Theoretical Study of Reagent Rotational Excitation effect on the Stereodynamics of H+LiF→HF+Li Reaction
Ting-xin Xie,Ying-ying Zhng,Ying Shi∗,Ming-xing Jin
a.Department of Physics,Dalian Jiaotong University,Dalian 116028,China
b.Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China
Theoretical Study of Reagent Rotational Excitation effect on the Stereodynamics of H+LiF→HF+Li Reaction
Ting-xian Xiea,Ying-ying Zhangb,Ying Shib∗,Ming-xing Jinb
a.Department of Physics,Dalian Jiaotong University,Dalian 116028,China
b.Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China
The reagent rotational excitation effect on the stereodynamics of H+LiF→HF+Li is calculated by means of the quasi-classical trajectory method on the Aguado-Paniagua2-potential energy surface(AP2-PES)constructed by Aguado et al.[J.Chem.Phys.106,1013(1997)]. The angular distributions of vector correlations between products and reactants,P(θr)and P(φr)are presented.Meanwhile,the four polarization-dependent generalized differential cross sections are computed.The results indicate that the reagent rotational quantum numbers have impact on the vector properties of the title reaction.In addition,the reaction probability has been calculated as well.
Stereodynamics,Quasi-classical trajectory,Polarization-dependent generalized differential cross sections
I.INTRODUCTION
During the past decades,numerous of experimental and theoretical studies for the H+LiF→HF+Li reaction have been reported[1-7]and the reaction has been a prototype of the heavy-heavy-light(HHL)system.Furthermore,the LiHF system has a rather deep van der Waals well in the entrance channel,which causes the long-lived collision complexes and narrow scattering resonances in the energy-dependent reaction probabilities [8].In the experimental aspects,for example,Loesch et al.have reported the crossed molecular beam studies on the Li+HF reaction[9,10].Hobel et al.have detected the double differential cross sections at different collision energies[11].Theoretical studies like the exact quantum probabilities for Li+HF and its isotopic variants have been reported by Lagan`a[12].In 2012,Li et al.have presented the reagent vibration effect for the reaction[13].
Aguado et al.constructed a potential energy surface of LiHF system based on 570 MRDCI ab initio energy points and carried out wave packet calculations [14].Later they carried out the fitting of the energy points under the same circumstance[15].Meanwhile, the three-dimensional time-dependent quantum method was used to investigate the dynamics of the LiHF system[15].To the best of our knowledge,investigations of experimental and theoretical aspects for its reverse reaction H+LiF→HF+Li have been seldom presented except that Weck et al.performed quantum scattering calculations for zero total angular momentum of vibrational excitation effect of the title reaction[8],due to the lack of experimental results with metastable hydrogen atom sources in crossed beams experiments.
In term of the theoretical calculation methods,the ab initio calculation has been used to compute the potential energy surface.By means of the ab initio calculation,Li et al.have carried out the double manybody expansion potential energy surface of NH3and NH2molecule[16,17].Varandas has calculated the ab initio potential energy surface of the O+OH reaction[18].In addition,the quasi-classical trajectory (QCT)method[19-21]has also been widely adopted in the stereodynamics f i eld to calculate a great deal of chemical reactions particularly for the atom-molecule [22-24],the ion-molecule[25-27],and the polyatomic molecules[28-30]reactions which have achieved a great success.Recently,it has been developed by Han and coworkers to propagate trajectories and obtain the stereodynamical quantities with simultaneous treatment[31, 32].
In our present work,in order to gain more dynamical information,we have studied the stereodynamics of the title reaction by using a developed QCT method. Meanwhile,the reagent rotational excitation effect on the vector properties of the reaction is performed and discussed as well.
II.THEORETICAL METHODOLOGY
A.PES and the QCT calculations
TheAguado-Paniagua2-potentialenergysurface (AP2-PES)given by Aguado et al.[15]is employed in the present work.The AP2-PES has a relatively deepwell in its entrance channel and a nonlinear transition state shifted into the exit channel.The Hamilton’s classical equations were integrated numerically in three dimensions.The QCT method[19-21,33,34]was also developed.In this work,the collision energy for the title reaction is chosen to be 5.0 kcal/mol.The trajectories are initially installed at an H-LiF internuclear separation of 10˚A,the vibrational and rotational quantum numbers of reactant molecules are taken to be v=0 and j=0-6,respectively.Batches of 5×104trajectories are run for each rotational level and the integration step size of 0.1 fs is used to ensure the conservation of total angular momentum and total energy.
