朝鲜淫羊藿叶的黄酮苷类成分研究*
李金玉, 李洪梅, 李蓉涛
(昆明理工大学 生命科学与技术学院, 云南 昆明 650500)
淫羊藿(Epimediumkoreanum)为小檗科(Berbfidaceae)淫羊藿属(Epimedium)植物,又名三枝九叶草等,亦称“仙灵脾”。淫羊藿属全世界大约有52个种[1],我国约有40个种,是传统补肾壮阳药,据明代李时珍《本草纲目》记载,淫羊藿具有“补肾阳、强筋骨、祛风湿”的作用。淫羊藿是我国传统中药,作为滋阴壮阳、预防类风湿的植物药使用已有2000余年的历史[1]。现代药理研究表明,淫羊藿具有抗骨质疏松[2]、抗炎[3]、抗衰老[4]、抗氧化[5]和抗肿瘤[6]等功效,此外,还具有雌、雄性激素作用[7]。近年来,由于具有很好的开发利用前景,淫羊藿已成为国内外医药界的研究热点。本文对朝鲜淫羊藿70%丙酮水提取物的正丁醇部分进行研究,得到了6个黄酮苷,分别被鉴定为:金圣草素-7-O-β-D-葡萄糖醛酸-6″-甲酯(1),芹菜素-7-O-β-D-葡萄糖醛酸-6″-甲酯(2),3′,5,7-三羟基-4′-甲氧基黄酮-3-O-α-L-吡喃鼠李糖基(1→6)-β-D-葡萄糖苷(3),山奈酚-3-O-α-L-吡喃鼠李糖基(1→6)-β-D-葡萄糖苷(4),芦丁(5)和槲皮素-3-O-β-D-半乳糖-7-O-β-D-葡萄糖苷(6)。化合物1和2为首次从淫羊藿属植物中分离得到,化合物3,4和6为首次从该植物中分离得到。
1 实验仪器和材料
1D和2D NMR谱在Brucker AM-400、DRX-500及Avance III 600型超导核磁共振仪上测定;ESI-MS用API QSTAR Pulsar质谱仪测定。HPLC使用安捷伦1200高效液相色谱仪,分析柱为Agilent ZORBAX SB-C18柱(4.6 × 250 mm,5 μm),半制备柱为Agilent ZORBAX SB-C18柱(9.6 × 250 mm,5 μm);MPLC C-605/C-601型中压液相色谱仪由瑞士BÜCHI公司生产;Dianion HP-20为日本三菱公司生产;Toyopeal HW-40C由日本TOSOH公司生产;Sephadex LH-20(20-100 μm)由瑞典Pharmacia公司生产;YMC*-GEL ODS-A 由日本YMC生产;TLC正相硅胶板(G和GF254),拌样用硅胶(80-100目)和柱层析用硅胶(200-300目),均为青岛海洋化工厂生产;显色剂为碘粉和5% H2SO4-EtOH溶液;展开剂:(1)氯仿:甲醇:水(8:2:0.2);(2)甲苯:甲酸甲酯:甲酸(1:5:2)。
朝鲜淫羊藿叶于2010年11月购买自吉林省通化市,经昆明理工大学李海舟博士鉴定为朝鲜淫羊藿(E.koreanum),凭证标本(KMUST-B-20101109)存放于昆明理工大学资源药物化学重点实验室。
2 提取与分离
干燥朝鲜淫羊藿叶(10 kg)经70%丙酮/水提取,减压浓缩至无丙酮味,然后依次用石油醚、乙酸乙酯和正丁醇进行萃取。正丁醇相(164 g)经Toyopeal HW-40柱层析,甲醇/水(0%、30%、60%、90%、100%)梯度洗脱,合并得到6段,A-F。C段(40 g)经ODS中压柱,25%-65%甲醇水梯度洗脱(流速:10 mL/min)分为4段,C1-C4。C3(10 g)经Sephadex LH-20(甲醇洗脱)被分为4段,C3-1~C3-4。C3-4(1.1 g)经ODS中压柱(50%甲醇,流速:10 mL/min)洗脱分为3段C3-4-1~C3-4-3。C3-4-3(200 mg)用80-100目硅胶样,200-300目硅胶柱层析,氯仿/甲醇10:1洗脱,得到5段,C3-4-3-1~ C3-4-3-5。