微脉冲半导体激光对兔视网膜色素上皮细胞阈值下光凝的光生物调制效应△
杨宝娣 宋艳萍 陈中山 丁琴
微脉冲半导体激光对兔视网膜色素上皮细胞阈值下光凝的光生物调制效应△
杨宝娣 宋艳萍 陈中山 丁琴
微脉冲激光;视网膜色素上皮细胞;血管内皮生长因子;色素上皮源性因子;碱性成纤维细胞生长因子
目的探讨微脉冲激光阈值下光凝对色素兔视网膜形态结构以及视网膜色素上皮细胞(retinal pigment epithelial cells,RPE)分泌细胞因子的影响。方法微脉冲810 nm激光阈值下光凝色素兔视网膜,光凝后1 d、3 d、7 d、14 d分别行眼底荧光血管造影(fluorescein angiography,FFA)和组织切片技术观察视网膜结构变化;免疫荧光技术观察视网膜血管内皮生长因子(vascular endothelial growth factor,VEGF)、色素上皮源性因子(pigment epithelium-derived factor,PEDF)、碱性成纤维细胞生长因子(basic fibroblast growth factor,b-FGF)的表达变化;RT-PCR检测视网膜组织匀浆中VEGF mRNA、PEDF mRNA、b-FGF mRNA的表达变化。结果光凝后FFA检查未见荧光素渗漏;组织切片各层结构基本完整。免疫荧光表明,光凝后VEGF、PEDF在视细胞层及RPE层表达明显增强;b-FGF在神经纤维层、节细胞层也有表达。RT-PCR表明,激光后3种细胞因子mRNA含量均增多,光凝后1 d PEDF mRNA(3.748±0.890)表达最高,3 d次之,与其他时间点比较差异均有统计学意义(均为P<0.05);b-FGF mRNA光凝后1 d(1.578±0.299)增高最明显,但与其他时间点比较差异均无统计学意义(均为P>0.05);光凝后3 d VEGF mRNA(2.301±0.378)表达最高,与其他时间点比较差异均有统计学意义(均为P<0.05)。光凝后14 d,3种细胞因子的升高幅度相近(1.283±0.310、1.662±0.409、1.310±0.184)。结论微脉冲810 nm激光阈值下光凝可刺激正常视网膜RPE细胞协调表达分泌3种细胞因子,且对视网膜组织结构无损伤。
[眼科新进展,2014,34(1):5-9]
自从眼底激光器问世,激光治疗已成为众多眼底病的主要治疗方法,包括视网膜血管性疾病、视网膜裂孔、眼内肿瘤以及青光眼等,尤其对于视网膜血管性疾病,眼底激光治疗是目前最主要的方法之一。传统激光主要通过激光的热损伤作用达到治疗的目的,包括破坏视网膜组织、改善残余视网膜的供养状态、改变视网膜内血管活性因子的产生等[1-2]。但是这种治疗为强激光治疗,会引起医源性视网膜损伤,限制了激光在黄斑区疾病的应用[3]。近年来,很多学者提出阈值下光凝,即光凝所采用的激光能量处于损伤阈值(细胞失活)水平以下,在达到治疗效果的同时将视网膜损伤降到最小。810 nm激光穿透性较强,主要作用于视网膜色素上皮(retinal pigment epithelium,RPE)层,采用微脉冲模式,调整合适的参数可进行阈值下光凝。目前研究表明,阈值下微脉冲810 nm 半导体激光可成功治疗糖尿病黄斑水肿[4-5]、中心性浆液性脉络膜视网膜病变[6-7]等,但治疗机制仍未完全明确。