Experimental Research on Pore Structure and Gas Adsorption Characteristic of Deformed Coal
Guo Deyong; Guo Li; Miao Xinhui
(School of Resource and Safety Engineering, China University of Mining and Technology (Beijing), Beijing 100083)
Experimental Research on Pore Structure and Gas Adsorption Characteristic of Deformed Coal
Guo Deyong; Guo Li; Miao Xinhui
(School of Resource and Safety Engineering, China University of Mining and Technology (Beijing), Beijing 100083)
The pore structure and gas adsorption property of deformed coal with different degrees of metamorphism were tested by low-temperature nitrogen adsorption and isothermal adsorption experiments. The fractal theory and the Langmuir adsorption theory were used to analyze the experimental data. The test results showed that the deformed coal had more heterogeneous pore structures and open pores, and its specific surface area (SSA) and fractal dimension (D) were higher. There is a polynomial relationship betweenDand specific surface area as well as gas adsorption capacity (VL). The gas adsorption capacity of deformed coal is influenced by pore structure, coal rank, deformation and stress together, among which the pore structure is the main influencing factor for the adsorption capacity of deformed coal. The test pressure could affect the accuracy of the adsorption constantsaandb, so the highest experiment pressure should be greater than the actual pressure of coal seam in order to reduce the deviation of adsorption constants.
deformed coal; pore structure; nitrogen adsorption; isothermal adsorption
1 Introduction
As a porous medium, coal has various pores of different sizes that are formed by organic matters and mineral matters on the surface and body of coal[1], which ranges from 10-10m to the micron level[2]. Nanometer-scale pores are generally studied in the adsorption experiments. The internal surface of coal pores is the main space available for methane adsorption[3]. The size, shape and development of coal pores directly affect the coal property for adsorption and desorption of methane[4-5]. The research on coal pore characteristics is an important approach for interpreting the gas adsorption of coal and ascertaining the mechanism of migration and flow of gas in coal seams.
Deformed coal is the coal whose primary structure is damaged by tectonism[6-7]. Because of suffering from the action of tectonic stress, the deformed coal is characterized by low strength, high energy storage, quick desorption, low permeability, etc., and its gas adsorption capacity is also changed. Coal and gas outburst occurs mainly in deformed coal development zones. Coal adsorptivity is affected comprehensively by multiple factors such as pore structure, coal rock composition, coal metamorphism degree, coal mass deformation, geologic structure, temperature, pressure, etc. The development of deformed coal reduces the gas permeability of coal seams and has an important impact on coal seam gas extraction[8]. The research on the pore structure and gas adsorption of deformed coal through low temperature liquid nitrogen adsorption experiments and isothermal adsorption experiments is of great guiding significance for the prediction of coal and gas outburst and coal seam gas extraction.
2 Experimental
2.1 Coal sampling and coal quality analysis
Coal samples were collected from Jiaozuo (JZ) Jiulishan Coalmine, Jiaozuo (JZ) Guhanshan Coalmine, Pingdingshan (PDS) No.8 Coalmine, Pingdingshan (PDS) No.10 Coalmine and Zhengzhou (ZZ) Chaohua Coalmine. Five sets of deformed coal samples and corresponding undeformed coal samples were selected for experimental study, with the coal quality analyzed (Table 1).
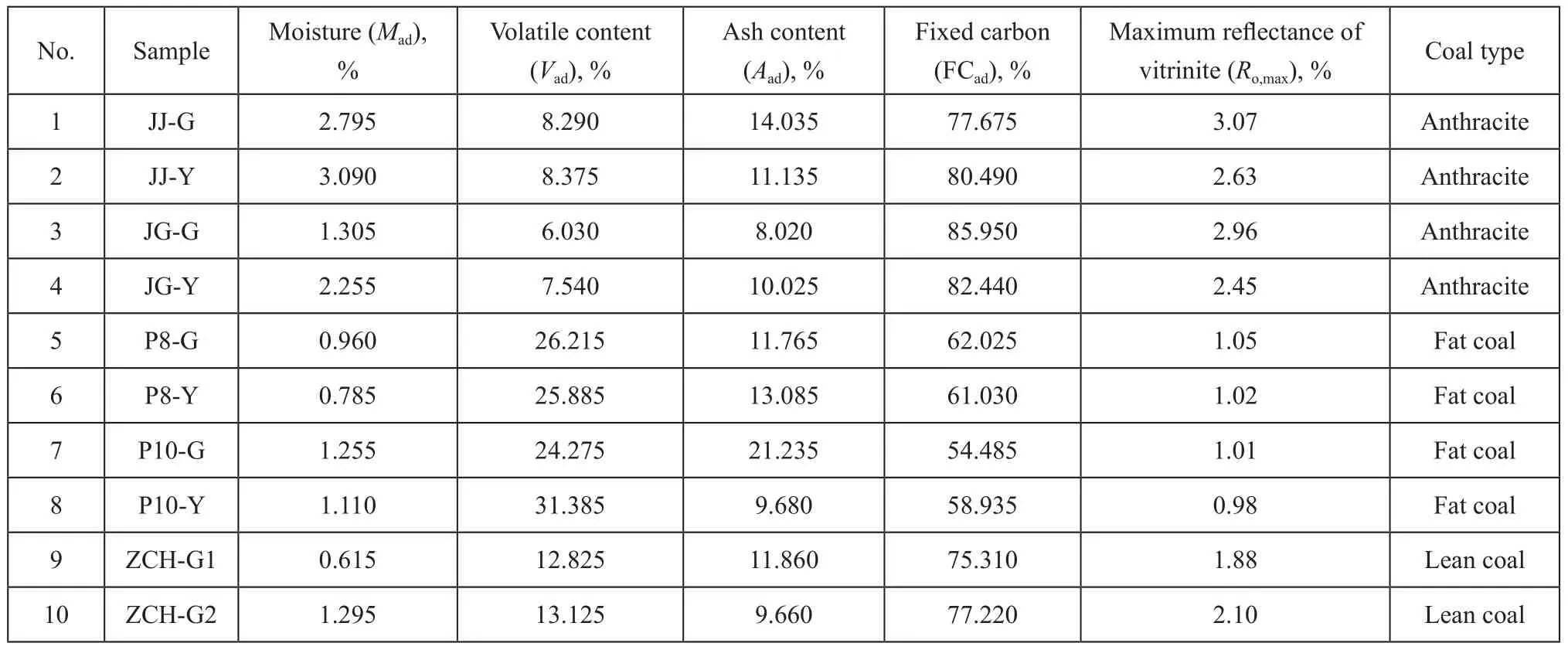
Table 1 Quality analysis parameters of coal samples used in experiments
2.2 Low temperature liquid nitrogen adsorption experiment
The instrument used in the experiment is an ASAP2000 type specific surface area and pore size distribution tester manufactured by the Micromeritics Instrument Corporation, USA. The coal samples before testing in the experiments were dried. The theoretical basis for this method is the law of gas adsorption on solid surface and actually the classical volumetric method[9]. The measured pore size range is from 1.43 nm to 160 nm. A classification from Hodot[10]for coal pore size is used in this work, which defines micropores (<10 nm), mesopores (10—100 nm), and macropores (>100 nm).