B.Rotational alignment parameter and PDDCSs
In this work,the centre-of-mass(CM)frame is chosen and shown in Fig.1.The z-axis is parallel to the reagent initial relative velocity vector k,while the yaxis is perpendicular to the x-z scattering plane which contains the initial and f i nal relative velocity vectors k and k0.θtis the angle between k0and k which is called the scattering angle.θris the angle between k and the rotational angular momentum vector j0,and φris the dihedral angle of the k-k0-j0correlation.The two angles also refer to the polar angle and the azimuth angle of the vector j0,respectively.All of these angles can be derived from the QCT stereodynamical calculations.
The angular distribution function P(θr)which describes the k-j0correlation can be expanded via the Legendre polynomials,the P(φr)describing the k-k0-j0correlation can be expanded in Fourier series,and the joint probability density function P(θr,φr)can be written as the following[35-41]:

while k=2 indicates the product rotational alignment

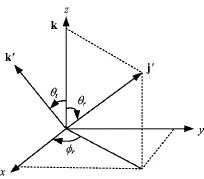
FIG.1 Centre-of-mass coordinate system used to describe the k,k0,and j0correlations.
The value of the polarization parameter is evaluated as follows:
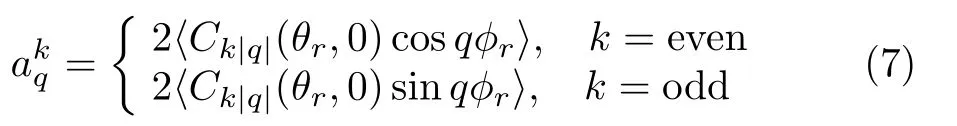
In our calculations,the P(θr,φr)is expanded up to k=7,which is sufficient for good convergence.Details on the dynamical information of P(θr),P(φr),and P(θr,φr)are discussed later.
The full three-dimensional angular distribution associated with k-k0-j0correlation can be represented by a set of PDDCSs in the CM frame.The fully correlated CM angular distribution is written as[42-45]:
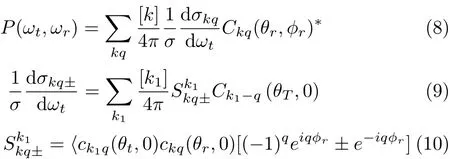
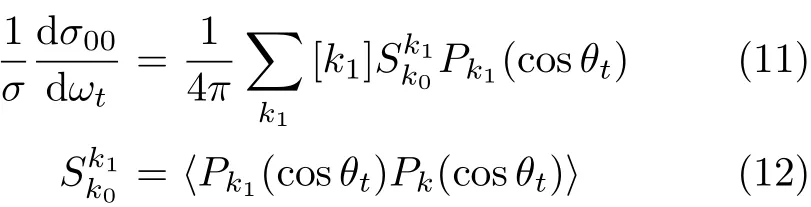
Many photon-initiated bimolecular reaction experiments will be sensitive to only those polarization momentswithk=0and2.Inthiswork,the four PDDCSs,(2π/σ)(dσ00/dωt),(2π/σ)(dσ20dωt), (2π/σ)(dσ22+/dωt),and(2π/σ)(dσ22-/dωt)are calculated.
III.RESULTS AND DISCUSSION

FIG.2 Distributions of P(θr)ref l ecting k-j0correlations at different rotational levels j=0-6 for the title reaction.
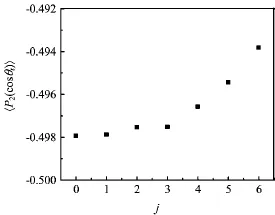
FIG.3Variationofrotationalalignmentparameter hP2(cosθr)i with rotational levels j=0 to j=6 for the title reaction.
In our calculations,we plot all the vector correlation functions and the PDDCSs in three dimensions. Figure 2 illustrates the product angular distributions P(θr)describing the k-j0correlation.The peaks are situated at θr=90◦and also symmetric with respect to θr=90◦at all calculated rotational levels,which reveal that the product rotational angular momentum vector j0is strongly aligned along the direction perpendicular to the direction of the reagent relative velocity k.Furthermore,the values of the peaks become lower and broader as the rotational level increases,indicating that influence of the product alignment becomes weaker at high rotational levels.The result can be ref l ected via the rotational alignment parameter hP2(cosθr)i depicted in Fig.3.We can obtain that with increasing of the rotational level,the negative values of the hP2(cosθr)i become smaller which indicates a weaker alignment as well.

