Theoretical Study on the High-Temperature P¯6 and P¯60Phases of Si3N4: A Tool to Aid in Ceramics Development
Ben-hai Yu,Dong Chen
College of Physics and Electronic Engineering,Xinyang Normal University,Xinyang 464000,China
Theoretical Study on the High-Temperature P¯6 and P¯60Phases of Si3N4: A Tool to Aid in Ceramics Development
Ben-hai Yu,Dong Chen∗
College of Physics and Electronic Engineering,Xinyang Normal University,Xinyang 464000,China
Atomistic modeling based on the density functional theory combined with the quasi-harmonic approximation is used to investigate the lattice parameters and elastic moduli of the P¯6 and P¯60phases of Si3N4.β-Si3N4is set as a benchmark system since accurate experiments are available.The calculated lattice constants and elastic constants of β-Si3N4are in good agreement with the experimental data.The crystal anisotropy,mechanical stability,and brittle behavior of P¯6-and P¯60-Si3N4are also discussed in the pressure range of 30-55 GPa. The results show that these two polymorphs are metallic compounds.The brittleness and elastic anisotropy increase with applied pressure increasing.Besides,the phase boundaries of the β→P¯60→δ transitions are also analysed.The β phase is predicted to undergo a phase transition to the P¯60phase at 40.0 GPa and 300 K.Upon further compression,the P¯60→δ transition can be observed at 53.2 GPa.The thermal and pressure effects on the heat capacity,cell volume and bulk modulus are also determined.Some interesting features are found at high temperatures.
First-principles,Nitrides,Brittleness,Phase diagram
I.INTRODUCTION
Silicon nitride(Si3N4)belongs to the group-IV nitrides exhibiting unique physical properties.As an important ceramic,Si3N4can be used as gas turbines, cutting tools,etch masks,solar cells,and energy conversion materials[1,2].Its low density,high strength, tunable electrical conductivity and high decomposition temperature lead to numerous applications[1,3].There are several Si3N4polymorphs,namely α phase(space group:P31c)[4],β phase(space group:P63/m)[4], willemite-II phase(wII-Si3N4,space group:I¯43d)[5],γ phase(space group:Fd¯3m)[6],δ phase(space group: P3)[7],and post-spinel phase(space group:BBMM) [8].In recent years,the α→β[9,10],β→wII[5],β→γ [7,11-13],α→γ[7],β→δ[7],and γ→post-spinel[8,14] phase transitions have been carefully investigated.
Xu et al.found new Si3N4polymorphs(the hexagonal P¯6 and P¯60phases,space group:P3),and observed the high-pressure β→P¯60→δ phase transitions[7].Although many efforts have been made on Si3N4,the P¯6 and P¯60phases are far less studied than their counterparts α-,β-,wII-,δ-and γ-Si3N4.Due to the difficulties in the preparation of polycrystalline samples[7],theoretical calculations,especially f i rst-principles method, can help.Using f i rst-principles calculation,we provide predictions of the thermal quantities,elastic properties, phase stabilities,and phase transition characters of the β,P¯6,P¯60,and δ phases.
II.BENCHMARK CALCULATION
Present calculations are performed using the f i rstprinciples plane-wave method[15]in combination with ultrasoft pseudo-potentials(US-PP)[16]to calculate the total energy of Si3N4.The Perdew-Burke-Ernzerhof functional[17]is used for the exchange-correlation potential.In consideration of accuracy,the plane-wave cut of fenergies of 500 eV(β-Si3N4),450 eV(P¯6-Si3N4), 450 eV(P¯60-Si3N4),and 450 eV(δ-Si3N4)are used in our calculation.The k-point meshes,based on the Monkhorst-Pack scheme[18],are 4×4×12 for β-Si3N4, 5×5×12 for P¯6-Si3N4,5×5×12 for P¯60-Si3N4,and 6×6×15 for δ-Si3N4,respectively.The internal coordinates of different atoms have been fully relaxed. Reference configurations for the valence electrons are Si3s23p2and N2s22p3.The calculated total energies of Si3N4are converged to less than 1µeV/atom.Besides, the crystal structures and atomic coordinates of P¯6-and P¯60-Si3N4can be found in Ref.[7].