2.3 Isothermal adsorption experiment
The isothermal adsorption experiment of coal is an important method for coal seam gas testing. A WY-98B adsorption constant tester was used in the experiments to simulate the adsorption of methane by coal when the specified temperature is 30 ℃. Coal adsorptivity is a function of the coal’s physical property and external temperature, pressure and adsorption medium. In case of a given temperature and adsorption medium, the law of gas adsorption by coal can be expressed in Langmuir equation:V=VL/ (PL+P). Leta=VLandb= 1/PL; then this equation is converted toP/V=P/a+1/(ab). As known from references[11-13], the value ofais a saturated adsorption quantity, reflecting that coal has the maximum adsorptivity; the value ofbis the ratio of adsorption rate to desorption rate, reflecting the velocity and difficulty of gas adsorption by the internal surface of coal.aandbare adsorption constants and are common parameters for calculating coal seam gas content in actual coalmine work.
3 Results and Discussion
3.1 Low temperature liquid N2adsorption
3.1.1 Low temperature liquid N2adsorption experiments
The test results obtained from the low temperature liquid nitrogen adsorption experiments are shown in Table 2.
According to Table 2, the BET specific surface area of deformed coal is 3.5—35 times that of un-deformed coal, and the percentage of micropores of deformed coal is 1.1—5.0 times that of un-deformed coal. It can be known that the specific surface area of deformed coal is much larger than that of un-deformed coal, and the proportion of micropores of deformed coal is also much higher than that of un-deformed coal. This is because coal structure is damaged after being affected by the action of tectonic stress, and therefore the micropores developed in the deformed coal would increase, whereas lots of microporescould increase the specific surface area of coal. The total pore volume of un-deformed coal in both JZ coal sample and PDS coal sample is slightly larger than that of deformed coal because of the high proportion of mesopores in the un-deformed coal.
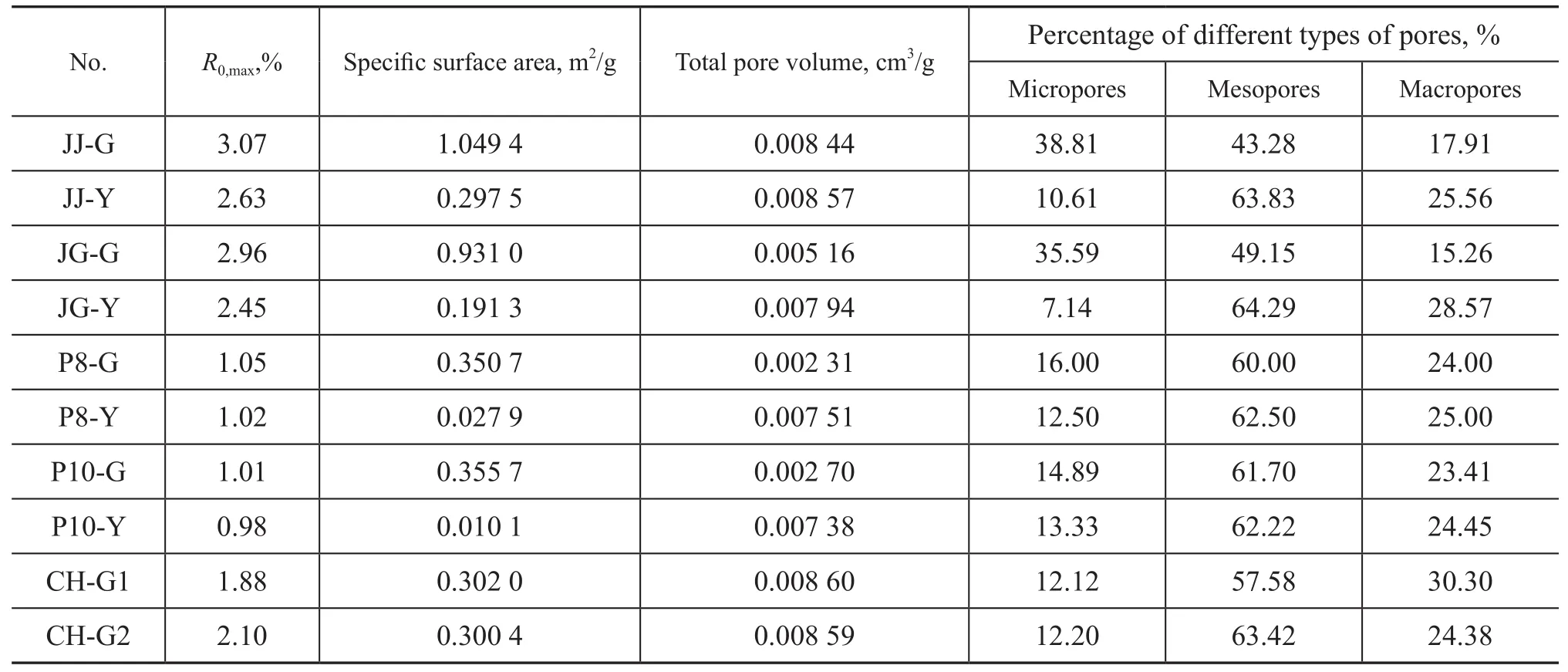
Table 2 Specific surface area and pore size distribution in coal measured by low temperature liquid nitrogen adsorption method
3.1.2 Analysis of the relationship between pore size and specific surface area
The experimental results indicate that the specific surface area of deformed coal is mainly composed of micropores and mesopores, but the specific surface area of the coal with different metamorphism degrees and structure types is slightly different. The pore size distribution (Figure 1 (a)) of JZ deformed coal shows a single-peak shape, in which micropores less than 5 nm in diameter provides the main specific surface area, and pores with a diameter of around 3.0 nm are quite developed. The main contributor to the specific surface area of ZZ Chaohua coal (Figure 1 (b)) comprises micropores with diameter < 10 nm, while mesopores also provide certain specific surface area. The pore size distribution (Figure 1 (c)) of PDS deformed coal is uniform, with micropores and mesopores jointly providing the main specific surface area, and the development degree of micropores is higher than that of mesopores. This indicates that the biggest contributor to gas adsorption in the deformed coal is micropores. Micropores are not only the main space for adsorbing gas but also function as gas motion media and connection channels of larger pores.