C3-4-3-2(60 mg)经半制备型HPLC进行分离(流动相:20%乙腈/水,流速:3 mL/min),得到化合物1(7.6 mg)和2(8.0 mg)。C3-4-2(180 mg)经Sephadex LH-20(氯仿/甲醇1:1)柱层析,后经半制备型HPLC进行分离(流动相:18%乙腈/水,流速:3 mL/min)得到化合物3(10 mg)和4(7.6 mg)。C3-4-1(500 mg),用80-100目硅胶拌样,200-300目硅胶柱层析,氯仿/甲醇(15:1,10:1,8:1,4:1)梯度洗脱得到化合物5(13 mg)。D段(28 g)经Dianion柱梯度洗脱(30%、60%、90%甲醇/水)分为5段,D1-D5。D3(8.0 g)先经Sephadex LH-20(氯仿/甲醇1:1)纯化,然后经硅胶(200-300目)柱层析(氯仿/甲醇 10:1,6:1,4:1,0:1洗脱),分为三段,D3-1~D3-3。D3-2(75 mg)通过半制备型HPLC进行分离(流动相:18%乙腈/水,流速:3 mL/min)得到化合物6(13 mg)。
3 结构鉴定
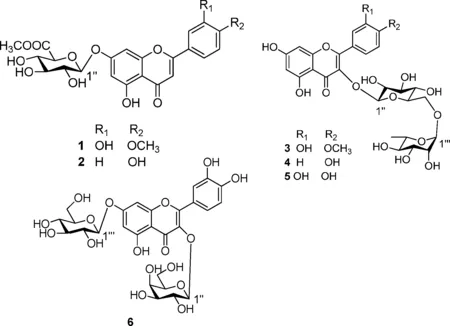
图1 化合物1-6的结构
化合物1:黄色无定形粉末,1H-NMR(500 MHz,DMSO-d6)δH:3.10-3.47(3H,m,H-2″,H-3″,H-4″),3.65(3H,s,COOCH3),3.88(3H,s,OCH3),4.18(1H,d,J=9.5 Hz,H-5″),5.30(1H,d,J=7.2 Hz,H-1″),6.46(1H,s,H-8),6.86(1H,s,H-6),6.93(1H,d,J=8.1 Hz,H-5′),6.97(1H,s,H-3),7.57(1H,s,H-2′),7.58(1H,d,J=8.1 Hz,H-6′);13C-NMR(125 MHz,DMSO-d6)δC:164.2(s,C-2),103.5(d,C-3),182.0(s,C-4),161.2(s,C-5),99.3(d,C-6),162.4(s,C-7),94.8(d,C-8),156.9(s,C-9),105.5(s,C-10),121.3(s,C-1′),110.4(d,C-2′),148.1(s,C-3′),151.0(s,C-4′),115.8(d,C-5′),120.5(d,C-6′),99.1(d,C-1″),72.1(d,C-2″),75.4(d,C-3″),71.3(d,C-4″),75.1(d,C-5″),169.2(s,C-6″),56.0(q,OCH3),52.0(q,COOCH3)。波谱数据与文献[8]报道一致,故结构鉴定为金圣草素-7-O-β-D-葡萄糖醛酸-6″-甲酯。
化合物2:黄色无定形粉末,1H-NMR(500 MHz,DMSO-d6)δH:3.13-3.40(3H,m,H-2″,H-3″,H-4″),3.65(3H,s,COOCH3),4.21(1H,d,J=9.5 Hz,H-5″),5.31(1H,d,J=7.1 Hz,H-1″),6.47(1H,s,H-8),6.87(1H,s,H-6),6.89(1H,s,H-3),6.94(2H,d,J=8.7 Hz,H-3′,5′),7.95(2H,d,J=8.7 Hz,H-2′,6′),12.98(1H,s,5-OH);13C-NMR(125 MHz,DMSO-d6)δC:164.1(s,C-2),103.5(d,C-3),182.3(s,C-4),161.6(s,C-5),99.6(d,C-6),162.8(s,C-7),95.0(d,C-8),157.4(s,C-9),105.9(s,C-10),121.3(s,C-1′),129.0(d,C-2′,6′),116.4(d,C-3′,5′),162.8(s,C-4′),99.5(d,C-1″),73.1(d,C-2″),75.8(d,C-3″),71.7(d,C-4″),75.6(d,C-5″),169.5(s,C-6″),52.3(q,COOCH3)。波谱数据与文献[9]报道一致,故结构鉴定为芹菜素-7-O-β-D-葡萄糖醛酸-6″-甲酯。
