Infrared Spectroscopy of COIsolated in Solid Nitrogen Matrix
Lei Wu,Ricardo Lambo,Yan Tan,An-wen Liu,Shui-ming Hu
Hefei National Laboratory for Physical Science at the Microscale,University of Science and Technology of China,Hefei 230026,China
Infrared Spectroscopy of COIsolated in Solid Nitrogen Matrix
Lei Wu,Ricardo Lambo∗,Yan Tan,An-wen Liu,Shui-ming Hu
Hefei National Laboratory for Physical Science at the Microscale,University of Science and Technology of China,Hefei 230026,China
The infrared absorption spectra of the CO monomer isolated in solid N2have been recorded at various temperatures between 4.5 and 30 K.The absorption features of the fundamental stretching mode show its linewidth and matrix-induced frequency shift to be weakly temperature-dependent.As the temperature of the matrix was raised,an increase in the linewidth together with a redshift in the central frequency was observed.These observations were explained in terms of the quenching of the CO rotational states by the N2matrix into closely-lying librational states.A quantitative model was then used to calculate the energy difference between these librational states.Results show that they can be thermally populated through the absorption of matrix phonons.
Phonon coupling,Vibrational relaxation,Energy gap law
I.INTRODUCTION
The temperature dependent behavior of infrared absorption spectra is one of the easiest ways to probe basic matrix-molecule interactions and relaxation processes and are therefore of considerable interest.Molecules,for which vibrational relaxation processes provide a dominant contribution to the absorption linewidths,have long been favored for their simplicity.The molecule is typically treated as a harmonic oscillator weakly coupled to a heat bath representing the matrix and its behavior can then be analyzed by solving the equations of motion arising from various model Hamiltonians[1-3]. A recent report on the H2O monomer isolated in N2matrices by our group conf i rmed the Lorentzian lineshape and temperature dependent line broadening predicted by these models[4].
Experimental and theoretical work on systems that decay through other processes have focused extensively on CO isolated in the rare gases(RGs).Fluorescence spectroscopy has demonstrated that its decay is largely radiative with a long lifetime of 16 ms in solid Ar [5].Meanwhile,absorption spectroscopy has demonstrated that its linewidths are inhomogenously broadened and strongly temperature-dependent[6].The principal source of this broadening has been determined to be librational relaxation,though there may be other effects such as the coupling of rotational motion to lattice vibrations[7].However,because of the large number of absorption features presented in CO/RG spectra, which include multiple trapping sites and CO-dimers, distinguishing these effects remains a challenge.
In the present work,we report on the temperaturedependent behavior of the CO monomer isolated in solid N2.The IR absorption spectra of its fundamental stretching mode in the range of 4.5-30 K have been recorded.In contrast to the CO/RG systems,only a single absorption peak is observed which we attribute to monomer occupation in a single substitutional site. Nonetheless,it manifests qualitatively similar temperature effects,i.e a homogeneously broadened linewidth which increases with temperature.We f i nd that these observations are best accounted for in terms of the librational motion of CO in its trapping site,thus providing support for the observations cited for CO/RG systems.
II.EXPERIMENTS
The experimental setup was essentially the same as those reported by our group in previous experiments[4] and only a brief description is given here.The principal apparatus for IR spectroscopy was a Fourier-transform (FT)spectrometer(Bruker IFS120 HR).Spectra in the range of 500-4000 cm-1with an unapodized resolution of 0.1 cm-1were obtained using an MCT detector together with a Globar light source.To minimize absorptions due to air appearing in the spectra,the FT chamber was isolated from the sample chamber and evacuated to a pressure of 40 Pa.
A BaF2window with diameter of 1 in.was selected as the substrate on which the matrix grew.It was thermally anchored to the coldhead of a closedcycle helium refrigerator(Sumitomo-205D)in a sample chamber evacuated to a pressure of 10-5Pa.Standard manometric techniques were used to produce a carbon monoxide/nitrogen gas mixture at a dilution of 1/2000from CO and N2(~99.9%pure,Nanjing Special Gas Inc.).This gas mixture was then deposited on the BaF2window at a flux of 25 mmol/h for 30 min.

FIG.1 Temperature dependent behavior of the matrix isolated CO stretching mode in the range of 4.5-30 K.