FIG.4 Dihedral angle distribution of P(φr)with respect to the k-k0plane,plotted at reagent rotational quantum numbers j=0,j=3,and j=6,respectively.
Figure 4 demonstrates the dihedral angle distributions P(φr)which represent the k-k0-j0correlation. From inside to the outside,the corresponding rotational state is j=0,j=3,and j=6,respectively.The incremental direction of φrmoves clockwise.It shows that the distributions of P(φr)are asymmetric with φr=180◦at all the rotational states which indicates a strong polarization of the angular momentum.The two major peaks are at φr=90◦and φr=270◦.Obviously,the peaks at φr=270◦are far stronger than the peaks at φr=90◦, which ref l ects that j0is not only aligned along the y axis but also oriented along the negative direction of the yaxis in the CM frame.This appearance of the result can be explained by the impulse model[37]about the A+BC→AB+C reaction[46-48].The product angular momentum vector j0is given by

where L is the reagent orbital angular momentum,rABand rCBare unit vectors.µBCis the reduced mass of molecule BC and R is the repulsive energy.The term J1plays a vital role in the product rotational orientation. The Lsin2β+j cos2β is symmetric,while for the effect of R,the term J1mA/mBshows a prioritized direction which leads to the left-handed HF product rotation in plane parallel to the k-k0scattering plane.
In order to obtain more stereodynamical information on the effect of rotational quantum numbers and have a better observation of the product angular momentum polarization which ref l ects the k-k0-j0correlation, the distributions of P(θr,φr)are depicted in Fig.5.In this work,we only show the distributions at rotational quantum numbers j=0,j=3,and j=6.The two peaks at(90◦,90◦)and(90◦,270◦)are in good agreement with the distributions of P(θr)and P(φr)mentioned above. Meanwhile,the plot of the distributions indicates that the HF products are preferentially polarized perpendicular to the scattering plane and the reaction is dominated by in-plane mechanisms[49].
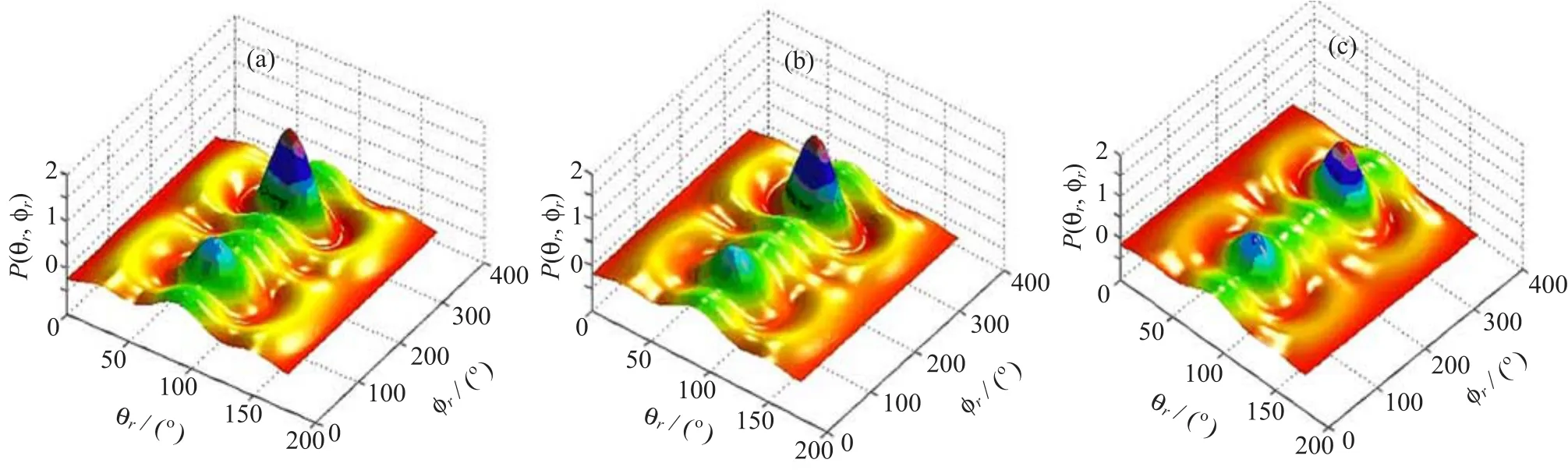
FIG.5 Polar plots of the P(θr,φr)distributions with peaks and valleys at different rotational states.(a)j=0,(b)j=3,and (c)j=6.