目前推测,阈值下微脉冲810 nm激光可选择性作用于视网膜RPE层,通过光生物调制效应刺激RPE 再生,增强其吞噬功能,并促进分泌各种血管活性因子[8-9],如血管内皮生长因子(vascular endothelial growth factor,VEGF)、碱性成纤维细胞生长因子(basic fibroblast growth factor,b-FGF)、色素上皮源性因子(pigment epithelium-derived factor,PEDF)等,使促血管生长因子与抗血管生长因子达到新的平衡,抑制新生血管的形成,改善视网膜屏障功能,达到治疗眼底血管性疾病的目的[10]。本研究我们观察微脉冲810 nm激光阈值下光凝对色素兔视网膜形态结构的影响以及激光后RPE细胞表达分泌VEGF、PEGF、b-FGF 的变化,进一步明确阈值下激光治疗的作用机制。
1 材料与方法
1.1材料
1.1.1实验动物健康青紫蓝兔12只24眼,体质量2.5~3.0 kg,雌雄不限,由广州军区武汉总医院动物实验中心提供,实验前双眼前节及眼底检查均正常。
1.1.2主要仪器与试剂810 nm半导体激光器(IRIDEX公司)、眼底荧光造影仪(Heidelberg公司);小鼠抗兔VEGF单克隆抗体(Abcam公司)、小鼠抗人PEDF单克隆抗体(Abcam公司)、小鼠抗人b-FGF单克隆抗体(Abcam公司)、荧光(Cy3)标记羊抗小鼠IgG(武汉博士德生物工程有限公司)、抗荧光淬灭封片剂(Southernbiotech公司)、Trizol液(Invitrogen公司)、cDNA反转录合成试剂盒(Fermentas公司)、SYBR Green荧光定量PCR试剂盒(Fermentas公司)。
1.2实验方法
1.2.1动物分组、激光造模、眼底荧光血管造影检查12只(24眼)青紫蓝兔随机分为两组:实验组8只(16眼),对照组4只(8眼)。100 g·L-1水合氯醛3.5 mL·kg-1腹腔注射麻醉,复方托吡卡胺眼液滴眼散瞳,盐酸奥布卡因眼液滴眼行表面麻醉。实验组16眼在眼前放置全视网膜镜,经裂隙灯用810 nm半导体激光,参照Sanislo等[11]微脉冲激光对兔眼视网膜形态学阈能量、组织学阈能量及阈下能量的比值关系和阈值下微脉冲半导体激光治疗糖尿病黄斑水肿的相关方法[12-13]确定激光参数:功率400 mV,光斑200 μm,时间(每次发射激光的总时间)200 ms,负载系数10%,避开视盘及有髓神经纤维,距离视盘垂直距离1 PD处上下方行视网膜光凝,每眼500点。对照组8眼不做激光光凝。激光光凝后1 d、3 d、7 d、14 d麻醉后行眼底荧光血管造影(fluorescein angiography,FFA)检查。随后深麻醉处死,摘取眼球,进行下一步实验操作。对照组每个时间点分别选取一只兔子进行实验组同样的操作。
1.2.2视网膜组织切片HE染色每个时间点各摘取兔右眼球,40 g·L-1多聚甲醛固定,酒精梯度脱水,二甲苯透明,石蜡包埋,切片厚4 μm,脱蜡,脱水,苏木素-伊红(HE)染色,中性树胶封片,显微镜下观察视网膜组织结构改变,采集分析图像。
1.2.3视网膜组织切片免疫荧光检测VEGF、PEDF和b-FGF表达视网膜组织切片脱蜡,抗原修复,山羊血清封闭抗原,分别滴加一抗过夜,即小鼠抗兔VEGF单克隆抗体(150)、小鼠抗人PEDF单克隆抗体(1100)、小鼠抗人b-FGF单克隆抗体(1300),在暗处滴加荧光(Cy3)标记羊抗小鼠IgG,核复染,封片,荧光显微镜观察采集图像。
