Hierarchical Radial Co3O4Microcrystal and Application in Gas Sensor
Xin-xin Yu,Xin-song Liu,Ming-zi Wu∗,Zho-qi Sun,Gung Li,Xio-shung Chen
a.School of Physics and Materials Science,Anhui University,Hefei 230039,China
b.The Shanghai Institute of Technical Physics,Chinese Academy of Sciences,Shanghai 200083, China
Hierarchical Radial Co3O4Microcrystal and Application in Gas Sensor
Xin-xin Yua,Xian-song Liua,Ming-zai Wua∗,Zhao-qi Suna,Guang Lia,Xiao-shuang Chenb
a.School of Physics and Materials Science,Anhui University,Hefei 230039,China
b.The Shanghai Institute of Technical Physics,Chinese Academy of Sciences,Shanghai 200083, China
Three-dimensional(3D)hierarchical Co3O4microcrystal with radial dendritic morphologies was prepared through hydrothermal reactions followed by subsequent annealing treatment. Structural and morphological characterizations were performed by X-ray diffraction,scanning electron microscopy and transmission electron microscopy.The gas sensing properties of the as-obtained microcrystal were investigated at 110◦C,which revealed that the 3D hierarchical porous Co3O4microcrystal exhibited high sensitivity to ammonia,as well as a short response time of 10 s.The response characteristic indicates that the sensor has a good stability and reversibility.Detections of toxic and f l ammable gases,such as ethanol, acetone and benzene were also carried out at a relative low temperature.The results indicate that such hierarchical Co3O4microcrystal would be a potential material in the f i eld of gas sensing.
Co3O4,Hierarchical,Gas sensor,Sensitivity
I.INTRODUCTION
Cobalt oxide(Co3O4),a promising functional p-type semiconductor,has been greatly investigated for its applications in the f i elds of heterogeneous catalysts,anode materials in Li ion rechargeable batteries,electrochemical devices,f i eld-emission materials,and so forth[1-3]. It is also a promising material for the preparation of solid-state gas sensor,due to the catalysis property of Co3O4,which is helpful to promote the sensitivity and improve the stability of the device.As is well known, the working mechanism of a solid-state metal oxide gas sensor is based on the conductivity change with the surrounding gas atmosphere.Typically,a depletion or accumulation layer would form at the surface of n-or p-type semiconductor as a result of the oxygen adsorption.When the atmosphere is switched to reducing gas, thinning of the layer would be brought up by surface reaction,which would result in the variation of the conductivity[4,5].Such process depends on the activity of the metal oxide,which can be controlled by the grain size and morphology[6].Therefore,controllable fabrication of the metal oxide attracts great attention for gas sensor applications.
In the past years,much effort has been made in the research of Co3O4-based gas sensor.For better performance in applications[7],Co3O4with various shapes have been synthesized,such as nanocubes,nanowires, nanorods,hollow sphere,nanoboxes,and porous structure[8-13].It is also reported dendrite-like nanostructure made up of nanorods has been successfully prepared[14].However,to our best knowledge,the synthesis of hierarchical dendritic Co3O4has not been reported,even the investigation of its gas sensing properties.
Moreover,despite the achievements in the research of Co3O4-based gas sensor,study of its response to ammonia is still at early stage.Ammonia is a penetrating and corrosive gas,which is harmful to health after an exposure to high concentration of ammonia.Measuring the concentration of ammonia is needed in environmental gas analysis,automotive industry,chemistry industry, and medical applications[15].
In this work,3D hierarchical radial Co3O4microcrystal with dendrite as the assembled unit has been prepared through hydrothermal reaction and subsequent heat treatment.The structure and morphology of the prepared Co3O4microcrystal were characterized by means of X-ray diffraction(XRD),scanning electron microscopy(SEM)and transmission electron microscopy(TEM).Moreover,a comprehensive study of its gas sensing properties was carried out with ammonia, ethanol,acetone,and benzene at a relative low temperature.
II.EXPERIMENTS
The synthesis of 3D hierarchical Co3O4microcrystal was based on our previous report on the synthesis of cobalt dendrites[16],which was used as the precursor ofCo3O4.In a typical procedure,CoCl2·6H2O(2 mmol) and NaOH(3 mmol)were f i rst dissolved in distilled water(30 mL)under magnetic stirring.Then ethylenediamine(1.0 mL)and hydrazine(1.0 mL)were added into the mixed solution drop by drop,followed by continual stirring for 15 min.The mixed solution was then transferred to a 50 mL Tef l on-lined autoclave,which was kept at 200◦C for 4 h,and then cooled to room temperature naturally.After thorough wash and dry, the hierarchical dendritic cobalt microcrystal was obtained.Then it was annealed in ambient air at 500◦C (heating rate of 4◦C/min)for 1 h to get crystalline Co3O4particles with the morphology unchanged.