The pore size distribution of both JZ un-deformed coal (Figure 2 (a)) and PDS un-deformed coal (Figure 2 (b)) is uniform, where the specific surface area is provided jointly by micropores and mesopores, and the development degree of mesopores is higher than that of micropores. The proportion of mesopores in the un-deformed coal is larger, as compared to the greater ratio of micropores in the deformed coal, indicating that tectonically destructive effect changes the size, quantity and size distribution of pores.
3.1.3 Adsorption-desorption curves of coal and pore type analysis
Figure 3 shows the coal’s adsorption-desorption isotherm curves obtained by the low temperature liquid nitrogen experiments, which conform to the adsorption-condensation theory[14-15]. The pore shape structure of coal samples can be analyzed according to the adsorption loop in the research on coal’s pore structure[16-19]. In general, open pores can generate an adsorption loop, and the pores with one end closed would not generate an adsorption loop. In addition, although one end is closed, “ink bottle” pores can generate an adsorption loop due to their particularity, and an inflection point will appear on the adsorption loop. The adsorption-desorption curves of JZ deformed coal are shown in Figure 3(a), and the adsorption loop is very obvious. The first half section of the adsorption curve shows an upward convex shape when the relative pressure is lessthan 0.2 and then slowly rises; when the relative pressure reaches 0.9, it rises sharply, indicating to a sharp increase in adsorption quantity. This can be interpreted as follows. When the relative pressure is less than 0.2, gas is adsorbed mainly on micropores’ wall and gradually reaches a saturated state of single-layer adsorption. After condensation, nitrogen can humidify the pore wall. Therefore, as the relative pressure of gas increases gradually, the gas adsorption layer thickness on each pore wall would gradually increase accordingly; moreover, in case of reaching the critical relative pressure that corresponds to a pore size, capillary condensation also occurs. Small radius pores will be firstly full of condensed liquid. As the relative pressure of gas increases, the pores