 10 h for reutilization.
2.3 Characterization and product analysis
Phenyl acetate was quantitatively analyzed by GC (gas chromatography). Phenyl acetate was measured quantitatively via the area normalization method, and the selectivity was calculated on the basis of phenol consumption. The selectivity of phenyl acetate was 100% as the sole product (phenyl acetate) obtained.
The conversion of phenol is defined as follows:
Conversion of phenol=(moles of phenol consumed/moles of phenol charged)×100%.
3 Results and Discussion
3.1 Effect of solvent
The effect of common solvents on the catalytic performance of TS-1-U was tested, and the result is presented in Figure 1.
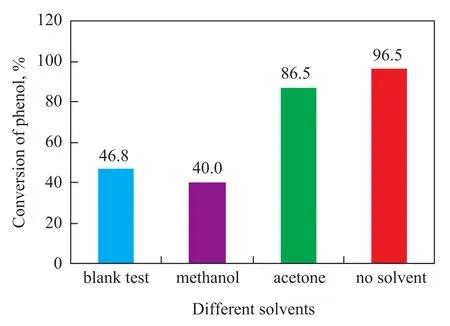
Figure 1 The influence of different solvents
Some common solvents including methanol and acetone were tested. Reaction was carried out at a reaction temperature of 70 ℃, a reaction time of 2.5 h, and a molar ratio of phenol to acetic anhydride of 1:1.2, with the mass fraction of TS-1-U equating to 6%. It is clear that the conversion of phenol while using methanol was inferior as compared to the blank test, which might be caused by the reaction of acetic acid on methanol. This reaction could reduce the concentration of acetic acid. And it is clear that the result of solvent-free condition was better than the outcome of reaction using the solvent. Phenyl acetate selectivity was 100% as there was only one product. There-fore, the following tests were all conducted without using the solvent.
3.2 Effect of reaction temperature
The effect of reaction temperature on the catalytic performance of TS-1-U was tested, and the test results along with the outcome of blank test are presented in Figure 2.
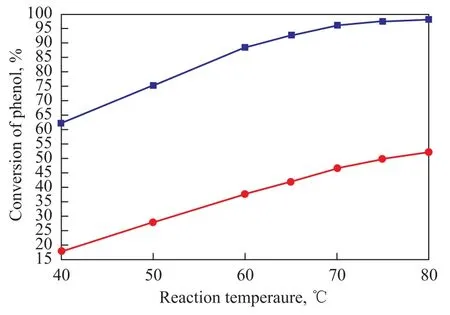
Figure 2 Influence of reaction temperature on the catalytic performance of TS-1-U
These results were obtained without using any solvent at a reaction time of 2.5 h, a phenol/acetic anhydride molar ratio of 1:1.2, and a mass fraction of TS-1-U equating to 6%.
It suggested that phenol conversion tended to go up with the reaction temperature increasing from 40 ℃ to 80 ℃, while the phenyl acetate selectivity still reached 100%. Compared with the blank test, these experiments conducted over TS-1-U were far more efficient. The conversion of phenol was about 65% at 40 ℃ but reached over 96% at 70 ℃, and the conversion almost did not increase when the temperature rose beyond 70 ℃. Therefore, the appropriate reaction temperature was 70 ℃ in this reaction when TS-1-U was used.
3.3 Effect of reaction time
Reaction time is also an important factor in many chemical reactions. Figure 3 shows the effect of reaction time on the catalytic performance of TS-1-U. And the blank experiments affected by the reaction time were also conducted, with the result shown in Figure 3.
This result was obtained without using any solvent under the following reaction conditions: a reaction temperature of 70 ℃, a phenol/acetic anhydride molar ratio of 1:1.2, and a TS-1-U amount of 6 m%. The phenyl acetate selectivity was still 100%. Phenol conversion rate increased rapidly at first, and then decreased slowly when the reaction time was beyond 2 h. The conversion rate of phenol was about 96.6% at 2.5 h and then had a slight increase to reach a maximum of 97.2% at 5 h. The result of blank test showed that the phenol conversion rate was rising at the beginning and then falling. However, the effect of catalyst was still obvious. In other words, there was a sharp rise of phenol conversion rate in the reaction mixture containing TS-1-U catalyst. Therefore, the suitable reaction time was chosen as 2.5 h.