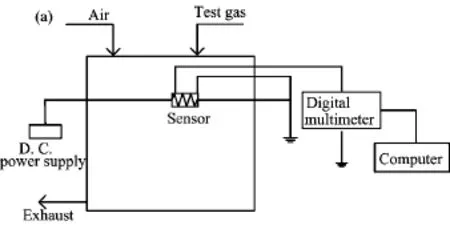
FIG.1 The scheme of the experimental setup.
The microstructure and morphology were characterized by XRD,SEM and TEM.The BET surface areas and the pore size distributions were characterized by measuring adsorption-desorption isotherms of nitrogen gas with a Micromeritics ASAP 2020 M+C.
Gas-sensing measurements were carried out in a homemade experimental setup(Fig.1)at working temperature of 110◦C with a constant f l ow rate of 500 sccm (standard cubic centimeter per minute).The asprepared Co3O4microcrystal was directly coated on the outer surface of an alumina tube-like substrate on which a pair of Au electrodes had been printed previously,followed by drying at 60◦C for about 2 h and subsequent annealing at 400◦C for about 3 h.Finally, a small Ni-Cr alloy coil was inserted into the tube as a heater,which provided the working temperature of the gas sensor.A stationary state gas distribution method was used for testing gas response in dry air.In the measurement of electric circuit for gas sensors,a load resistor was connected in series with a gas sensor.The circuit voltage was set at 5 V,and the output voltage (Vout)was the terminal voltage of the load resistor.The resistance of a sensor in air or test gas was measured by monitoring Vout.The test was operated in a measuring system of ART-2000A(Art Beijing Science and Technology Development Co.,Ltd.,China).
III.RESULTS AND DISCUSSION
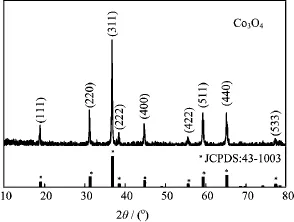
FIG.2 XRD of the as-obtained sample.The standard JCPDS card of Co3O4with cubic spinel structure is also given.
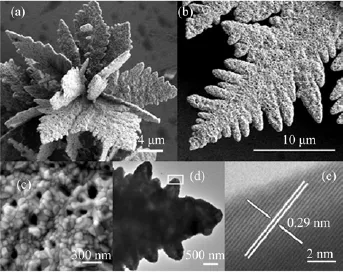
FIG.3(a)SEM image of the as-prepared Co3O4microcrystal,(b)SEM image of a single dendrite,(c)high magnif ication SEM image of the surface of the dendrite,(d)TEM image of a single dendrite structure,(e)lattice fringes image of the part labeled by the rectangle in(d).
The XRD of the as-obtained sample is shown in Fig.2. All the diffraction peaks can be well indexed to Co3O4with the cubic spinel structure.The narrow full-width at half-maximum(FMWH)of the diffraction peaks indicates the perfect crystallize character of the Co3O4powder.The crystallite size is estimated to be 37.7 nm from the full-width at half-maximum of the(311)peak using the Scherrer equation.Lattice parameter is calculated to be 8.082˚A using the d-spacing value and the corresponding(hkl)index,which is very close to that of the bulk Co3O4(8.084˚A).

FIG.4(a)Response(Rg/Ra)of the Co3O4hierarchical nanostructure with the increasing gas f l ow.(b)Response time(open square)and recovery time(solid square)toward NH3of the sensor based on the Co3O4hierarchical nanostructure.
The size and morphology of the as-prepared product were characterized by SEM,as shown in Fig.3.Due to the big size,Figure 3(a)only shows a single radial microcrystal composed of many dendrites,all of which radiate from the center with length about 10µm and width about 7µm.For the reason of annealing,many pores were produced on both the main branch and the secondary branches of the dendrite.A higher resolution SEM image of a rectangular area reveals that the surface is composed of many pores with size averaged to be 100 nm and crystallites with size averaged to be 80 nm.The big difference between the crystallite size and the microcrystal size suggests that the Co3O4microcrystal is polycrystalline.In order to further investigate the microstructure,HRTEM characterization was performed on a part of single dendrite.The lattice spacing is about 0.29 nm,consistent with the(220)crystal planes of Co3O4.The perfect fringes are supposed to be an evidence for the good crystalline quality of the Co3O4microcrystal.