The k-point mesh should be described in order to avoid the unclearness.In fact,the k-points are determined by the equation(1/a:1/b:1/c),where a,b,and c are the lattice constants.For β-Si3N4,the experimental values of the lattice constants are a=b=0.7607 nm and c=0.2907 nm(see Table I),thus 1/a:1/b:1/c≈1:1:2.62. The k-point mesh can be chosen as 3×3×8,4×4×12,5×5×14,etc.According to our convergence tests,the plane-wave cutof f500 eV and the k-points 4×4×12 can generate good results for β-Si3N4.For the hexagonal P¯6-and P¯60-Si3N4,we do not know the experimental lattice constants.The only known parameters are the internal coordinates of atoms[7].We have built the unit cell for P¯60-Si3N4using a=b=1.0 nm and c=0.5 nm. After geometry optimization and full relaxation of internal coordinates with very high cut-of fenergy and k-points,the equilibrium lattice constants can be obtained.According to the equation(1/a:1/b:1/c)and the convergence tests,the adequate parameters for P¯60-and P¯6-Si3N4are found to be cut-of fenergy of 450 eV and k-points 5×5×12.
Then,we apply the quasi-harmonic approximation (QHA)scheme[19,20],in which the non-equilibrium Gibbs function G∗(V;P,T)can be determined by

where E(V)is the total energy,pV represents the hydrostatic pressure condition,Avibis the vibrational term,which can be written as[21]:
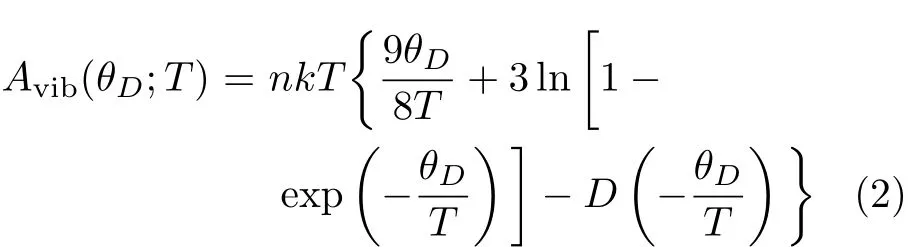
where n,θD,T,and D(θD/T)are the number of atoms, the Debye temperature,the temperature and the Debye integral,respectively.Accordingly,G∗(V;P,T)can be minimized by
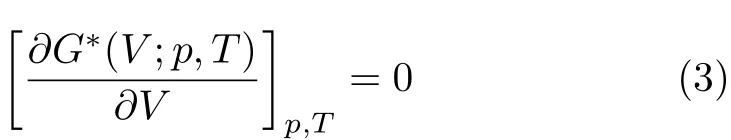
By solving Eq.(3),some thermal properties such as constant-volume heat capacity(CV),isobaric heat capacity(CP)and isothermal bulk modulus(BT)can be obtained by


FIG.1 All-known polymorphs of Si3N4and the transition paths among these phases.The dashed lines denote the β→P¯6→δ transitions.

TABLEI Lattice constants a,c,and cell volume V of the β,P¯60,P¯6,and δ phases.
where V0is the equilibrium volume,kBthe Boltzmann’s constant,α the thermal expansion coefficient,B0the zero-pressure bulk modulus,γ the Gr¨uneisen parameter,pspthe spinodal pressure,L∗the fitting parameter, and B00is the f i rst-order derivative of B0,respectively. A detailed expression of QHA can be found in Refs.[19, 20].
III.RESULTS AND DISCUSSION
The experimentally conf i rmed and theoretically hypothesized transition paths of the Si3N4polymorphs are illustrated in Fig.1.The β→P¯6→δ transitions(dashed line)have not been verif i ed by experiments or theoretical studies.In order to have a deep insight into the fundamental properties of Si3N4,we have calculated the pressure dependences of lattice constants,cell volumes, elastic constants,and elastic moduli.The results are shown in Tables I and II.