Before recording the IR spectra,the matrix was annealed by heating to 30 K over 30 min to reduce defects in the crystal as well as monomer occupation in thermally unstable sites.We were able to stabilize the temperature of the BaF2window within 0.2 K between 4.5 and 30 K using a resistive heater mounted on the coldhead and controlled by a proportional-intergral derivative(PID)loop.The upper limit of this temperature range was chosen to avoid the phase transition that occurred in solid N2at 35 K causing it to change from an fcc crystal to an hcp crystal.These temperatures were monitored by a silicon diode sensor(DT-470)mounted on the baseplate holding the BaF2window and read offa Lake Shore 331S controller.
III.RESULTS
As shown in Fig.1,the spectrum of the CO/N2system at 4.5 K consists of a single absorption peak centered at 2139.8 cm-1.Based on its proximity to the gas phase value of 2413.3 cm-1,we attribute it to the stretching mode of the isolated CO molecule.Previous work on the CO isolated in solid Ar provides a strong clue to the nature of its trapping site.The lattice parameters of the Ar crystal(5.33˚A)and that of N2crystal(5.61˚A)are similar enough that CO is expected to have the same type of occupation in both matrices[7].Furthermore, for the doping of the CO/Ar system with high concentrations of N2,no evidence of CO-N2complexation was found[8].The absorption features shown in Fig.1 are therefore conf i dently assigned to the stretching mode of the CO monomer in a single substitutional site.
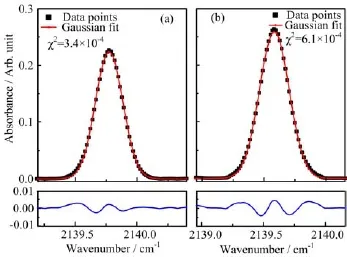
FIG.2 Gaussian fits of the absorption profiles of CO/N2spectra at 4.5 K(a)and 30 K(b)with their respective residuals shown below.
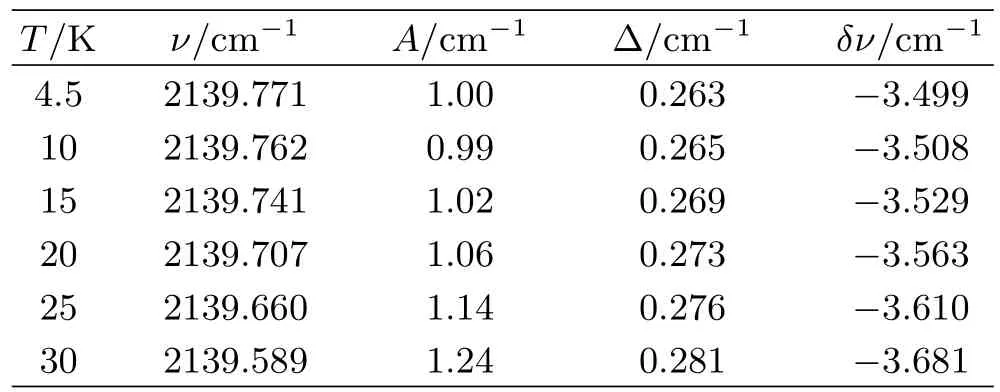
TABLEI Principal features of the absorption spectrum of the CO/N2system.Here T refers to the substrate temperature,ν refers to the measured line position,A refers to area under the peak at temperature T normalized against its area at 4.5 K,∆refers to the transition linewidth(FWHM) obtained from a Gaussian fit of the optical line shape,δν refers to the matrix frequency shift relative to the gas phase transition frequency.
The absorption peaks of the CO/N2spectra all show good fits to Gaussian distributions as illustrated in Fig.2.We therefore conclude that the broadening of the linewidth is mainly due to inhomogeneous processes.A further distinctive feature of this spectrum is the temperature-dependence of its absorption features.As the temperature was increased from 4.5 K to 30 K,the absorption peak frequency was redshifted from 2139.8 cm-1to 2139.5 cm-1together with an increase in the amplitude by 16%.There was also a small broadening of the linewidth from 0.26 cm-1to 0.31 cm-1.All these effects were reversible with temperature and,on cooling to 4.5 K,the original absorption profile was recovered.The main details of the CO absorption spectra are summarized in Table I.