1.2.4荧光定量RT-PCR检测视网膜VEGF、PEDF和b-FGF的mRNA表达采用Trizol一步法提取总RNA,紫外分光光度计检测RNA的纯度与浓度。按照M-MLV逆转录酶试剂盒说明书进行逆转录反应合成第一链cDNA。按照SYBR Green荧光染料试剂盒说明书建立20 μL反应体系,用ABI7900型荧光定量PCR仪进行扩增反应。PCR热循环数:50 ℃ 2 min,95 ℃ 10 min循环1次,接着变性95 ℃ 30 s,退火和延伸60 ℃ 30 s,循环40次。所用引物序列:VEGF上游引物5’-CTACCTCCACCATGCCAAGT-3’,下游引物5’-GCACTCCAGGCTTTCATCAT-3’;PEDF上游引物5’-ATCACAGGCAAGCCCATCAA-3’,下游引物5’-GCTGGTTCAGGTGGTAGTCC-3’;b-FGF上游引物5’-GTGCAAACCGTTACCTTGCT-3’,下游引物5’-ACTGCCCAGTTCGTTTCAGT-3’;内参β-actin上游引物5’-AGTGCGACGTGGACATCCG-3’,下游引物5’-TGGCTCTAACAGTCCGCCTAG-3’。每个样本分别重复3次,以提高实验的重复性和可信度。目标mRNA的相对含量用相对Ct值法(△△Ct)。

2 结果
2.1光凝前后FFA检查结果光凝前后,FFA检查无明显的荧光素渗漏,光凝后即刻可见不明显的激光斑样透见荧光,光凝后14 d透见荧光减弱(图1)。
2.2光凝前后视网膜组织结构变化光镜下,正常色素兔视网膜层次结构清晰完整,RPE层连续规则。光凝后实验组兔视网膜各层结构完整,但光凝后1 d,视网膜RPE层局灶性小隆起、欠规整;光凝后3 d RPE层仍欠规整,局灶性小隆起较前好转;光凝后14 d视网膜各层结构恢复正常(图2)。

Figure 1 FFA results of rabbit’s retina before and after photocoagulation.A:FFA before photocoagulation;B:FFA after photocoagulation instantly;C:FFA at 14 days after photocoagulation 光凝前后兔眼视网膜FFA检查结果。A:光凝前FFA;B:光凝后即刻FFA;C:光凝后14 d FFA
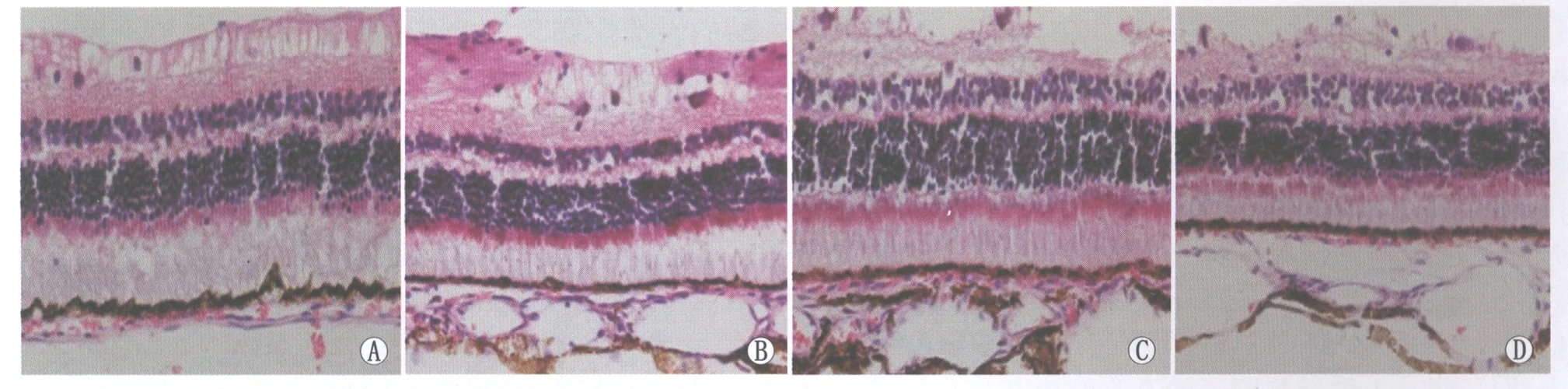
Figure 2 Changes of retinal tissue of rabbits (HE,×400).A:One day after photocoagulation;B:Three days after photocoagulation;C:Seven days after photocoagulation;D:Fourteen days after photocoagulation 实验组兔视网膜组织学改变(HE,×400)。A:光凝后1 d;B:光凝后3 d;C:光凝后7 d;D:光凝后14 d
2.3光凝前后视网膜内VEGF、PEDF、b-FGF的表达对照组VEGF在神经纤维层(nerve fiber layer,NFL)、节细胞层(ganglion cell layer,GC)、内丛状层(inner plexiform layer,IPL)、内核层(inner nuclear layer,INL)、外丛状层(outer plexiform layer,OPL)、视锥视杆细胞层(rods or cones cell layer,RC)、RPE层表达;实验组光凝后VEGF在RC及RPE层表达明显增强,且光凝后3 d表达最强,以后逐渐减弱,光凝后14 d基本恢复正常(图3)。对照组PEDF在NFL、GC、IPL、INL、RPE层表达;实验组光凝后PEDF在RC及RPE层表达明显增强,光凝后1 d表达最强,3 d次之,以后逐渐减弱,但光凝后14 d仍较激光光凝前表达增强(图4)。对照组b-FGF在RC、RPE层表达;实验组光凝后b-FGF在NFL、GC也有表达,且光凝后1 d表达更强,此后逐渐减弱,光凝后14 d基本恢复正常(图5)。