with slightly larger radius will also be filled with condensed liquid successively, and thereby the wall adsorption layer thickness of the pores with larger radius continues to increase. This is manifested by the fact that the second half section of the curve rises sharply at the position with a relative pressure being in the range of 0.9—1.0, indicating that capillary condensation occurs in the relatively large pores, resulting in sharp increase in adsorption quantity.
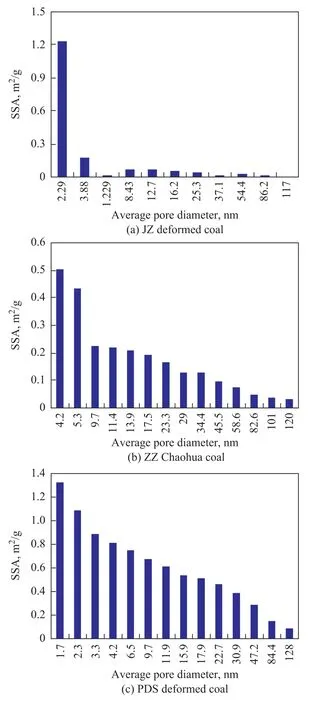
Figure 1 Relationship between specific surface area and pore size distribution of deformed coal
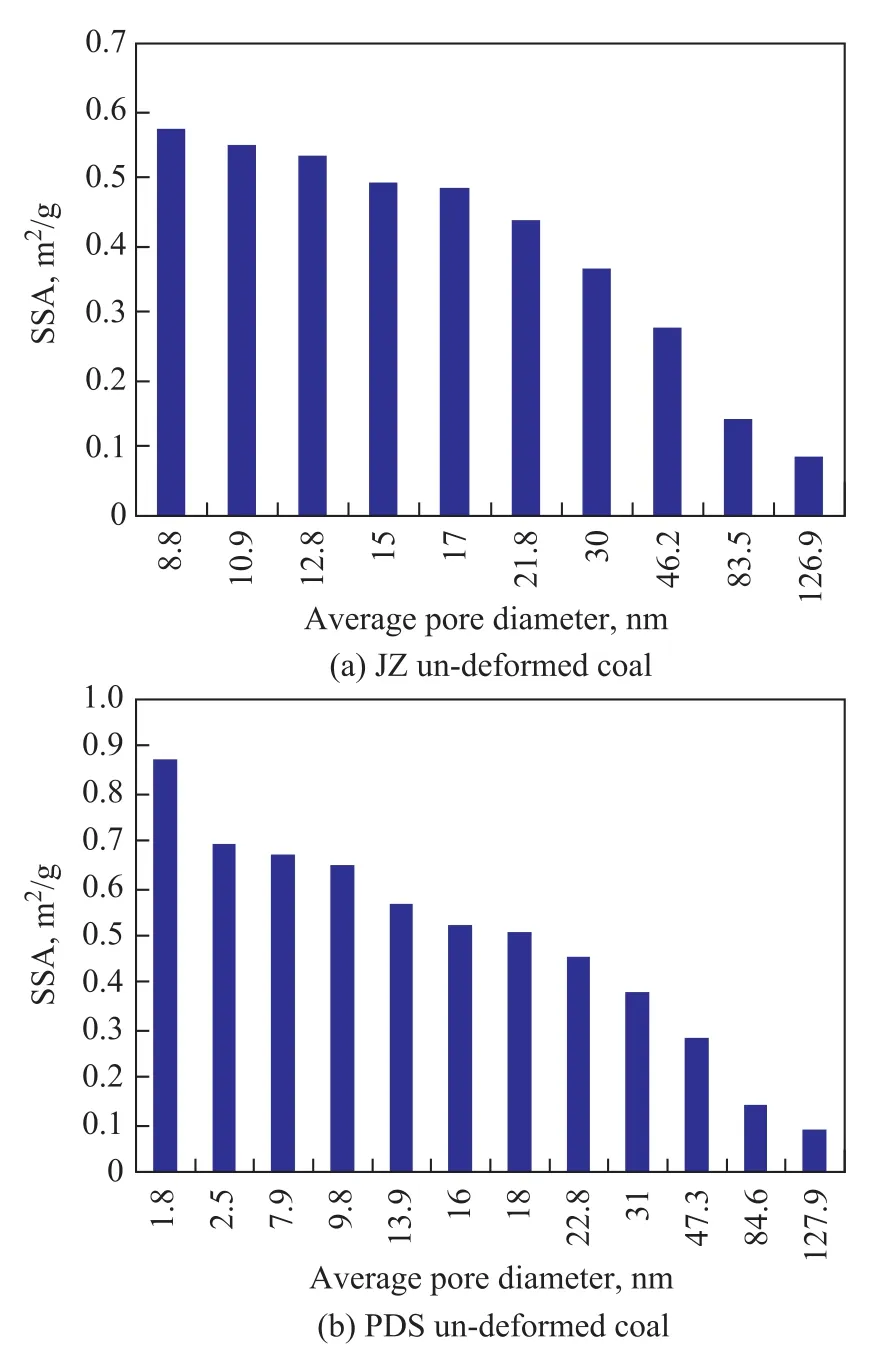
Figure 2 Relationship between specific surface area and pore size distribution of un-deformed coal
The adsorption loop (b) of ZZ Chaohua deformed coal is obvious, indicating that open pores and fine flasktype pores are developed. The adsorption and desorption curves (c) of other coal samples are separated at a relative pressure in the range of 0.60—0.98. The pore radius corresponding to a relative pressure of 0.60 calculated as per Kelvin equation is 1.86 nm, reflecting that the relativelysmall pores are gas-proof pores with one end closed. The adsorption loop has small separation scope and short distance, indicating that most of the pores with a radius exceeding 1.86 nm are the semi-closed pores which cannot generate an adsorption loop, and a part of them are the open pores which can generate an adsorption loop.
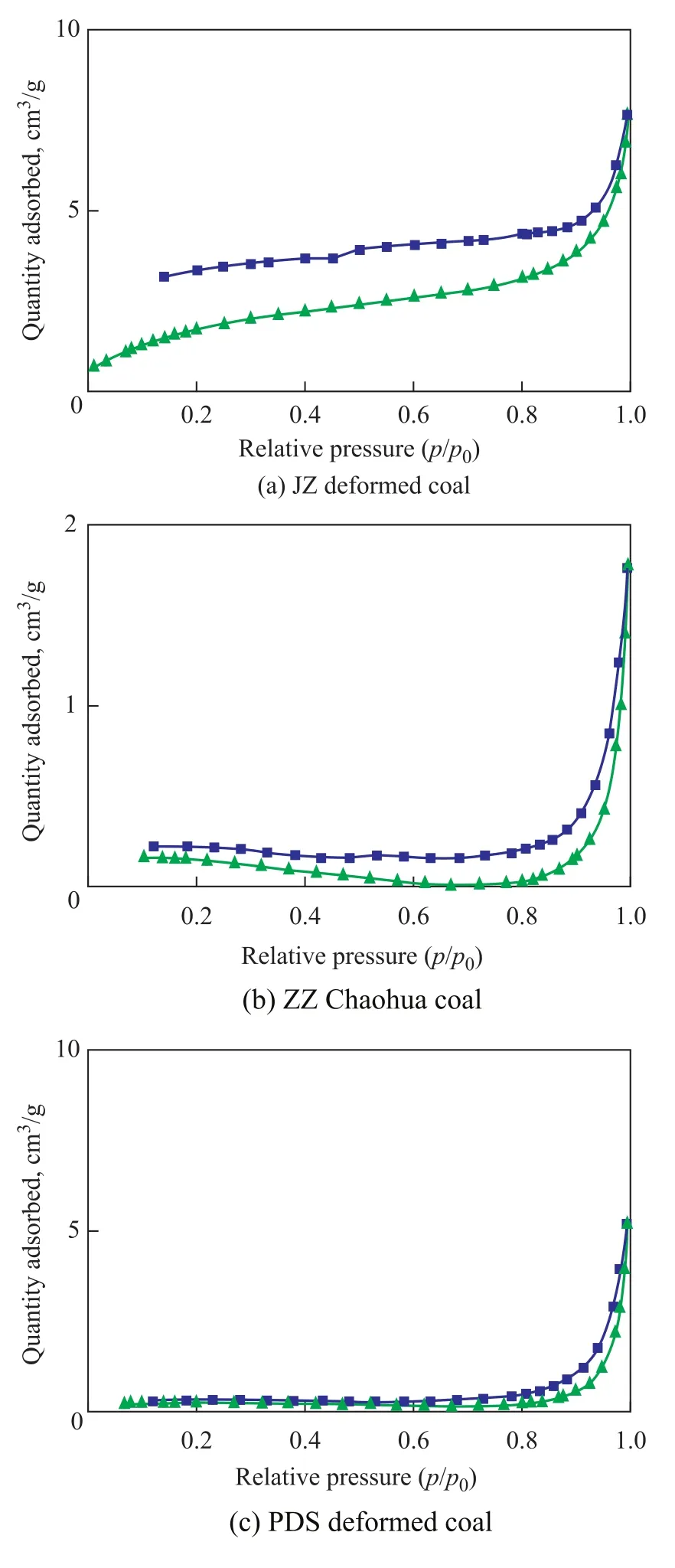
Figure 3 Low temperature liquid nitrogen adsorptiondesorption curves of the coal with different structures
3.1.4 Fractal characteristics of pores
Fractal dimensionD(hereinafter abbreviated asD) is a tool for analyzing the geometric and structural characteristics of surface and pore structure[20-23].Dis often used to describe the complexity of coal surface and pore characteristics: 2≤D≤3. WhenD=2, this indicates that coal surface is smooth. WhenD=3, this indicates that coal surface is so curly that its volume is filled.Din all other cases is meaningless[24].
According to the liquid nitrogen adsorption data, the fractal dimension results obtained using the Frenkel-Halsey-Hill (FHH) method[25-26]are shown in Table 3.