Since the experimental data of β-Si3N4are available, we have calculated the fundamental properties of theβ phase.As shown in Table I,the predicted lattice constants and cell volumes are in excellent agreement with the experimental data and the theoretical results. The calculated elastic constants of β-Si3N4given in our previous work[10]were also in satisfactory agreement with the results in Refs.[7,22].For P¯6-and P¯60-Si3N4, a,c,and V decrease with the pressure increasing.The cell volume of the P¯6 phase is larger than that of the P¯60phase.Therefore,the channels in the P¯60phase are larger than those in P¯60-Si3N4.

TABLE II Calculated elastic constants Cij(in GPa),bulk modulus BH(in GPa),shear modulus GH(in GPa),Young’s modulus YH(in GPa),Poisson ratio σ and anisotropy factor A of P¯6-and P¯60-Si3N4.
It is well known that the elastic constants are calculated by means of Taylor expansion of the total energy, E(V,δ),for the system with respect to a small strain δ of the cell volume V.The energy of a strained system can be expressed as follows[28]:

where,E(V0,T)is the energy of the unstrained system with equilibrium volume V0at different temperatures,τiis an element in the stress tensor,and ξiis a factor to consider Voigt index[28].The total energy E(V0,T)at a certain temperature T and the f i nite temperature lattice constant can be obtained by the vibrational Debye-like model.According to the Voigt-Reuss-Hill approximation,the bulk modulus BH,shear modulus GH,Young’s modulus YH,Poisson ratio σ and anisotropy factor A can be obtained by[29]


where the subscripts V,R,and H are the Voigt index, the Reuss index,and the Hill index,respectively.These quantities are listed in Table II.
For a hexagonal lattice,there are f i ve independent elastic constants C11,C12,C13,C33,and C44(2C66=C11-C12).As listed in Table II,the elastic constants Cij,elastic moduli BH,GH,and YHof P¯60-Si3N4increase monotonously with applied pressure,but the slopes are different.The pressure effect on Cijis signif i cant.The decrease of C44ref l ects the shear resistance decreases in the{010}or{100}plane in the h001i direction.The value of the Poisson ratio σ for covalent materials is quite small(about 0.1),whereas for metallic materials a typical σ is 0.33[30].The calculated σ is 0.320-0.336,showing moderate lateral expansion when compressed.The P¯60phase is a metallic compound in the whole pressure range of 30-55 GPa. The anisotropy factor A=1 represents completely elastic isotropy while any value smaller or larger than 1 indicates elastic anisotropy.A increases as the pressure increases f i rst,then the elastic anisotropy of P¯60-Si3N4is quite small at 40 GPa.When P>40 GPa,A will gradually strengthen with the pressure increasing.Besides,the BH/GHratio ref l ects the brittle and ductile behaviors of polycrystalline materials since solids behave in brittle manners if BH/GH>2[31].P¯60-Si3N4remains brittle in the pressure range of 30-55 GPa. The brittleness increases with the pressure increasing. More importantly,the chemical bonds in P¯60-Si3N4are ionic due to the fact that the typical B/G ratios for covalent and ionic solids are 0.91 and 1.67,respectively.
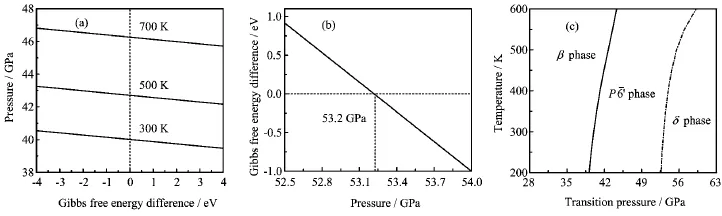
FIG.2(a)Gibbs free energy difference(GP¯60-Gβ)as a function of pressure.(b)Critical pressure as a function of Gibbs free energy difference of the P¯60→δ transition at 300 K.(c)Relationships of the transition pressures of the β→P¯60→δ transitions with temperature.