Figure 3 Expression of VEGF in each retinal layer before and after photocoagulation.A:Expression of VEGF in NFL,GC,IPL,INL,OPL,RC and RPE layers in control group;B:Expression of VEGF in RC and RPE layers enhanced obviously at 3 days after photocoagulation in experimental group 光凝前后VEGF在视网膜各层的表达。A:对照组VEGF在NFL、GC、IPL、INL、OPL、RC、RPE层表达;B:实验组光凝3 d后VEGF在RC及RPE层表达明显增强

Figure 4 Expression of PEDF in retina before and after photocoagulation.A:Expression of PEDF in control group;B:Expression of PEDF at 1 day after photocoagulation in experimental group;C:Expression of PEDF at 3 days after photocoagulation in experimental group 光凝前后PEDF在视网膜的表达。A:对照组PEDF表达;B:实验组光凝后1 d PEDF表达;C:实验组光凝后3 d PEDF表达(×200)
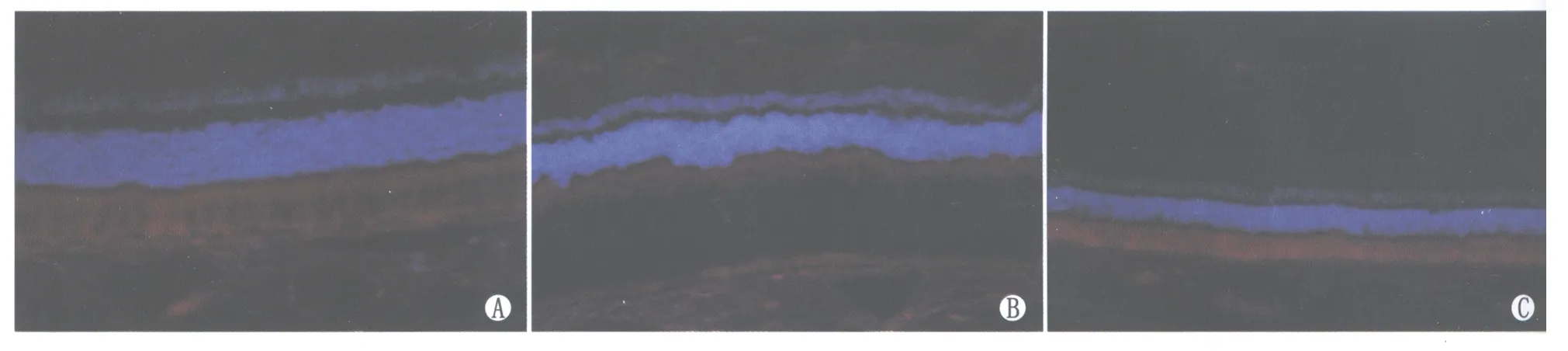
Figure 5 Expression of b-FGF in each retinal layer before and after photocoagulation.A:Expression of b-FGF in RC and RPE layers in control group;B:Expression of b-FGF in NFL and GC layers at 1 day after photocoagulation in experimental group;C:Expression of b-FGF at 7 days after photocoagulation in experimental group 光凝前后b-FGF在视网膜各层的表达。A:对照组b-FGF在RC、RPE层表达;B:实验组光凝后1 d b-FGF在NFL、GC也有表达;C:实验组光凝后7 d b-FGF表达
2.4光凝后VEGF、PEDF和b-FGF的mRNA表达微脉冲激光光凝后视网膜组织中VEGF、PEDF和b-FGF mRNA表达均增多,其中VEGF mRNA激光后3 d表达最强,与其他时间点比较差异均有统计学意义(均为P<0.05),激光后7 d及14 d表达逐渐减弱;PEDF mRNA光凝后1 d表达最强,3 d次之,与其他时间点比较差异均有统计学意义(均为P<0.05),此后表达逐渐减弱;b-FGF mRNA光凝后表达增强,但各个时间点统计学差异不显著(均为P>0.05)。光凝后14 d 3种细胞因子的升高幅度相近(表1)。