Table 3 shows thatDof the deformed coal is 2.46—2.67 and 2.530 8 on an average;Dof the un-deformed coal is 2.12—2.49 and 2.2656 on the average;Dof the deformed coal is larger than that of the corresponding un-deformed coal. As shown in Figure 4, the relationship betweenDand specific surface area (SSA) is a quadratic polynomial (R2=83%);Dfirstly increases quickly with an increasing specific surface area and then decreases slowly, and the maximum value ofDappears at the position of about 0.7 m2/g for the specific surface area. This is because both SSA andDhave their relationship with pore diameter distribution and the amount of the pores, thus SSA andDare highly relevant to each other. As shown in Figure 5, the relationship betweenDand gas adsorption quantity is a cubic polynomial (R2=86%). WhenVLis less than 42 mL/g or larger than 51 mL/g,Dincreases with anincreasingVL; whenVLis 42—51 mL/g,Dis decreased with an increasingVL. The inner surface is the main space of gas desorption and is also influenced by pore size and amount of pores. The relationship betweenDand gas adsorption amount can be explained by the restriction of pore structure together. By taking into account the previous research conclusions, SSA can be predicted byD, and the gas adsorption amount of the coal seam can be also estimated byD.

Table 3 Fractal dimension of different types of coal
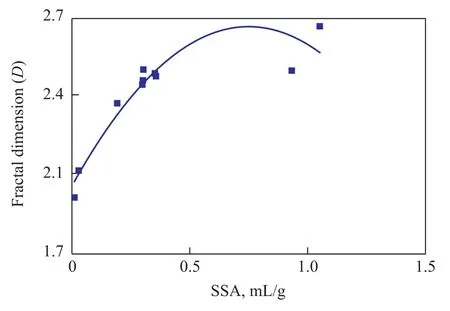
Figure 4 The relationship between specific surface area and fractal dimension
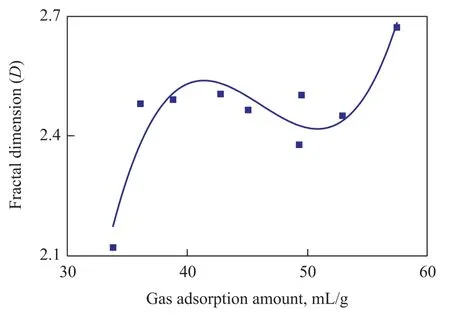
Figure 5 The relationship between gas adsorption quantity and fractal dimension
3.2 Isothermal adsorption
3.2.1 Effect of the maximum experiment pressure
The isothermal adsorption experiments on different types of deformed coal indicate that if the maximum experimental pressure fails to reach coal adsorption saturation, there would be large error in the obtained Langmuir adsorption constants; in addition, the law of the pressure variation needed for reaching saturated adsorption by the coal of different metamorphism degrees has been found.
Table 4 shows the Langmuir adsorption model parameters of 10 coal samples with different metamorphism degrees at different maximum pressures, andVL,PLand adsorption quantity of the 10 coal samples all increase with an increasing experimental pressure, indicating that pressure is one of the main influencing factors for coal adsorptivity. The value ofain JZ deformed coal is larger than that of the corresponding un-deformed coal, while the value ofbof the deformed coal is slightly larger than that of the corresponding un-deformed coal. The value ofain PDS deformed coal is larger than that of the corresponding undeformed coal, and the value ofbin the deformed coal is slightly less than that of the corresponding un-deformed coal. This indicates that the destructive effect of geologic structure has a little impact on the adsorption constants. The destructive effect of geologic structure slightly increases the adsorption constanta, i.e.: the gas adsorption quantity slightly increases. In different coalmine areas, tectonism has different influence on the adsorption constantb; but the influence is not significant.
The higher the pressure is, the closer the coal samples capable of reaching the saturation state and the more accurate the Langmuir adsorption constants would be. According to Table 4, it cannot be judged whether the maximum adsorption quantity of the coal samples at 6 MPa reaches the adsorption quantity at the original geostatic pressure. Therefore, in order to obtain more precise adsorption constants, the maximum pressure in this experiment should reach or be larger than the actual reservoir pressure as far as possible.
To discuss the accuracy of the maximum experimental pressure, an error analysis of the Langmuir adsorption model parameters at 4 MPa and 6 MPa, respectively, has been made. The relative error is based on the adsorption parameters obtained at 6 MPa. The result is shown in Table 5.
According to Table 5, when the experimental pressure is 4 MPa and 6 MPa, respectively, the absolute error of the obtained Langmuir adsorption model parameterVLis 0.04—1.06 cm3/g and 0.39 cm3/g on an average and its relative error is 1.10%—3.90% and 2.27% on an average; the absolute error ofPLis 0—0.48 MPa and 0.11 MPa on an average, and its relative error is 0—7.90% and 3.73% on the average. Therefore, the experimental pressure should be higher than the actual pressure of coal seamsduring the isothermal adsorption experiment by simulating the temperature and pressure conditions of deep coal seams in order to obtain more accurate experimental data.