FIG.6 Four PDDCSs of the reagent rotational quantum numbers with(a)(k,q)=(0,0),(b)(k,q)=(2,0),(c)(k,q)=(2,2+), and(d)(k,q)=(2,1-),respectively.
The PDDCSs describing the k-k0-j0correlation and the scattering direction of the HF product molecule have been displayed in Fig.6.In our present work, the four PDDCSs,(2π/σ)(dσ00/dωt),(2π/σ)(dσ20dωt), (2π/σ)(dσ22+dωt),and(2π/σ)(dσ22-dωt)are computed at all rotational levels from j=0 to j=5.The PDDCS(2π/σ)(dσ00dωt)which only describes the k-k0correlation or the scattering direction of the product is a simple differential cross section and has no ties to the orientation and alignment of the product rotational angular momentum vector j0.As shown in Fig.6(a),with increasing of the rotational quantum numbers,the forward scattering of product HF becomes weaker while the backward scattering turns stronger.The PDDCS (2π/σ)(dσ20/dωt)which shows an opposite trend to the PDDCS(2π/σ)(dσ00/dωt)is the expectation value of the second Legendre moment drawn in Fig.6(b). Clearly,the values of PDDCS(2π/σ)(dσ20/dωt)are negative which is probably associated with the alignment moment hP2(cosθr)i.
The PDDCSs with q6=0 are shown in Fig.6(c)and(d).In some manners,the distributions of the PDDCSs with q6=0 are necessarily zero at the extremely forward and backward scattering.Figure 6(c)illustrates the distributions of PDDCS(2π/σ)(dσ22+/dωt). We can obtain that the values of the distributions are negative at all scattering angles which correspond to the product rotational alignment that shows a preference along the y-axis.There are two stronger polarizations ranging from 35◦-40◦and 136◦-141◦,respectively.In addition,it also ref l ects that the product angular momentum distribution is anisotropic.The PDDCS(2π/σ)(dσ21-/dωt)ref l ects the product rotational angular momentum vector j0is along the direction of vector x-z or x+z.Figure 6(d)displays a strong negative peaks ranging from 21◦to 26◦which indicates the rotational alignment of the HF products is along the x+z direction.Moreover,the distributions of(2π/σ)(dσ21-/dωt)are all in such small values which implie that the product angular momentum distribution is isotropic.

TABLEI Reaction probability as a function of the impact parameter at rotational level j=0 to j=6.
In order to obtain more about the title reaction,the rotational excitation effect of its scalar properties is computed as well.Table I displays the reaction probability as a function of the impact parameter with the rotational quantum numbers ranging from j=0 to j=6. Generally speaking,the values of the reaction probability are small and change little at all rotational levels and impact parameters which means that the reaction probability doesn’t strongly depend on the rotational levels.The similar phenomenon has also been observed in reactions like H+O2[50-52],O+H2[53]and other reactions[54,55]which may attribute to the deep well on the potential energy surface in this type of reactions.
IV.CONCLUSION
Investigationsonthestereodynamicsofthe H+LiF→HF+Li reaction have been performed by the QCT method on the AP2-PES at rotational levels from j=0 to j=6.The collision energy for the title reaction is taken to be 5.0 kcal/mol.We have studied the angular distributions of P(θr)and P(φr).The distributions of P(θr)reveals a strong product rotational alignment and the distribution P(φr)shows a asymmetric behavior to the scattering plane and exhibits two peaks at φr=90◦and φr=270◦and peaks at φr=270◦are stronger than those at φr=90◦which can be explained by the implosive model which implies that j0is not only aligned but also oriented along the negative direction of the y-axis.In addition,we have calculated the four PDDCSs.The PDDCS(2π/σ)(dσ00/dωt)shows a weakly forward scattering and strongly backward scattering of the product HF with the increase of the rotational states. The PDDCS(2π/σ)(dσ20/dωt)indicates that j0is strongly aligned the direction perpendicular to k.The PDDCS(2π/σ)(dσ22+/dωt)reveals that the product angular momentum distribution is anisotropic while the PDDCS(2π/σ)(dσ21-/dωt)ref l ects its isotropy. Finally,the reaction probability indicates that the influence of the deep well on the potential energy surface to the initial rotational states of the reagent molecule can’t also be ignored.