IV.DISCUSSION
The absorption spectrum of the CO/N2system is markedly different from that of other molecules recently studied in solid N2.The ν2mode of the isolated H2O monomer,for instance,has a Lorentzian lineshape due to such homogeneous processes as matrixinduced vibrational relaxation,whereby high frequencyintramolecular modes decay by exciting matrix phonons [4].In the case of matrix-isolated CO,however, fl uorescence experiments have shown that the predominant decay channel is radiative so that the contribution of vibrational relaxation processes to the linewidth is negligible[6].

FIG.3 Illustration of the vibrational(ν)and librational(n) energy levels for CO in solid N2.The solid lines represent radiative transitions,while the dashed lines represent nonradiative transitions.
Following Dubost et al.[6,9],we consider librational motion of CO molecules in their trapping sites as the origin of the Gaussian lineshape and temperaturedependent frequency shift.If one models the crystal field of the trapping site as a potential well of depth V0, two limiting cases with respect to the rotational motion of the isolated molecule arise.In the fi rst,the energy states of the molecule are large compared to V0(i.e. kT?V0)and correspond closely to the rotational states of the free molecule,so that it can be said to undergo quasi-free rotation.In the second,the molecules occupy energy states near the bottom of the potential well (kT¿V0)and have discrete orientations determined by the potential minima.Molecular rotation is then limited to an oscillatory(or librational)motion around equilibrium positions.As illustrated in Fig.3,the energy levels,which are doubly degenerate,are those of a harmonic oscillator:

where ω0is the fundamental librational frequency.
The selection rules for librational transitions have been exposed in detail by Hexter and Dows[10].The strongest transition are those for which∆n=0,though anharmonicity means that weaker∆n=±1,±2 transitions are also allowed.In the CO/RG systems,for example,a small satellite peak corresponding to the ν=0, n=0→ν=1,n=1 is always visible to the blue of the dominant ν=0,n=0→ν=1,n=0 transitions[6].Since these satellite peaks are not visible for CO isolated in N2,the broadening of the central peak is predominantly due to∆n=0 librational transitions originating from the n=1 level.

FIG.4Least-squares fits of the temperature-dependent linewidths,∆,for CO isolated in N2.The errors bars are only minimally visible,but from the Gaussian fits the error on each value of∆is±0.0002 cm-1.
The broading of an ν→ν0vibrational transition due to the librational relaxation rate,kn→n0,in the excited (ν0)and ground vibrational(ν)state at temperature T is given by the following equation:

Theselibrationalstatesarepopulatedthermally through coupling to lattice vibrations.We therefore use the equation derived by Gerber et al.[11]for the transition rate between n=0 and n=1 levels involving the absorption of a phonon:

where D is a constant derived from the dynamical matrix[12],ωDis the Debye frequency,andn¯ωis the phonon population at the transition frequency.As defi ned in Ref.[13],|0i is the matrix element of the dynamical part of the potential describing the interaction of the isolated molecule with the ith matrix atom.
Figure 4 shows the result of a least-squares fi t of the linewidths∆(Table I)to Eq.(2).Here,the librationalphonon coupling was assumed to be sufficiently weak that the lowest measured value of∆could be used as the zero temperature linewidth(i.e.,∆(4.5)≈∆(0)).A value of 15±1 cm-1was obtained for ω0,comparable to value cited for the CO/Ar system(11 cm-1)[13]. The slightly larger value of ω0for the CO/N2system is also consistent with the greater ability of N2matrices to quench rotational states[14],reducing them to librational states with large energy gaps between them.
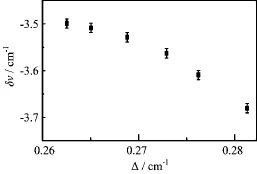
FIG.5 Graph showing the correlation between linewidth∆and frequency shift δν for CO isolated in N2.
The redshift observed in central frequency of the absorption peak with increasing temperature can also be explained in terms of librations of the isolated CO molecule.At low temperature,the ground ν=0,n=0 librational state is predominantly populated,but as temperature increases,the ν=0,n=1 state becomes increasingly populated.The above model has made the simplifying assumption that the libration states have negligible ν dependence i.e.that ω0is independent of ν. However,the experimental evidence suggests that the energy separation between these states is on average smaller in the excited vibrational(ν0)level than that in the ground vibrational(ν)level.The predominance of ν=0,n=1→ν=1,n=1 transitions and others originating from excited librational states at high temperature will therefore lead to a small redshift together with line broadening.The correlation between the linewidth and the redshift can be seen in Fig.5.