Co3O4is an important functional p-type semiconductor that can be used in chemical gas sensors.Since the structure and morphology of materials play an important role in the gas sensors properties[17],it is expected that such polycrystalline Co3O4would have good performance in gas detection.Based on the as-prepared Co3O4,we fabricated a solid-state gas sensor and investigated its response and recovery behaviors.In the typical measurement,the Co3O4sensor was exposed to the target gas at 110◦C.The response of the sensor is def i ned as the ratio of the sensor’s resistance in target gases(Rg)to that in dry synthetic air(Ra).The response time and the recovery time are def i ned as the time taken to reach 90%of the f i nal equilibrium value. It is found that the sensor takes characteristic of a ptype semiconductor.From the real-time responses toward ammonia,the resistance increases rapidly as the target gases are injected and returns to the initial state when the detected gases are released by dry air f l ow,as marked in Fig.4(a).The response characteristics sensitively depend on the ammonia concentration and gradually reach saturation with the increasing ammonia concentration.

FIG.5Nitrogen adsorption-desorption isotherms of the Co3O4microcrystal at 77 K.
The response values are about 1.33,1.63,1.68,and 1.78 to 100,200,300,and 500 ppm ammonia.The unique morphology of the microcrystal composed of pores and small crystallites is supposed to be favorable to the efficient charge depletion in such small grains upon interaction with oxygen and thus explains the large response when exposed to ammonia.More importantly,the baseline is f i xed even after several detection circles,which demonstrates a good stability and reversibility.It is supposed that the low temperature (110◦C)required for the operation can inhibit the reaction of grain growth,thus benefits the stability of the material.
In addition to the sensitivity,response time is another important parameter of gas sensor.Figure 4(b) displays the calculated response time and recovery time of the radial Co3O4gas sensors at 110◦C based on Fig.4(a).Specif i cally,the response time to ammonia is about 10 s,and the recovery time is no more than 2 min.The improved response performance of the radial Co3O4may be attributed to its unique morphology. The surface areas of the products will be enhanced with the increase of the number of folds and cribriform dendrites on the surfaces of the products,which can decrease the time of the gas adsorption and diffusion to the sensing surface via such porous hierarchical structure[18].
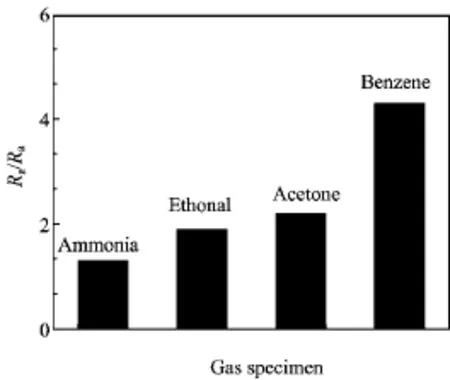
FIG.6 Gas responses of the Co3O4hierarchical nanostructure to ammonia,ethanol,acetone and benzene at 110◦C.
Figure 5 shows the nitrogen adsorption-desorption isotherms of Co3O4microcrystals at 77 K and the BET surface area is calculated to be 1.22 m2/g.Although it is much smaller than the reported value in Ref.[19],it is good enough for the response time.A longer recovery time than response time requires further and more detailed study.However,we give a tentative explanation here.The response process involves in-diffusion of target gas and oxidation reaction,while the recovery process involves the counter-diffusion of oxidized product,the in-diffusion of oxygen gas to the surface,as well as the adsorption,dissociation and ionization of oxygen.Since the operation temperature is relative low,the formation of oxygen ionization should be slowed down. Meanwhile,considering the fact that cobalt oxides are commonly used as a catalytic activator in oxidation reaction,the oxidation of the reducing gas could remain to be effective and keep a relative high response speed, all of which should be responsible for the relatively long recovery time.For further understanding,the gas sensor was also tested toward 100 ppm ethanol,acetone and benzene at 110◦C.The responses of the sensors are 1.31,2.36,3.28 and 4.31 to ammonia,ethanol,acetone and benzene respectively,which are all acceptable for individual gas detection without interfering gas(Fig.6).
IV.CONCLUSION
In summary,3D hierarchical radial Co3O4microcrystal with a size about 24µm has been successfully fabricated by a sequential process of hydrothermal reaction and heat treatment.The 3D Co3O4microcrystal is composed of dozens of cobalt oxide dendrites radiating from the center and exhibits good sensing performance to ammonia detection,such as quick response,good sensitivity,stability and reversibility, which are supposed to result from its unique morphology and porous structure.The operation temperature (110◦C)is much lower than the usual operation temperature(500-800 K)of metal oxide gas sensor. Thus such gas sensor would consume less energy and is more proper for applications.