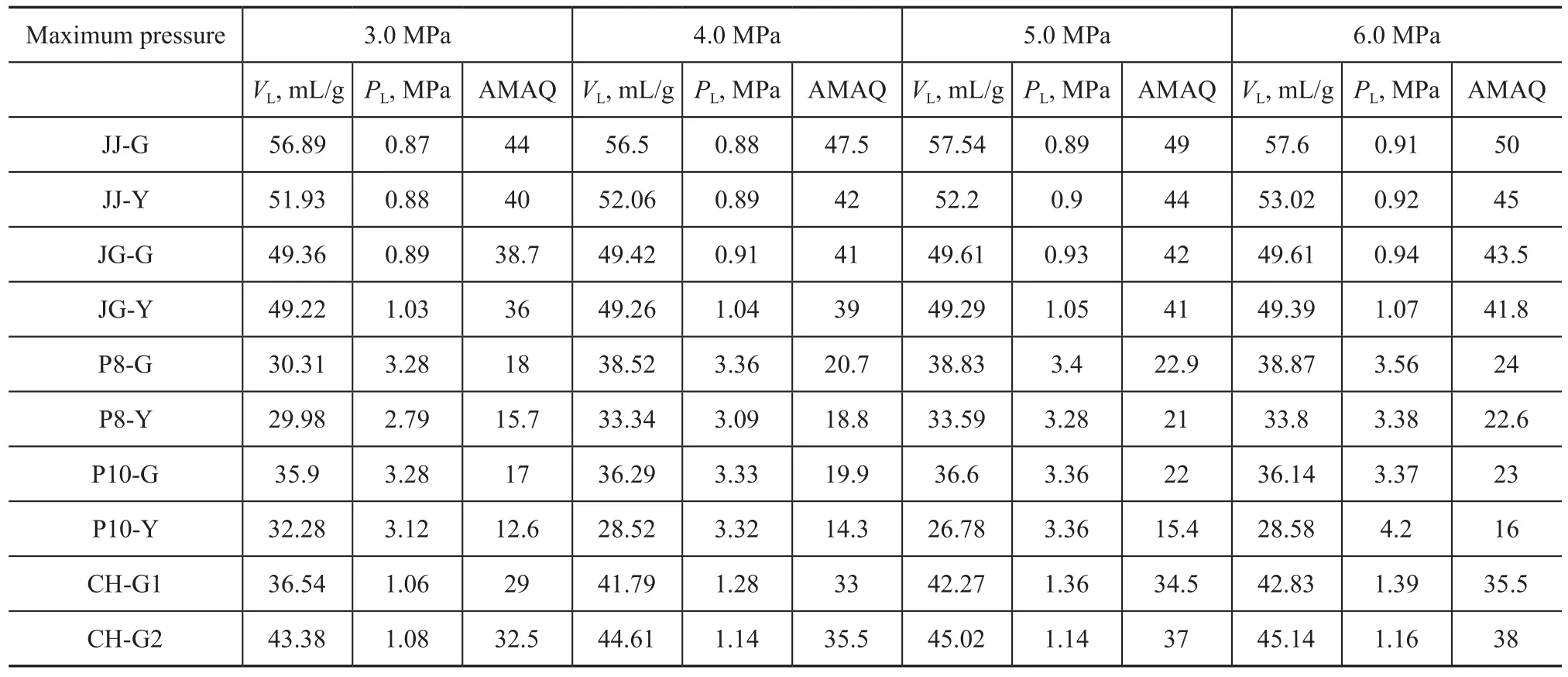
Table 4 Langmuir adsorption model parameters of coal at different maximum pressures
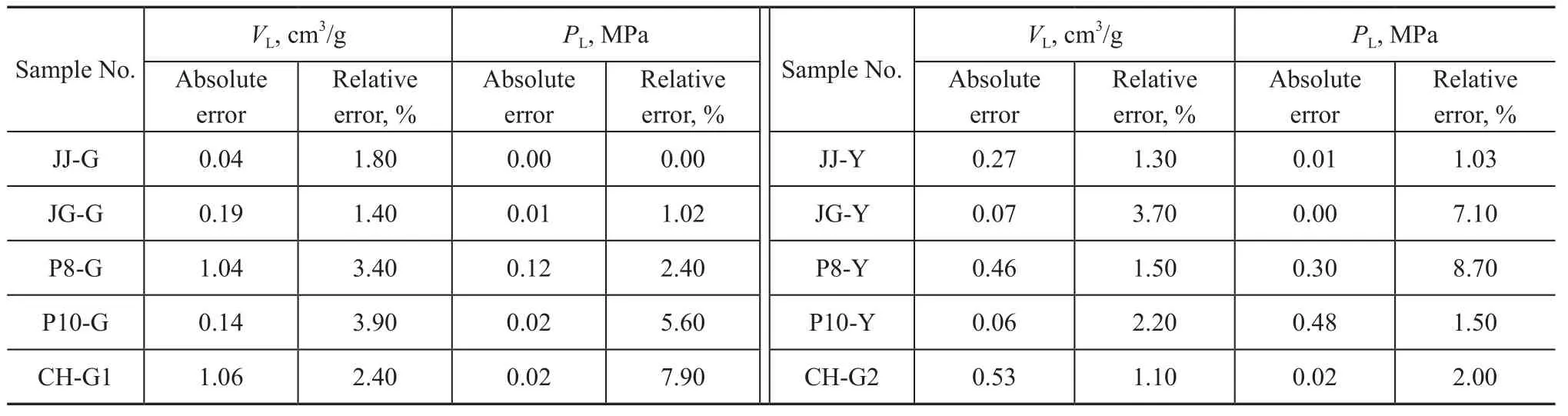
Table 5 Errors of Langmuir adsorption model parameters of deformed coal at 4 MPa and 6 MPa
3.2.2 Impact of coal rank on coal adsorptivity
Coal rank has an important impact on coal adsorptivity. There is different recognition of the impact of the coal’s metamorphism degree on coal adsorptivity. Some scholars deem that coal adsorptivity tends to increase as coal rank increases[27-28]. Some scholars point out that they are of “U”-shaped relation, i.e.: coal adsorptivity is a minimum for the bituminous coal with a medium volatiles content[29]. Some scholars indicate that coal adsorptivity looks like a three-segment variation relation as coal rank increases; i.e.: as the degree of coalification increases,VLfirstly reaches a maximum and then decreases[30-31].
The results depicted in Table 4 indicate that the coal rank plays a main controlling role in coal adsorptivity, and as coal rank increases, coal adsorptivity tends to increase. Different coal ranks will lead to the variation in pore structure, chemical composition and structure in coal accordingly and especially will have a large impact on pore structure. As coal rank increases, the pore structure of coal varies regularly, mesopores and micropores increase gradually, and lots of mesopores and micropores provide the space of methane adsorption by coal and also increase coal adsorptivity.
R0of coal is 0.98%—3.07% in this experiment. Within this range, coal adsorptivity would increase as coal rank increases. WhenR0is larger than 3.07%, the rule on variation of coal adsorptivity needs to be further studied.
3.3 Impact of coal pore structure parameters on adsorptivity
Pores provide the main space for methane gas adsorption, so pore structure characteristics are closely related to gasadsorption. The pore structure parameters (total specific surface area, total pore volume, proportion of micropores) obtained from the low-temperature liquid nitrogen adsorption process can be used to analyze the relationship between pore structure and methane adsorption quantity of coal (Figures 6—9).
As shown in Figure 6, the determination coefficient(R2) of total specific surface area withVLis about 0.5, which can properly interpret the positive linear correlation of total specific surface area withVL. Figures 7 and 8 show that theR2value of total pore volume and micropores percentage withVLis less than 0.3, which is too low to interpret the relationship between total pore volume and micropores percentage withVL. Figure 9 shows that the absolute value of the R2ofVLwith porosity is 0.72 and the negative correlation is remarkable, so it can be interpreted as the fact thatVdecreases as porosity increases.