V.ACKNOWLEDGMENTS
This work was supported by the Jilin University,China(No.419080106440),the Chinese National Fusion Project for ITER(No.2010GB104003),and the National Natural Science Foundation of China (No.10974069).Many thanks to Prof.Ke-li Han for providing the stereodynamics program.
[1]T.J.Odiorne,P.R.Brooks,and J.V.V.Kasper,J. Chem.Phys.551,980(1971).
[2]Z.Karny,R.C.Estler,and R.N.Zare,J.Chem.Phys. 69,5199(1978).
[3]F.E.Bartoszek,B.A.Blackwell,J.C.Polanyi,and J. J.Sloan,J.Chem.Phys.74,3400(1981).
[4]M.Baer,I.Last,and H.J.Loesch,J.Chem.Phys.101, 9648(1994).
[5]D.R.Herschbach,Adv.Chem.Phys.10,319(1996).
[6]F.J.Aoiz,E.Verdasco,R.V.S´aez,H.J.Loesch,M. Men´endez,and F.Stienkemeier,Phys.Chem.Chem. Phys.2,541(2000).
[7]R.S.Tan,X.G.Liu,and M.Hu,Chin.Phys.Lett.29, 123101(2012).
[8]P.F.Weck and N.Balakrishnan,J.Chem.Phys.122, 234310(2005).
[9]H.J.Loesch,S.Stenzel,and B.W¨ustenbecker,J. Chem.Phys.95,38419(1991).
[10]H.J.Loesch and F.Stienkemeier,J.Chem.Phys.99, 9598(1993).
[11]O.Hobel,A.Paladini,A.Russo,R.Bobbenkamp,and H.J.Loesch,Phys.Chem.Chem.Phys.62,198(2004).
[12]A.Lagan`a,A.Bolloni,and S.Crocchianti,Phys.Chem. Chem.Phys.2,535(2000).
[13]S.J.Li,Y.Shi,T.X.Xie,and M.X.Jin,Chin.Phys. B 21,013401(2012).
[14]A.Aguado,C.Suf i rez,and M.Paniagua,Chem.Phys. 201,107(1995).
[15]A.Aguado and M.Paniagua,J.Chem.Phys.106,1013 (1997).
[16]Y.Q.Li,Y.Z.Song,P.Song,Y.Z.Li,Y.Ding,M.T. Su,and F.C.Ma,J.Chem.Phys.136,194705(2012).
[17]Y.Q.Li,J.C.Yuan,M.D.Chen,F.C.Ma,and M. T.Sun,J.Comp.Chem.34,1686(2013).
[18]A.J.C.Varandas,J.Chem.Phys.138,134117(2013). [19]F.J.Aoiz,M.T.Martınez,M.Men´endez,R.V.S´aez, and E.Verdasco,Chem.Phys.Lett.299,25(1999).
[20]T.G.Yang,J.C.Yuan,D.H.Cheng,and M.D.Chen, Commun.Comput.Chem.1,15(2013).
[21]M.L.Wang,K.L.Han,J.P.Zhan,V.W.K.Wu,G.Z. He,and N.Q.Lou.Chem.Phys.Lett.278,307(1997). [22]A.J.Alexander,F.J.Aoiz,M.Brouard,and J.R. Simons.Chem.Phys.Lett.256,561(1996).
[23]M.D.Chen,K.L.Han,and N.Q.Lou,Chem.Phys. Lett.357,483(2002).
[24]T.S.Chu,H.Zhang,S.P.Yuan,A.P.Fu,H.Z.Si,F. H.Tian,and Y.B.Duan,J.Chem.Phys.A 113,3470 (2009).
[25]L.Yao,H.Y.Zhong,Y.L.Liu,and W.W.Xia,Chem. Phys.359,151(2009).
[26]X.H.Li,M.S.Wang,I.Pino,C.L.Yang,and L.Z. Ma,Chem.Phys.11,10438(2009).
[27]N.Bulut,J.F.Castillo,F.J.Aoiz,and L.Ba˜nares, Chem.Phys.10,821(2008).
[28]A.J.C.Varandas,P.J.S.B.Caridade,Z.H.Zhang, Q.Cui,and K.L.Han,J.Chem.Phys.125,064312 (2006).