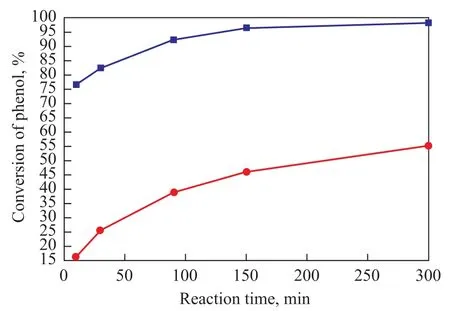
Figure 3 Influence of reaction time on the catalytic performance of TS-1-U
3.4 Effect of catalyst amount
The effect of catalyst amount on the synthesis of phenyl acetate was tested, with the result presented in Figure 4.
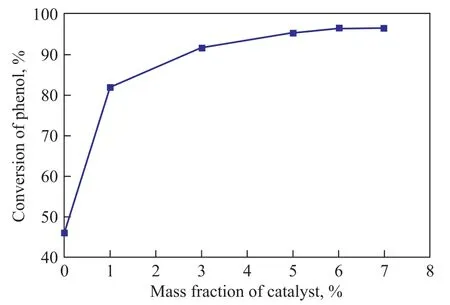
Figure 4 Influence of catalyst amount on the reaction for synthesis of phenyl acetate
This result was obtained without using any solvent under the following reaction conditions: a reaction temperature of 70 ℃, a reaction time of 2.5 h, a phenol/acetic an-hydride molar ratio of 1:1.2, and a TS-1-U amount of 6% (where 1% was equal to 0.09 g of catalyst). The phenyl acetate selectivity was still 100%. It can be identified that the phenol conversion rose with an increasing mass fraction of TS-1-U used, and reached its highest value of 96.1% at a catalyst mass fraction of 6%. Then a maximum phenol conversion was reached, when the mass fraction of TS-1-U increased beyond 6%. Economic considerations would suggest the viable mass fraction of TS-1-U to be 6%.
3.5 Effect of molar ratio of phenol to acetic anhydride
Figure 5 illustrates the effect of molar ratio of phenol to acetic anhydride on the catalytic performance of TS-1-U.
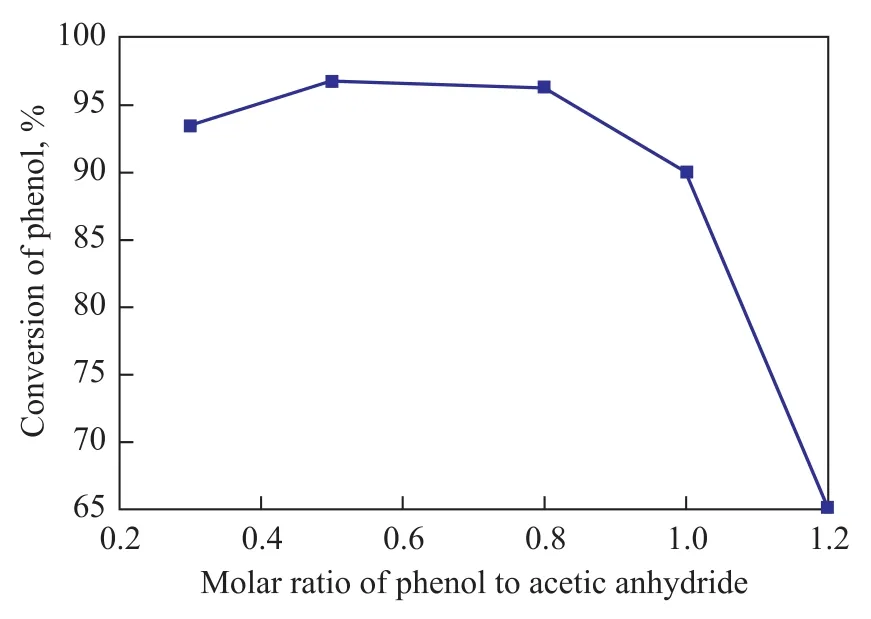
Figure 5 Influence of the molar ratio of reactants on the performance of TS-1-U
This result was obtained without using any solvent under the following reaction conditions: a reaction temperature of 70 ℃, a reaction time of 2.5 h, and a mass fraction of TS-1-U equating to 6%. It is clear that when the molar ratio of phenol/acetic anhydride was greater than 1:1, a lower phenol conversion was identified due to the fact that the concentration of acetic anhydride was not enough. The theoretic proportion of phenol versus acetic anhydride was 1:1 as shown in Equation 1. The phenol conversion increased with a decreasing molar ratio of phenol/acetic anhydride and reached the maximum at a molar ratio of 1:1.2. The phenol conversion decreased slightly with a further decrease in the molar ratio of phenol/acetic anhydride, which occurred possibly because the equilibrium reaction between acetic anhydride and acetic acid proceeded along the direction of Equation 2 as shown below. This reaction could also take place owing to the reaction between the alkaline template in TS-1-U and an excessive amount of acetic acid. A large amount of acetic anhydride could result in more acetic acid because of the hydrolysis of acetic anhydride, which would inhibit the conversion of phenol.