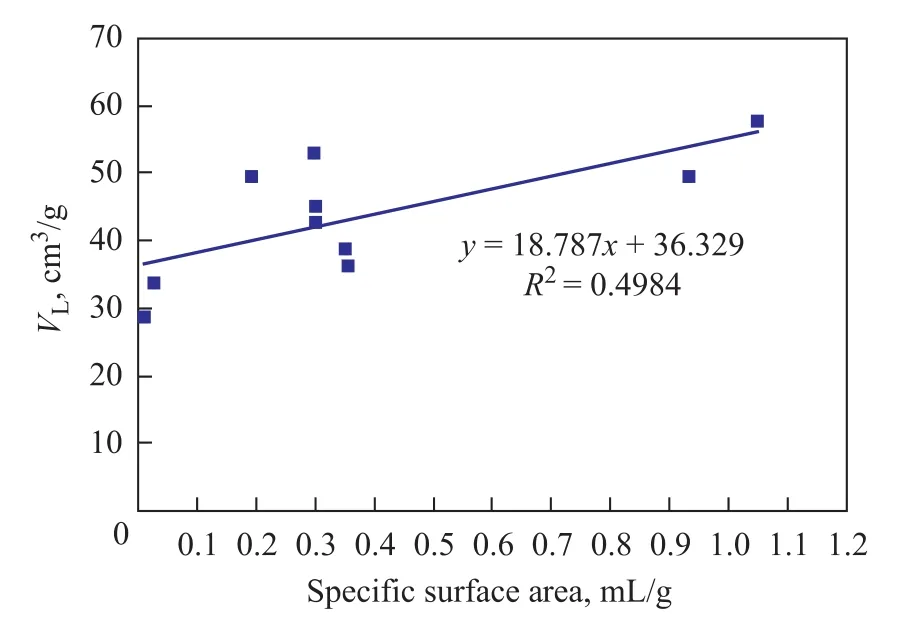
Figure 6 The relation between specific surface area and VL
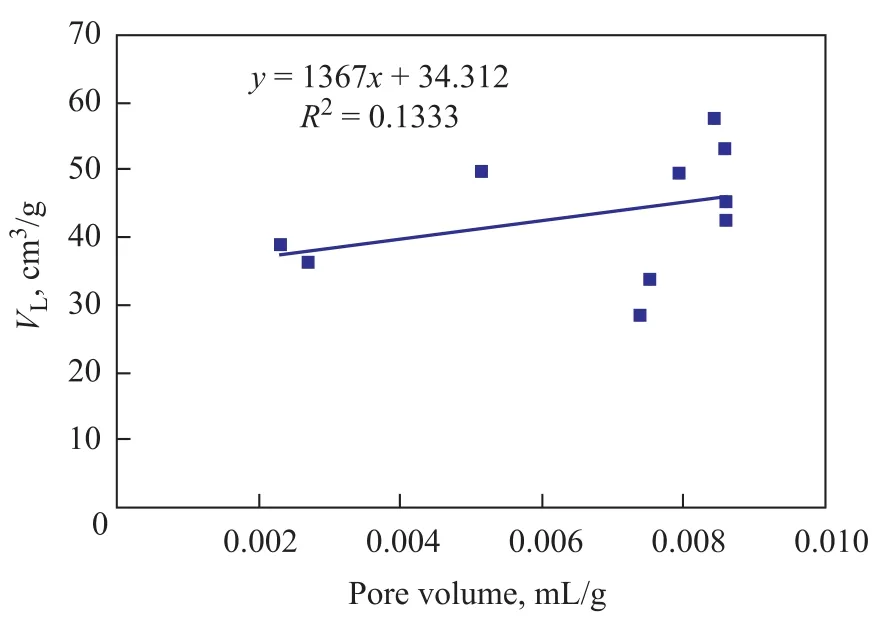
Figure 7 The relation between pore volume and VL
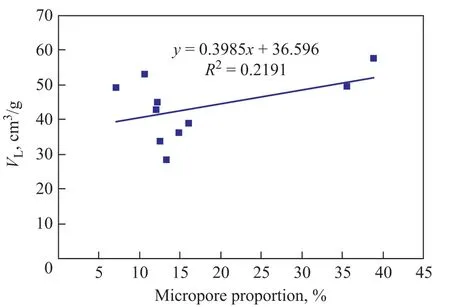
Figure 8 The relation between micropores proportion and VL
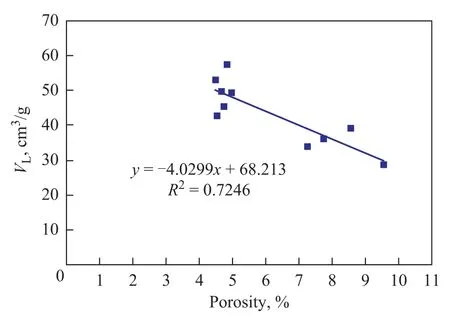
Figure 9 The relation between porosity and VL
4 Conclusions
(1) The adsorption loop of deformedcoal obviously indicates that most pores of deformed coal are open pores, the micropores are developed in deformed coal, the speci fic surface area is large,Dis high, and the relationship betweenDand speci fic surface area is a quadratic polynomial, and the relationship between gas adsorption quantity andDis a cubic polynomial.
(2) The adsorptivity of deformed coal is affected jointly by the factors such as pore structure, coal quality, coal deformation, pressure, etc. Gas adsorption quantity (VL) has a positive linear correlation with speci fic surface area. WhenR0of coal is 0.98%—3.07%, coal adsorptivity is increased as coal rank increases; the geologic tectonism damages coal structure and increases coal adsorptivity.
(3) Pressure is one of main external conditions that can affect coal adsorptivity. Before gas adsorption by coal is saturated, the higher the experimental pressure, the larger the adsorption quantity. Adsorption constants are also affected by the maximum experimental pressure. The maximum experimental pressure causes coal adsorptivity to be saturated and can directly affect the accuracy of coal adsorption constants.
Acknowledgements:The project is financially supported by the National Natural Science Foundation of China (No. 41172144)and supported by the Key (Key Grant) Project of Chinese Ministry of Education(No. 311022).