In Table II,we can see that the elastic moduli of P¯6-Si3N4do not follow the same trend.C12,C13,and BHincrease with the increasing pressure whereas GHand YHshow the opposite trends.C11,C33,and C44increase f i rst,and then decrease with the increasing pressure.The calculated σ is 0.347-0.399,which indicates that P¯6-Si3N4is a metallic compound.The variation of A with pressure(P¯6-Si3N4)is similar to that of P¯6-Si3N4.The anisotropy of P¯6-Si3N4is stronger than that of P¯60-Si3N4since|A(P¯60)-1|>|1-A(P¯6)|. According to the BH/GHratio,P¯6-Si3N4is more brittle than P¯60-Si3N4.For a hexagonal structure,the mechanical stability can be determined by the Born’s criteria[32]:C11-|C12|>0,C44>0,(C11+C12)C33-2C213>0. At 0 K,P¯6-and P¯60-Si3N4are stable in the pressure range of 30-55 GPa since all the elastic constants satisfy these criteria.Besides,the bulk moduli of the two phases are greater than the bulk moduli of α,β,and γ phases[33].
The transition pressures among different phases of solids can be obtained by comparing the Gibbs free energy G of different phases.We have calculated the Gibbs free energy difference between β-Si3N4and P¯60-Si3N4,as shown in Fig.2(a).The thermodynamic requirement,for the equality of G,at the critical points, suggests that the transition pressures of the β→P¯60transition are 40.0,42.7,and 46.2 GPa at temperatures of 300,500,and 700 K,respectively.β-Si3N4has the lower free energy at low pressures,which indicates that this phase is the low-temperature phase of Si3N4.The Gibbs free energy difference obtained,Gδ-GP¯60,as a function of pressure is given in Fig.2(b).It is clearly seen that the transition pressure between the P¯60phase and the δ phase is 53.2 GPa(at 300 K),at which the P¯60phase will automatically transform into δ-Si3N4.P¯60-Si3N4has the lower free energies when P<53.2 GPa. The δ phase has the lower free energies at higher pressure,i.e.δ-Si3N4would be favored at sufficiently high pressures.The δ phase boundaries of the β→P¯60→δ transitions are illustrated in Fig.2(c).
As shown in Fig.2(c),the slopes of the β→P¯60and P¯60→δ transitions are both positive,which suggests that at higher temperatures it will require higher pressures to synthesize P¯60-Si3N4/δ-Si3N4.It is found that the transition pressure from the β phase to the P¯60phase is 40.0 GPa(at 300 K).The critical pressure of the β→P¯60transition is about 13 GPa higher than that of the P¯60→δ transition.According to the Clausius-Clapeyron relation[11],the slope of the phase boundary can be determined by∆S/∆V,where∆S and∆V are the entropy change and the volume variation,respectively.Therefore,the β→P¯60→δ transitions are accompanied by the shrinkage of volume,which is in agreement with the cell volumes listed in Table I(the calculated volume of δ-Si3N4at 55 GPa is 109.6˚A3).
One of the most important vibrational properties of solids is the temperature dependence of heat capacity. Figure 3(a)and(b)give the evolutions of the constantpressure heat capacity CPwith temperature for P¯6-and P¯60-Si3N4,respectively.As shown in Fig.3(a),CPis very small at low temperature.From 0 to 500 K,CPincreases exponentially with the temperature increasing.At high temperatures,CPfollows a linear increase, which is not similar to the constant-volume heat capacity CV(CVfollows the Dulong-Petit’s law,i.e.3R for monoatomic solids).Although CVgives direct information on the intrinsic anharmonic effects,it is CPthat is experimentally measured,and it contains both anharmonic effects and quasi-harmonic contributions.In Fig.3(b),it is clearly seen that CPincreases rapidly at low temperatures,and reaches a plateau region at high temperatures.The heat capacity of P¯6-Si3N4is larger than that of P¯60-Si3N4at the same temperature.