表1 光凝后VEGF mRNA、PEDF mRNA 和b-FGF mRNA 不同时间点的表达变化Table 1 Expression of VEGF,PEDF,b-FGF mRNA at different time points after laser photocoagulation
3 讨论
新生血管的形成是很多眼底病共同的病理改变,如湿性年龄相关性黄斑变性、病理性近视、增殖期糖尿病视网膜病变等,它是机体对缺血、缺氧及炎症等刺激的一种适应性反应,但极易造成出血、渗出及过度增生等病理改变,损伤眼部结构,引起视功能的严重障碍甚至致盲。大量研究表明新生血管形成的直接原因是促血管生长因子与抗血管生长因子之间的平衡失调[10,14-15]。其中VEGF和b-FGF在促血管生长因子中作用显著,而PEDF在抗血管生长因子中作用显著。在体内RPE有多种生理功能,包括遮光、构成视网膜-脉络膜屏障、物质转运、视黄醛的转运存储、吞噬降解脱落的感光细胞外节、清除氧自由基以及合成多种细胞因子等[16]。正常生理状态下,体内的RPE相对静止,无增殖现象,但是受到炎症、理化因素及光化学因素的刺激将发生增殖,并分泌多种细胞因子,如VEGF、PEDF和b-FGF等[10,17]。通过调整合适的激光参数,可使阈值下微脉冲810 nm激光主要作用于RPE层,对内层神经视网膜和外层脉络膜的影响很小。本研究主要通过观察阈值下微脉冲激光光凝前后VEGF、PEDF、b-FGF的变化,探讨阈值下激光对视网膜RPE细胞的光生物学效应,进一步为视网膜新生血管疾病的治疗提供新的思路。
VEGF可诱导内皮细胞的增殖、促进细胞分裂,抑制细胞凋亡,增加血管通透性[18],具有很强的促血管生长作用。b-FGF属于肝素结合生长因子家族成员,具有比VEGF更强的促内皮细胞分裂的作用[19-20],然而,Ozaki等[21]研究表明b-FGF是发生视网膜新生血管的非必需非充分因素。Wong等[22]实验结果表明,兔眼玻璃体内同时注射VEGF和b-FGF可使视网膜新生血管长得更快,渗漏更明显,而VEGF与b-FGF单独作用均不会出现这种效果。此外,b-FGF可诱导Müller胶质细胞分泌VEGF,并促进细胞增殖[23]。以上研究说明,b-FGF对VEGF有协同刺激作用。本研究显示光凝后1 d b-FGF升高最明显,VEGF在光凝后3 d升高最明显,这与Hu等[14]的研究结果一致,推测在视网膜新生血管的形成过程中,b-FGF可能对VEGF功能的发挥起着启动和允许作用。PEDF具有诱导培养的Y79视网膜母细胞瘤细胞神经分化作用。后来的研究表明,PEDF具有神经保护[24]和很强的抑制新生血管生长的作用[25-26]。
本研究RT-PCR结果显示,光凝后1 d、3 d、7 d、14 d,三种细胞因子mRNA表达均升高,其中PEDF、b-FGF光凝后1 d升高最显著,VEGF光凝后3 d升高最显著,这与Hu等[14]及Ogata等[8]的实验结果一致。光凝后14 d三种因子的升高幅度相似,说明阈值下激光可刺激视网膜RPE细胞分泌细胞因子,并保持促血管生长因子与抗血管生长因子的内在平衡,可能起到预防或治疗视网膜新生血管的作用。本研究FFA检查未见血管荧光素渗漏,仅见不明显的激光斑样透见荧光,视网膜激光部位病理切片HE染色视网膜各层结构未见明显损伤,说明采用本实验激光参数进行阈值下光凝安全可行,并可以通过及时荧光造影观察定位激光作用部位。