化合物3:黄色无定形粉末,1H-NMR(600 MHz,CD3OD)δH:1.10(3H,d,J=6.0 Hz,H-6‴),3.81(1H,d,J=10.5 Hz,H-5″),3.95(3H,s,OCH3),4.53(1H,s,H-1‴),5.31(1H,d,J=7.1 Hz,H-1″),6.20(1H,d,J=2.4 Hz,H-8),6.40(1H,d,J=2.4 Hz,H-6),6.90(1H,d,J=8.4 Hz,H-5′),7.62(1H,d,J=1.8 Hz,H-2′),7.95(1H,dd,J=1.8,8.4 Hz,H-6′);13C-NMR(150 MHz,CD3OD)δC:159.0(s,C-2),135.4(s,C-3),179.4(s,C-4),163.2(s,C-5),100.2(d,C-6),166.4(s,C-7),95.1(d,C-8),158.7(s,C-9),105.8(s,C-10),123.1(s,C-1′),114.6(d,C-2′),148.4(s,C-3′),150.9(s,C-4′),116.2(d,C-5′),124.1(d,C-6′),104.5(d,C-1″),76.0(d,C-2″),78.3(d,C-3″),69.2(d,C-4″),77.5(d,C-5″),68.6(t,C-6″),102.3(d,C-1‴),72.2(d,C-2‴),72.4(d,C-3‴),73.9(d,C-4‴),68.7(d,C-5‴),18.0(q,C-6‴)。波谱数据与文献[10]报道一致,故结构鉴定为3′,5,7-三羟基-4′-甲氧基黄酮-3-O-α-L-吡喃鼠李糖基(1→6)-β-D-葡萄糖苷。
化合物4:黄色无定形粉末,1H-NMR(400 MHz,CD3OD)δH:1.13(3H,d,J=6.2 Hz,H-6‴),3.65(1H,d,J=10.2 Hz,H-5″),4.51(1H,s,H-1‴),5.22(1H,d,J=6.8 Hz,H-1″),6.18(1H,d,J=2.4 Hz,H-8),6.38(1H,d,J=2.4 Hz,H-6),6.88(2H,d,J=8.6 Hz,H-3′,H-5′),8.03(2H,d,J=8.6 Hz,H-2′,H-6′);13C-NMR(100 MHz,CD3OD)δC:159.3(s,C-2),135.5(s,C-3),179.3(s,C-4),163.0(s,C-5),100.2(d,C-6),166.8(s,C-7),95.1(d,C-8),158.6(s,C-9),105.5(s,C-10),122.7(s,C-1′),132.6(d,C-2′,C-6′),116.1(d,C-3′,C-5′),161.5(s,C-4′),104.7(d,C-1″),75.7(d,C-2″),78.1(d,C-3″),71.4(d,C-4″),77.2(d,C-5″),68.5(t,C-6″),102.4(d,C-1‴),72.3(d,C-2‴),72.1(d,C-3‴),73.9(d,C-4‴),69.7(d,C-5‴),18.0(q,C-6‴)。波谱数据与文献[11]报道一致,故结构鉴定为山奈酚-3-O-α-L-吡喃鼠李糖基(1→6)-β-D-葡萄糖苷。