As a f i nal observation,we note that the area under the CO/N2absorption peak increases with temperature,as given in Table I.The amplitudes of Fig.1 also clearly illustrate this effect as they increase by 16%from 4.5 K to 30 K,indicating that the absorption and decay processes do not form an energetically closed cycle. This is in contradistinction to the CO/RG absorption peaks which uniformly decrease in amplitude as a consequence of the coupling between the librational motion and the lattice vibrations at high temperature[7]. While the temperature-dependence of the CO/N2absorption amplitudes is still not yet fully understood,we take its behavior as a strong evidence of the very weak librational-phonon coupling in this system.
V.CONCLUSION
The infrared spectra of the CO monomer isolated in solid N2have been recorded.The absorption profile corresponding to the fundamental stretching mode is found to be homogeneously broadened and has a reversible dependence on the temperature of the matrix. As the temperature increases,there is a mild increase in the linewidth together with a redshift in the position of the central frequency.The quenching of rotational motion by the anisotropy of the N2matrices is wellknown from studies of the H2O/N2system[15]and is used here to explain the libration of the CO molecule. The temperature-dependence of the absorption spectra can then be understood in terms of the thermal population of closely-lying librational states through the absorption of matrix phonons.A quantitative model for librational-phonon coupling give the average separation of these states to be 0.2 cm-1.The temperature dependence of the absorption features of the CO monomer isolated in other molecular matrices are currently being explored.These should provide a deeper understanding of the complex matrix-molecule interactions for the CO molecule.
VI.ACKNOWLEDGMENTS
This work was supported by the Young International Scientist Fellowship from the Chinese Academy of Sciences,the National Natural Science Foundation (No.21225314 and No.11150110457),the National Basic Research Program of China(No.2010CB923300),and the Fundamental Research Funds for the Central Universities.
[1]A.Nitzan and J.Jortner,Mol.Phys.25,713(1973).
[2]A.Nitzan and S.Silbey,J.Chem.Phys.60,4070 (1974).
[3]A.Nitzan,S.Mukamel,and J.Jortner,J.Chem.Phys. 60,3929(1974).
[4]L.Wu,R.Lambo,Y.Tan,A.W.Liu,and S.M.Hu, J.Chem.Phys.138,114303(2013).
[5]H.Dubost,L.Abouaf-Marguin,and F.Legay,Phys. Rev.Lett.34,145(1972).
[6]H.Dubost,Chem.Phys.12,139(1976).
[7]J.Lindgren,A.Olbert-Majkut,M.Pettersson,and T. Kiljunen,Low Temp.Phys.38,708(2012).
[8]H.Abe,H.Takeo,and K.M.T.Yamada,Chem.Phys. Lett.311,153(1999).
[9]H.Dubost and L.Abouaf-Marguin,Chem.Phys.Lett. 17,269(1972).
[10]R.M.Hexter and D.A.Dows,J.Chem.Phys.25,1956 (1956).
[11]R.B.Gerber,M.Berkowitz,and V.Yakhot,Mol.Phys. 36,355(1978).
[12]M.Berkowitz and R.B.Gerber,Chem.Phys.Lett.49, 260(1977).
[13]H.Dubost,A.Lecuyer,and R.Charneau,Chem.Phys. Lett.66,191(1979).
[14]X.Michaut,A.M.Vasserot,and L.Abouaf-Marguin, Vib.Spectrosc.34,83(2004).
[15]J.Tursi and E.R.Nixon,J.Chem.Phys.52,1521 (1970).
ceived on May 23,2013;Accepted on May 27,2013)
∗Author to whom correspondence should be addressed.E-mail:lambo@mail.ustc.edu.cn
 CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Experimental and Theoretical Investigation on Excited State Intramolecular Proton Transfer Coupled Charge Transfer Reaction of Baicalein
- Theoretical Study of Reagent Rotational Excitation effect on the Stereodynamics of H+LiF→HF+Li Reaction
- effects of Rotational Isomerism and Bond Length Alternation on Optical Spectra of FTC Chromophore in Solution
- High Pressure Structural Instability and Thermal Properties of Rutile TiO2from First-principles
- Kinetic Implication from Temperature effect on Hydrogen Evolution Reaction at Ag Electrode
- Anisotropy of Thermal-expansion for β-Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine:Quantum Chemistry Calculation and Molecular Dynamics Simulation