[1] Guo Deyong, Song Guangtai, Ku Mingxin. Research on coal structure indices to coal and gas outbursts in Pingdingshan Mine Area, China[J]. Journal of Coal Science & Engineering(China), 2002, 8(1): 1-6
[2] Mallikarjun P, Satya H. Gas diffusion behavior of coal and its impact on production from coalbed methane reservoirs[J]. International Journal of Coal Geology, 2011, 86(5): 342-348
[3] Zhang Songhang, Tang Shuheng, Tang Dazhen. The characteristics of coal reservoir pores and coal facies in Liulin district, Hedong coal field of China[J]. International Journal of Coal Geology, 2009, 81(5): 117-127
[4] Gan H, Nandi S P, Walkeer P L. Nature of the porosity of American coals[J]. Fuel, 1972, 51(2): 272-277
[5] Chen Ping, Tang Xiuyi. Low-temperature nitrogen adsorption method and the study of the microporous characteristics of coal[J].Journal of China Coal Science, 2001, 26(5): 552-556 (in Chinese)
[6] Yuan Chongfu. Deformed coal and coal and gas outburst[J]. Coal Science and Technology, 1986(1): 32-33 (in Chinese)
[7] Guo Deyong, Zhou Xinquan. Coal seam methane distribution and its significance in Pingdingshan mining area[J]. Journal of Coal Science & Engineering(China), 2002, 8(2): 17-22
[8] Yu Bufan. Technical Manuals for the Prevention and Utilization of Coal Mine Gas Disaster (revised edition)[M]. Beijing: China Coal Industry Publishing House, 2005 (in Chinese)
[9] Kenneth S. The use of nitrogen adsorption for the characterisation of porous materials[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 3(9): 187-188
[10] Hobot B B. Coal and Gas Outburst[M]. Translated by Song Shizhao, et al. Beijing: China Industry Press, 1966 (in Chinese)
[11] Yan Jimin, Zhang Peiyuan. The Adsorption and Condensation[M]. Beijing: Science Press, 1979 (in Chinese)
[12] de Boer J H. The shape of capillaries[M]//Everett D H, Stone F S. The Structure and Properties of Porous Materials. London: Butterworth, 1958
[13] Zhao Hong, Guo Fei, Yang Jianguo. Adsorption characteristic of Indonesia lignite and dewater experiment[J]. Journal of China Coal Science, 2008, 33(7): 137-140
[14] Cai Yidong. Pore structure and its impact on CH4adsorption capacity and flow capability of bituminous and subbituminous coals from Northeastern China[J]. Fuel, 2013, 103(8): 258-268
[15] Guo Deyong, Li Fei, Liu Wenge. Methane adsorption study using activated carbon fiber and coal based activated carbon[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(4): 20-25
[16] Qu Zhenghui. Study of Tectonized Coal Texture and Its Controlling Mechanism upon Gas Properties[D]. Xuzhou: China University of Mining, 2010
[17] Mandelbrot B B. Stochastic models for the earth’s relief, the shape and the fractal dimension of the coastlines, and the number-area rule for islands[J]. Proceedings of the National Academy of Sciences, 1975, 72(10): 3825-3828
[18] Yao Yanbin, Liu Dameng, Tang Dazhen, et al. Fractal characterization of adsorption-pores of coals from North China: An investigation on CH4adsorption capacity of coals[J]. International Journal of Coal Geology, 2008, 73(8): 27-42
[19] Pyun S I, Rhee C K. An investigation of fractal characteristics of mesoporous carbon electrodes with various pore structures[J]. Electrochemical Acta, 2004, 49: 4171-4180
[20] Wang Chengyang, Hao Shixiong, Sun Wenjing. Fractal dimension of coal particles and their CH4adsorption[J]. International Journal of Mining Science and Technology, 2012, 22: 855-858
[21] Wang Cong, Jiang Chengfa, Chu Wei. Fractal dimension of coals and analysis of its influencing factors[J]. Journal of China University of Mining & Technology, 2013, 42(6): 27-30
[22] Ismail M K, Ismail, Peter Pfeifer. Fractal analysis and surface roughness of nonporous carbon fibers and carbon blacks[J]. Langmuir, 1994, 10(5): 1532-1538
[23] Qi Hao, Ma Jian, Wong Po-zen. Adsorption isotherms of fractal surfaces[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 206(1): 401-407
[24] Zhang Can, Guo Deyong. Study on pore structure of coal and the adsorption on CH4[C]//Guo Deyong, Zhang Zimin, Zhang Zixu (editors). Research Progress of Gas Geology. Beijing: China University of Mining and Technology Press, 2013: 122-128
[25] Chikatamarla L, Peter J C. Role of coal type and rank on methane sorption characteristics of Bowen Basin, Australia coals[J]. International Journal of Coal Geology, 1999, 40: 309-325
[26] Guo Li. Experimental Research on Gas Adsorption Characteristic of Tectonic Coal[D]. Beijing: China University of Mining and Technology (Beijing), 2010
[27] Éttinger I L. Diffusion field in coal stratum[J]. Journal of Mining Science, 1991, 27(4): 368-370
[28] Zhang Qun, Yang Xilu. The study of coal to methane isothermal adsorption characteristics under the condition of equilibrium moisture[J].Journal of China Coal Science, 1999, 24(6): 35-40 (in Chinese)
[29] Laxminara C, Crosdae P. Role of coal type and rank on methane sorption characteristics of Bowen Basin, Australia coals[J]. International Journal of Coal Geology, 1999, 40(4): 309-325.
[30] Su Xianbo, Zhang Liping, Lin Xiaoying. Effects of coal rank on coal adsorption capacity[J]. Natural Gas Industry, 2005, 25(1): 19-21 (in Chinese)
[31] Chen Zhenhong, Jia Chengzao, Song Yan, et al. Differences and origin of physical properties of low-rank and high-rank coal-bed methane[J]. Acta Petrolei Sinica, 2008, 29(2): 15-18 (in Chinese)
Received date: 2014-10-10; Accepted date: 2014-11-14.
Prof. Guo Deyong, Telephone: +86-10-62331517; E-mail: kjkfg@cumtb.edu.cn.
- 中国炼油与石油化工的其它文章
- Comparative Studies on Low Noise Greases Operating under High Temperature Oxidation Conditions
- A Method for Crude Oil Selection and Blending Optimization Based on Improved Cuckoo Search Algorithm
- Mathematical Model of Natural Gas Desulfurization Based on Membrane Absorption
- Ni2P-MoS2/γ-Al2O3Catalyst for Deep Hydrodesulfurization via the Hydrogenation Reaction Pathway
- Effects of Airflow Field on Droplets Diameter inside the Corrugated Packing of a Rotating Packed Bed
- Synthesis of Phenyl Acetate from Phenol and Acetic Anhydride over Synthetic TS-1 Containing Template