本研究初步证实了阈值下微脉冲810 nm激光对视网膜RPE层的生物调制效应,促进其分泌细胞因子,并调节各种因子的内在平衡,发挥预防或治疗视网膜新生血管作用的同时避免造成医源性视网膜损伤,逆转了对传统激光破坏性治疗视网膜血管性疾病的观点,为黄斑部疾病尤其是血管性疾病的治疗提供了新的思路。
1 Lange CA,Stavrakas P,Luhmann UF,Silva DJ,Ali RR,Gregor ZJ,etal.Intraocular oxygen distribution in advanced proliferative diabetic retinopathy[J].AmJOphthalmol,2011,152(3):406-412.
2 Blumenkranz MS.Optimal current and future treatments for diabetic macular oedema[J].Eye,2010,24(3):428-434.
3 Shah AM,Bressler NM,Jampol LM.Does laser still have a role in the management of retinal vascular and neovascular disease[J]?AmJOphthalmol,2011,152(3):332-339.
4 Laursen ML,Moeller F,Sander B,Sjoelie AK.Subthreshold micropulse diode laser treatment in diabetic macular oedema[J].BrJOphthalmol,2004,88(9):1173-1179.
5 Luttrull JK,Dorin G.Subthreshold diode micropulse laser photocoagulation(SDM) as invisible retinal phototherapy for diabetic macular edema:a review[J].CurrdiabetesRev,2012,8(4):274-284.
6 Ricci F,Missiroli F,Regine F,Grossi M,Dorin G.Indocyanine green enhanced subthreshold diode-laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy[J].ClinExpOphthalmol,2009,247(5):597-607.
7 Gupta B,Elagouz M,McHugh D,Chong V,Sivaprasad S.Micropulse diode laser photocoagulation for central serous chorio-retinopathy[J].ClinExpOphthalmol,2009,37(8):801-805.
8 Ogata N,Tombran-Tink J,Jo N,Mrazek D,Matsumura M.Upregulation of pigment epithelium-derived factor after laser photocoagulation[J].AmJOphthalmol,2001,132(3):427-429.