[29]D.Troya and E.Garcia-Molina,J.Phys.Chem.A 109, 3015(2005).
[30]M.A.Horst,G.C.Schatz,and L.B.Harding,J.Chem. Phys.105,558(1996).
[31]X.Zhang and K.L.Han,Int.J.Quantum.Chem.106, 1815(2006).
[32]M.D.Chen,K.L.Han,and N.Q.Lou,Chem.Phys. 283,463(2002).
[33]K.L.Han,G.Z.He,and N.Q.Lou,Chin.J.Chem. Phys.2,323(1989).
[34]Q.Wei,X.Li,and T.Li,Chin.J.Chem.Phys.22,523 (2009).
[35]W.W.Xu,X.G.Liu,S.X.Luan,S.S.Sun,and Q.G. Zhang,Chin.Phys.B 18,339(2009).
[36]M.Brouard,H.M.Lambert,S.P.Rayner,and J.P. Simons,Mol.Phys.89,403(1996).
[37]M.L.Wang,K.L.Han,and G.Z.He,J.Chem.Phys. 109,4463(1998).
[38]K.L.Han,L.Zhang,D.L.Xu,G.Z.He,and N.Q. Lou,J.Phys.Chem.A 105,2956(2001).
[39]J.Zhang,T.S.Chu,S.L.Dong,S.P.Yuan,A.P.Fu, and Y.B.Duan,Chin.Phys.Lett.28,093403(2011). [40]M.H.Ge and Y.J.Zheng,Chem.Phys.392,185 (2012).
[41]C.X.Yao and G.J.Zhao,Can.J.Chem.91,387 (2013).
[42]W.L.Li,M.S.Wang,C.L.Yang,W.W.Liu,C.Sun, and T.Q.Ren,Chem.Phys.337,93(2007).
[43]F.J.Aoiz,M.Brouard,and P.A.Enriquez,J.Chem. Phys.105,4964(1996).
[44]M.L.Wang,K.L.Han,and G.Z.He,J.Chem.Phys. 109,5446(1998).
[45]L.Z.Wang,C.L.Yang,J.J.Liang,J.Xiao,and Q.G. Zhang,Chin.J.Chem.Phys.24,686(2011).
[46]A.Zanchet,O.Roncero,T.Gonz´alez-Lezana,A. Rodr´ıguez-L´opez,A.Aguado,C.Sanz-Sanz,and S. G´omez-Carrasco,J.Phys.Chem.A 113,14488(2009).
[47]K.L.Han,G.Z.He,and N.Q.Lou,J.Chem.Phys. 105,8699(1996).
[48]C.Suarez,A.Aguado,C.Tablero,and M.Paniagua, Int.J.Quantum Chem.52,935(1994).
[49]M.D.Chen,K.L.Han,and N.Q.Lou,Chem.Phys. Lett.357,483(2002).
[50]C.Leforestier and W.H.Miller,J.Chem.Phys.100, 733(1993).
[51]R.T.Pack,E.A.Butcher,and G.A.Parker,J.Chem. Phys.99,9310(1993).
[52]J.Q.Dai and J.Z.H.Zhang,J.Phys.Chem.100,1898 (1996).
[53]T.Peng,D.H.Zhang,J.Z.H.Zhang,and R.Schinke, Chem.Phys.Lett.248,37(1996).
[54]D.Kuang,T.Y.Chen,W.P.Zhang,N.J.Zhao,and D.J.Wang,Bull.Korean.Chem.Soc.31,2841(2010).
[55]Q.T.Meng,J.Zhao,Y.Xu,and D.G.Yue,Chem. Phys.362,65(2009).
ceived on June 9,2013;Accepted on October 14,2013)
∗Author to whom correspondence should be addressed.E-mail:shi-ying@jlu.edu.cn
 CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Experimental and Theoretical Investigation on Excited State Intramolecular Proton Transfer Coupled Charge Transfer Reaction of Baicalein
- Infrared Spectroscopy of COIsolated in Solid Nitrogen Matrix
- effects of Rotational Isomerism and Bond Length Alternation on Optical Spectra of FTC Chromophore in Solution
- High Pressure Structural Instability and Thermal Properties of Rutile TiO2from First-principles
- Kinetic Implication from Temperature effect on Hydrogen Evolution Reaction at Ag Electrode
- Anisotropy of Thermal-expansion for β-Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine:Quantum Chemistry Calculation and Molecular Dynamics Simulation