V.ACKNOWLEDGMENTS
This work was supported by the 211 project of Anhui University,the National Natural Science Foundation of China(No.11374013,No.61290301,No.51072001, No.51272001,and No.51272003),Anhui Provincial Natural Science Fund(No.11040606M49),Higher Educational Natural Science Foundation of Anhui Province (No.KJ2012A007),and the PhD Start-up Fund of Anhui University(No.33190209).Ming-zai Wu thanks Dr. Fan-li Meng and Miss Hui-hua Li from the Institute of Intelligent Machines,CAS for the help with gas sensing experiment.
[1]A.C.Co,J.B.Liu,I.Serebrennikova,C.M.Abel,and V.I.Birss,J.Mater.Sci.40,4039(2005).
[2]W.Zhang,M.Han,Z.Jiang,Y.Song,Z.Xie,Z.Xu, and L.Zheng,Chem.Phys.Chem.8,2091(2007).
[3]B.Varghese,Y.Zhang,L.Dai,V.B.C.Tan,C.T. Lim,and C.H.Sow,Nano.Lett.8,3226(2008).
[4]N.D.Cuong,T.T.Hoa,D.Q.Khieu,N.D.Hoa,and N.V.Hieu,Curr.Applied.Phys.12,1355(2012).
[5]J.W.Yoon,J.K.Choi,and J.H.Lee,Sens.Actuators B 161,570(2012).
[6]J.H.Lee,Sens.Actuators B 140,319(2009).
[7]C.Zhang,J.Chen,Y.Zeng,X.Rui,J.Zhu,W. Zhang,C.Xu,T.M.Lim,H.Huey Hoon,and Q.Yan, Nanoscale 4,3718(2012).
[8]R.Al-Tuwirqi,A.A.Al-Ghamdi,N.A.Aal,A.Umar, and W.E.Mahmoud,Superlatt.Microstruc.49,416 (2011).
[9]S.C.Petitto,E.M.Marsh,G.A.Carson,and M.A. Langell,J.Mol.Catal.A 281,49(2008).
[10]A.Gulino,P.Dapporto,P.Rossi,G.Anastasi,and I. Fragala,J.Mater.Chem.14,2549(2004).
[11]Y.C.Chen,Y.G.Zhang,and S.Q.Fu,Mater.Lett. 61,701(2007).
[12]T.Li,S.G.Yang,L.S.Huang,B.X.Gu,and Y.W. Du,Nanotechnology 15,1479(2004).
[13]T.Zhu,J.S.Chen,and X.W.Lou,J.Mater.Chem. 20,7015(2010).
[14]H.Pang,F.Gao,Q.Chen,R.M.Liu,and Q.Y.Lu, Dalton Trans.41,5862(2012).
[15]B.Timmer,W.Olthuis,and A.Van den Berg,Sens. Actuators B 107,666(2005).
[16]M.Z.Wu,Z.W.Pang,X.S.Liu,G.Li,Y.Q.Ma,Z. Q.Sun,L.D.Zhang,and X.S.Chen,J.Alloy.Compd. 513,245(2012).
[17]A.M.Cao,J.S.Hu,H.P.Liang,W.G.Song,L.J. Wan,X.L.He,X.G.Gao,and S.H.Xia,J.Phys. Chem B 110,15858(2006).
[18]J.Y.Liu,Z.Guo,F.L.Meng,T.Luo,M.Q.Li,and J.H.Liu,Nanotechnology 20,125501(2009).
[19]Z.P.Xu and H.C.Zeng,Chem.Mater.12,3459(2000).
ceived on August 16,2013;Accepted on October 29,2013)
∗Author to whom correspondence should be addressed.E-mail:mingzaiwu@gmail.com
 CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Experimental and Theoretical Investigation on Excited State Intramolecular Proton Transfer Coupled Charge Transfer Reaction of Baicalein
- Theoretical Study of Reagent Rotational Excitation effect on the Stereodynamics of H+LiF→HF+Li Reaction
- Infrared Spectroscopy of COIsolated in Solid Nitrogen Matrix
- effects of Rotational Isomerism and Bond Length Alternation on Optical Spectra of FTC Chromophore in Solution
- High Pressure Structural Instability and Thermal Properties of Rutile TiO2from First-principles
- Kinetic Implication from Temperature effect on Hydrogen Evolution Reaction at Ag Electrode