In Fig.3(c),it is clearly seen that the volumes of P¯6-and P¯60-Si3N4decrease smoothly as pressure increases.The V/V0ratio of P¯6-Si3N4decreases faster than that of P¯60-Si3N4.At high pressures,the difference between the two curves can be clearly seen.This means that the P¯6 phase is more compressible than the P¯60phase.Figure 3(d)shows the evolutions of bulk moduli with pressure at 300 K.The bulk modulus increases with the increasing pressure but the rate of increase is moderate.The f i rst sticking feature is that the bulk modulus of P¯60-Si3N4is greater than that of P¯6-Si3N4.The second feature is that the slopes of the two curves are different.At 50 GPa and 300 K,the calculated bulk modulus and heat capacity are 391.4 GPa (343.2 GPa)and 44.8 J/(mol K)(77.2 J/(mol K))forP¯60-Si3N4(P¯6-Si3N4),respectively.

FIG.3 The constant-pressure heat capacity CP(at 50 GPa)for(a)P¯6-Si3N4,(b)P¯60-Si3N4,(c)the normalized cell volume V/V0(V0is the equilibrium cell volume at 40 GPa),and(d)bulk modulus of Si3N4at 300 K.
IV.CONCLUSION
First-principlescalculationsarecarriedouton Si3N4in the recently-discovered P¯60and P¯6 phases to investigate their stability and physical properties, which have not yet been established experimentally.As a benchmark system(β-Si3N4),the calculated lattice constants and elastic moduli are in agreement with the experimental data.The lattice parameters,cell volume,elastic constants,elastic moduli,anisotropy factor and Poisson ratio of P¯6-and P¯60-Si3N4are also predicted in the pressure range of 30-55 GPa. β-Si3N4is predicted to undergo a f i rst-order phase transition to the P¯60phase at 40.0 GPa and 300 K. Upon further compression,the P¯60→δ transformation can be observed at 53.2 GPa.The positive slopes of the β→P¯60→δ transitions mean that the two phase transformations are accompanied by the shrinkage of volume.The two polymorphs are brittle compounds with little metallic character,which is not similar to the β phase.The anisotropy of P¯60-Si3N4increases with the increasing pressure while the anisotropy of P¯6-Si3N4shows the opposite trend.The heat capacity increases rapidly at low pressures,and reaches a plateau at high pressures.Furthermore,the P¯6 phase is more compressible than the P¯60phase.
V.ACKNOWLEDGMENTS
This work was supported by the National NaturalScienceFoundationofChina(No.U1204501, No.11105115,and No.11304141),the Project of Basic and Advanced Technology of Henan Province of China (No.112300410021),and the Key Project of Henan Educational Committee(No.12A140010).The authors are grateful to Prof.M.A.Blanco from the Departamento de Qu´ımica F´ısicay Analitica,Faculatad de Qu´ımica, Universidad de Oviedo for the Gibbs code.
[1]S.Y.Ren and W.Y.Ching,Phys.Rev.B 23,5454 (1981).
[2]W.X.Wang,D.H.Li,Z.C.Liu,and S.H.Liu,Appl. Phys.Lett.62,321(1993).
[3]Y.N.Xu and W.Y.Ching,Phys.Rev.B 51,17379 (1995).
[4]S.D.Mo,L.Z.Ouyang,W.Y.Ching,I.Tanaka, Y.Koyam,and R.Riedel,Phys.Rev.Lett.83,5046 (1999).
[5]P.Kroll,J.Solid State Chem.176,530(2003).
[6]A.Zerr,G.Miehe,G.Serghiou,M.Schwarz,E.Kroke, R.Riedel,H.Fueß,P.Kroll,and R.Boehler,Nature (London)400,340(1999).
[7]B.Xu,J.J.Dong,P.F.McMillan,O.Shebanova,and A.Salamat,Phys.Rev.B 84,014113(2011).
[8]K.Tatsumi,I.Tanaka,and H.Adachi,J.Am.Ceram. Soc.85,7(2002).