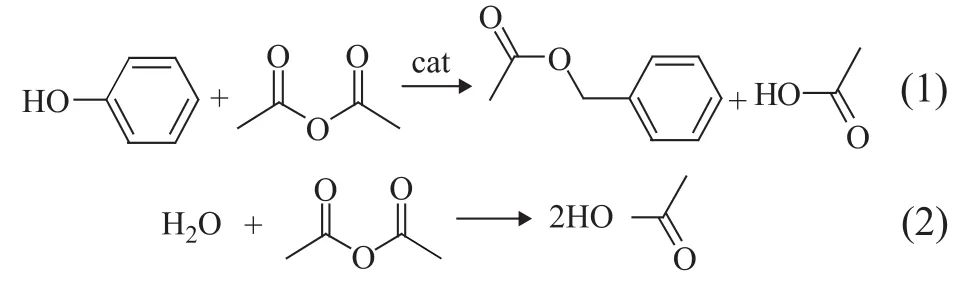
In conclusion, during the synthesis of phenyl acetate catalyzed by TS-1-U, the proper reaction conditions should be: a reaction temperature of 70 ℃, a reaction time of 2.5 h, a TS-1-U mass fraction of 6%, and a molar ratio of phenol to acid anhydride of 1:1.2 without using any solvent. The conversion of phenol was 96.5%, and the selectivity of phenyl acetate was 100%.
3.6 Recycle of TS-1-U
After completion of the reaction, the reaction mixture was filtered and washed with distilled water and dried at 100 ℃ for 12 h to recover the catalyst. The phenol conversion achieved over the recovered TS-1-U was 67.3% through this process. Compared with the result of blank test (46.1%), the phenol conversion was still higher. The results in Table 1 shows that the recovered TS-1-U can be used once more but there was a significant loss in catalytic performance of TS-1-U, especially in the second time for application of recycled catalyst. The loss of catalytic performance may be resulted from the loss of surface hydroxyl ions of templating agent which was not so stable compared with the internal hydroxyl ions. It is wellknown that the template is a kind of organic alkali and the esterification reaction can be catalyzed by both acids and bases. Some OH- ions of template on the surface of TS-1-U can be easily lost because the different chemical environments surrounding the internal template can affect the catalytic activity.
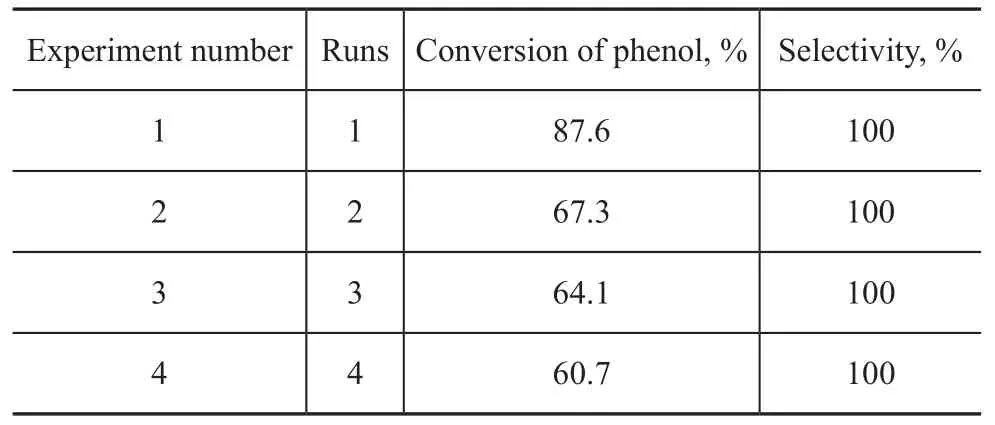
Table 1 Reuse of TS-1-U
4 Conclusions
Phenyl acetate was synthesized from phenol and acetic anhydride over the TS-1-U catalyst. By investigating different reaction conditions, a most suitable process regime was obtained as follows. When the molar ratio of phenol to acetic anhydride was 1:1.2, the quantity of catalyst adopted should be 6%, and the reaction temperature was chosen as 70 ℃ without using any solvent. And under these conditions, 96.5% of phenol was converted to phenyl acetate without any byproducts after 2.5 h of reaction. Furthermore, the catalytic effect of TS-1-U showed a tendency to decline but was still superior to the performance achieved in the blank test. And there was no significant loss in the catalytic performance except for the first run of recycled catalyst. With regard to this study, the outcome of reaction could provide valuable background information for further research, and would be beneficial to both of the pharmaceutical industry and the application of TS-1 zeolite.