9 Yu AK,Merrill KD,Truong SN,Forward KM,Morse LS,Telander DG.The comparative histologic effects of subthreshold 532-and 810-nm diode micropulse laser on the retina[J].InvestOphthalmolVisSci,2013,54(3):2216-2224.
10 Schlingemann RO.Role of growth factors and the wound healing response in age-related macular degeneration[J].GraefesArchClinExpOphthalmol,2004,242(1):91-101.
11 Sanislo K,Kelsoe D,Blumenkran Z.The selective effect of micropulse diode laser upon the retina[J].InvestOphthalmolVisSci,1996,37(3):S779.
12 Takatsuna Y,Yamamoto S,Nakamura Y,Tatsumi T,Arai M,Mitamura Y.Long-term therapeutic efficacy of the subthreshold micropulse diode laser photocoagulation for diabetic macular edema[J].JpnJOphthalmol,2011,55(4):365-369.
13 Nakamura Y,Mitamura Y,Ogata K,Arai M,Takatsuna Y,Yamamoto S.Functional and morphological changes of macula after subthreshold micropulse diode laser photocoagulation for diabetic macular oedema[J].Eye,2010,24(5):784-788.
14 Hu W,Criswell MH,Fong SL,Temm CJ,Rajashekhar G,Cornell TL,etal.Differences in the temporal expression of regulatory growth factors during choroidal neovascular development[J].ExpEyeRes,2009,88(1):79-91.
15 张忠红,栾洁.眼内新生血管与细胞因子[J].国际眼科杂志,2006,6(1):158-163.
16 张承芬.眼底病学[M].北京:人民卫生出版社,1998:1-3.
17 Perraud AL,Takanishi CL,Shen B,Kang S,Smith MK,Schmitz C,etal.Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels[J].JBiolChem,2005,280(7):6138-6148.
18 牛彤彤,徐艳春.VEGF与眼内新生血管[J].眼科新进展,2008,28(5):394-398.
19 Zubilewicz A,Hecquet C,Jeanny JC,Soubrane G,Courtois Y,Mascarelli F.Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells:a comparative study with VEGF[J].Oncogene,2001,20(12):1403-1413.
20 Wang YS,Eichler W,Friedrichs U,Yafai Y,Hoffmann S,Yasukawa T,etal.Impact of endostatin on bFGF-induced proliferation,migration,and matrix metalloproteinase-2 expression/secretion of bovine choroidal endothelial cells[J].CurrEyeRes,2005,30(6):479-489.
21 Ozaki H,Okamoto N,Ortega S,Chang M,Ozaki K,Sadda S,etal.Basic fibroblast growth factor is neither necessary nor sufficient for the development of retinal neovascularization[J].AmJPathol,1998,153(3):757-765.
22 Wong CG,Rich KA,Liaw LH,Hsu HT,Berns MW.Intravitreal VEGF and bFGF produce florid retinal neovascularization and hemorrhage in the rabbit[J].CurrEyeRes,2001,22(2):140-147.
23 Rosenthal R,Malek G,Salomon N,Peill-Meininghaus M,Coeppicus L,Wohlleben H,etal.The fibroblast growth factor receptors,FGFR-1 and FGFR-2,mediate two independent signalling pathways in human retinal pigment epithelial cells[J].BiochemBiophysResCommun,2005,337(1):241-247.
24 Houenou LJ,D’Costa AP,Li L,Turgeon VL,Enyadike C,Alberdi E,etal.Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons[J].Science,1999,412(3):506-514.
25 Stellmach V,Crawford SE,Zhou W,Bouck N.Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor[J].ProcNatlAcadSciUSA,2001,98(5):2593-2597.