[9]N.V.Danilenko,G.S.Oleinik,V.D.Dobrovol’skii,V. F.Britun,and N.P.Semenenko,Sov.Powder Metal. Met.Ceram.31,1035(1992).
[10]B.H.Yu and D.Chen,Chin.Phys.B 21,060508(2012).
[11]A.Kuwabara,K.Matsunaga,and I.Tanaka,Phys.Rev. B 78,064104(2008).
[12]A.Togo and P.Kroll,J.Comput.Chem.29,2255 (2008).
[13]H.L.He,T.Sekine,T.Kobayashi,and H.Hirosaki, Phys.Rev.B 62,11412(2000).
[14]P.Kroll and J.von Appen,Phys.Stat.Sol.B 226,R6 (2001).
[15]P.Hohenberg and W.Kohn,Phys.Rev.136,B864 (1964).
[16]D.Vanderbilt,Phys.Rev.B 41,7892(1990).
[17]J.P.Perdew,K.Burke,and M.Ernzerhof,Phys.Rev. Lett.77,3865(1996).
[18]H.J.Monkhorst and J.D.Pack,Phys.Rev.B 13,5188 (1976).
[19]M.A.Blanco,E.Francisco,and V.Lua´na,Comput. Phys.Commun.158,57(2004).
[20]A.Otero-de-la-Roza and V.Lua´na,Comput.Phys. Commun.182,1708(2011).
[21]M.Fl´orez,J.M.Recio,E.Francisco,M.A.Blanco,and A.Martin Pend´as,Phys.Rev.B 66,144112(2002).
[22]C.M.Marian,M.Gastreich,and J.D.Gale,Phys.Rev. B 62,3117(2000).
[23]W.Y.Ching,L.Z.Ouyang,and J.D.Gale,Phys.Rev. B 61,8696(2000).
[24]M.Yashima,Y.Ando,and Y.Tabira,J.Phys.Chem. B 111,3609(2007).
[25]H.F.Priest,F.C.Burns,G.L.Priest,and E.C.Skaar, J.Am.Ceram.Soc.56,395(1973).
[26]N.Hirosaki,S.Ogata,C.Kocer,H.Kitagawa,and Y. Nakamura,Phys.Rev.B 65,134110(2002).
[27]W.Y.Ching,Y.N.Xu,J.L.D.Gale,and M.R¨uhle, J.Am.Ceram.Soc.81,3189(1998).
[28]L.Fast,J.M.Wills,B.Johansson,and O.Eriksson, Phys.Rev.B 51,17431(1995).
[29]M.B.Kanoun,S.Goumri-Said,A.H.Reshak,and A. E.Merad,Solid State Sci.12,887(2010).
[30]J.Haines,J.M.Leger,and G.Bocquillon,Annu.Rev. Mater.Res.31,1(2001).
[31]S.F.Pugh,Philos.Mag.Ser.45,823(1954).
[32]M.Born and K.Huang,Dynamical Theory of Crystal Lattics,Oxford:Clarendon,(1956).
[33]C.Zhang,J.X.Sun,R.G.Tian,and S.Y.Zou,Acta Phys.Sin.56,5969(2007).
ceived on August 15,2013;Accepted on October 14,2013)
∗Author to whom correspondence should be addressed.E-mail:chchendong2010@163.com,Tel.:+86-376-6391731,FAX:+86-376-6391731
 CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2014年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Experimental and Theoretical Investigation on Excited State Intramolecular Proton Transfer Coupled Charge Transfer Reaction of Baicalein
- Theoretical Study of Reagent Rotational Excitation effect on the Stereodynamics of H+LiF→HF+Li Reaction
- Infrared Spectroscopy of COIsolated in Solid Nitrogen Matrix
- effects of Rotational Isomerism and Bond Length Alternation on Optical Spectra of FTC Chromophore in Solution
- High Pressure Structural Instability and Thermal Properties of Rutile TiO2from First-principles
- Kinetic Implication from Temperature effect on Hydrogen Evolution Reaction at Ag Electrode