Acknowledgement:The project is financially supported by the SINOPEC (Contact No. S413025).
[1] Bian W Y, Chen X Q. Zeolite HL phenyl acetate catalyzed Fries rearrangement[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2006, 22(S): 304-307 (in Chinese)
[2] Wang S Q, Gao C. The synthesis of medical intermediate benzyl acetate[J]. Advances in Fine Petrochemicals, 2007, 8(5):41-43 (in Chinese)
[3] Xu K X. Fine Organic Chemical Raw Materials and Intermediates Notebook[M]. Beijing: China Petrochemical Publishing House, 1988: 377-379 (in Chinese)
[4] Chang Y, Liu X W, Xu H. The synthesis of medical intermediate benzyl acetate[J]. Chemical Production and Technology, 2009, 16(4):13-15 (in Chinese)
[5] Wu D Y, Liu X H, Xing J, et al. Research on the new methods of phenyl acetate synthesis[J]. Applied Science and Technology, 2002, 29(6):54-55 (in Chinese)
[6] Yuan G. Solid super acid SO42-/TiO2catalyzes the synthesis of phenyl acetate[J]. Industrial Catalysis, 2009, 17(9): 62-66 (in Chinese)
[7] Anastas T, Bartlett B, Kirchhoff M, et al. The role of catalysis in the design, development, and implementation of green chemistry[J]. Catalysis Today, 2000, 55(1): 18-22
[8] Gang L, Xinwen G, Xiangsheng W, et al. Synthesis of titanium silicalites in different template systems and their catalytic performance[J]. Applied Catalysis A: General, 1999, 185(1): 17-20
[9] Taramasso M, Perego G, Notari B. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides: The United States, US 4410501[P]. 1983
[10] Taramasso M, Perogo G, Notari B. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides: The United States, US 4410501[P], 1983-11-18
[11] Khomane R B, Kulkarni B D, Paraskar A, et al. Synthesis, characterization and catalytic performance of titanium silicalite-1 prepared in micellar media[J]. Mater Chem Phys, 2002, 76(1): 99-103
[12] Gabriele Centi, Siglinda Perathoner. Oxidation catalysts: New trends[J]. Current Opinion in Solid State and Materials Science, 1999, 4(1): 60-75
[13] Davia J, Liu Zhufang. Titania-silica: A model binary oxide catalyst system[J]. Chem Mater, 1997, 9(11): 2310-2330
[14] Lin Min, Li Hua, Wang Wei, et al. The preparation of propylene oxide by propylene epoxidation with hydrogen peroxide in 1.0 kt/a pilot plant[J]. Petroleum Processing and Petrochemicals, 2013, 44(3): 1-5 (in Chinese)
[15] Yang Hui, Jin Peng, Zheng Xiaoguang, et al. Preparation of tianium silicalite, characterization and its application in propylene epoxidation with hydrogen peroxide[J]. Petroleum Processing and Petrochemicals, 2013, 44(6): 59-63 (in Chinese)
[16] Wu Ting, Wen Yiqiang, Liu Meng, et al. Characterization and performance evaluation of large crystal size ts-1 molecular sieve prepared from inorganic materials for ammoximation of cyclohexanone[J]. Petroleum Processing and Petrochemicals, 2013, 44(3): 54-59 (in Chinese)
[17] Li Yichuan, Shen Benxian, Xiao Weiguo, et al. Commercial test of kilo-ton scale direct propylene epoxidation[J]. Petroleum Processing and Petrochemicals, 2013,V44(4): 8-12 (in Chinese)
Received date: 2014-03-10; Accepted date: 2014-10-22.
Dr. Lin Min, Telephone: +86-10-82368801; E-mail: linmin.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Comparative Studies on Low Noise Greases Operating under High Temperature Oxidation Conditions
- A Method for Crude Oil Selection and Blending Optimization Based on Improved Cuckoo Search Algorithm
- Experimental Research on Pore Structure and Gas Adsorption Characteristic of Deformed Coal
- Mathematical Model of Natural Gas Desulfurization Based on Membrane Absorption
- Ni2P-MoS2/γ-Al2O3Catalyst for Deep Hydrodesulfurization via the Hydrogenation Reaction Pathway
- Effects of Airflow Field on Droplets Diameter inside the Corrugated Packing of a Rotating Packed Bed