26 金姬,关明.色素上皮细胞衍生因子:一种高效的新生血管抑制因子[J].眼科新进展,2002,22(1):68-70.
date:Aug 10,2013
National Natural Science Foundation of China(No:61378084);Scientific Research Foundation of Health Department of Hubei Province(No:JX6C-22)From theOphthalmologyCenterofEntireArmyofClinicalCollegeofWuhanofSouthernMedicalUniversity,WuhanGeneralHospitalofGuangzhouMilitaryRegion,Wuhan430070,HubeiPro-vince,China
Photobiological effects of micropulsed subthreshold 810 nm diode laser photocoagulation on retinal pigment epithelial cells of rabbits
YANG Bao-Di,SONG Yan-Ping,CHEN Zhong-Shan,DING-Qin
micropulsed diode laser;retinal pigment epithelial cell;vascular endothelial growth factor;pigment epithelium-derived factor;basic fibroblast growth factor
Objective To investigate the photobiological effects of micropulsed subthreshold 810 nm diode laser photocoagulation on retinal structure and cell factor secretion under retinal pigment epithelial cells of chinchilla rabbits.Methods Micropulsed subthreshold 810 nm diode laser photocoagulated retina of chinchilla rabbits.By fluorescein angiography (FFA),HE staining of retina,the changes of morphology and vascular of retina were observed at 1 day,3 days,7 days,14 days after photocoagulation.The expression changes of vascular endothelial growth factor (VEGF),pigment epithelium-derived factor (PEDF),basic fibroblast growth factor (b-FGF) in retina were observed by immunofluorescence.The mRNA expression changes of VEGF,PEDF,b-FGF were analyzed in retinal tissue homogenates by RT-PCR.Results After photocoagulation,FFA examination showed no vascular fluorescence leakage;Retinal tissue sections stained with HE showed retina structure was in order generally;Immunofluorescence showed that after laser photocoagulation,the expression of VEGF and PEDF were enhanced significantly in rods or cones cell layer and retinal pigment epithelial layer;b-FGF was also expressed in nerve fiber layer and ganglion cell layer.RT-PCR results showed that after laser photocoagulation the mRNA content of three cytokines increased.PEDF mRNA expression increased to the maximum at 1 day after photocoagulation (3.748±0.890),followed by that at 3 days,which both had significantly difference compared with the other time points(allP<0.05).b-FGF mRNA expression increased to the maximum at 1 day after photocoagulation (1.578±0.299),but for each time point,the difference was not statistically significant(allP>0.05).VEGF mRNA expression increased to the maximum at 3 days after photocoagulation (2.301±0.378),which had significantly difference compared with other time points(allP<0.05).At 14 days after photocoagulation,the increased amplitude of three cytokines was near to each other (1.283±0.310,1.662±0.409,1.310±0.184).Conclusion Micropulsed subthreshold 810 nm diode laser can stimulate normal RPE cellsinvivoto secrete VEGF,PEDF,b-FGF in perfect union with no damage on retinal structure.
杨宝娣,女,1988年11月出生,河南开封人,在读硕士研究生。联系电话:13986005204;E-mail:yangbaodi_8651@163.com
AboutYANGBao-Di:Female,born in November,1988.Postgraduate student.Tel:13986005204;E-mail:yangbaodi_8651@163.com
2013-08-10
国家自然科学基金资助(编号:61378084);湖北省卫生厅科学研究基金资助(编号:JX6C-22)
430070 湖北省武汉市,南方医科大学附属武汉临床学院,广州军区武汉总医院,全军眼科中心
宋艳萍,E-mail:songyanping@medmail.com.cn
杨宝娣,宋艳萍,陈中山,丁琴.微脉冲半导体激光对兔视网膜色素上皮细胞阈值下光凝的光生物调制效应[J].眼科新进展,2014,34(1):5-9.
��
10.13389/j.cnki.rao.2014.0002
修回日期:2013-09-19
本文编辑:方红玲
Accepteddate:Sep 19,2013
Responsibleauthor:SONG Yan-Ping,E-mail:songyanping@medmail.com.cn
[RecAdvOphthalmol,2014,34(1):5-9]