化合物5:黄色无定形粉末,1H-NMR(500 MHz,DMSO-d6)δH:1.00(3H,d,J=6.2 Hz,H-6‴),3.70(1H,d,J=10.5 Hz,H-5″),4.38(1H,s,H-1‴),5.31(1H,d,J=7.5 Hz,H-1″),6.14(1H,s,H-8),6.33(1H,s,H-6),6.82(1H,d,J=8.4 Hz,H-5′),7.52(1H,d,J=2.1 Hz,H-2′),7.54(1H,dd,J=8.5,2.1 Hz,H-6′),12.51(1H,s,5-OH);13C-NMR(100 MHz,DMSO-d6)δC:157.4(s,C-2),134.1(s,C-3),177.9(s,C-4),162.0(s,C-5),100.0(d,C-6),166.4(s,C-7),94.7(d,C-8),157.3(s,C-9),104.3(s,C-10),121.8(s,C-1′),116.1(d,C-2′),145.7(s,C-3′),149.6(s,C-4′),116.9(d,C-5′),122.5(d,C-6′),102.3(d,C-1″),74.9(d,C-2″),77.4(d,C-3″),70.9(d,C-4″),76.8(d,C-5″),67.9(t,C-6″),101.6(d,C-1‴),71.4(d,C-2‴),71.2(d,C-3‴),72.7(d,C-4‴),69.1(d,C-5‴),18.6(q,C-6‴)。波谱数据与文献[12]报道一致,故结构鉴定为芦丁。
化合物6:黄色无定形粉末,1H-NMR(400 MHz,DMSO-d6)δH:5.33(1H,dJ=7.6 Hz,H-1‴),5.43(1H,d,J=7.2 Hz,H-1″),6.14(1H,d,J=2.4 Hz,H-8),6.34(1H,d,J=2.4 Hz,H-6),6.80(1H,d,J=8.5 Hz,H-5′),7.53 (1H,d,J=2.0 Hz,H-2′),7.62(1H,dd,J=2.0,8.5 Hz,H-6′);13C-NMR(100 MHz,DMSO-d6)δC:156.4(s,C-2),133.3(s,C-3),177.3(s,C-4),161.2(s,C-5),99.1(d,C-6),165.6(s,C-7),93.8(d,C-8),156.0(s,C-9),103.5(s,C-10),121.1(s,C-1′),116.1(d,C-2′),148.6(s,C-3′),144.9(s,C-4′),115.2(d,C-5′),121.6(d,C-6′),101.9(d,C-1″),71.2(d,C-2″),73.2(d,C-3″),67.9(d,C-4″),75.8(d,C-5″),60.1(t,C-6″),100.9(d,C-1‴),74.1(d,C-2‴),76.5(d,C-3‴),70.0(d,C-4‴),77.5(d,C-5‴),60.9(t,C-6‴)。波谱数据与文献[13]报道一致,故结构鉴定为槲皮素-3-O-β-D-半乳糖-7-O-β-D-葡萄糖苷。
参考文献:
[1]Ma H P,He X R,Yang Y,et al.The genus Epimedium:An ethnopharmacological and phytochemical review[J].Journal of Ethnopharmacology,2011,134,519-541.
[2]Zhang D W,Cheng Y,Wang N L.et al.Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts[J].Phytomedicine,2008,15,55-61.
[3]Luo G J,Ci X X,Ren R,Wu Z Y,et al.Isolation of Two New Prenylflavonols from Epimedium brevicornum and their Effects on Cytokine Production in vitro[J].Planta Med.,2009;75,843-847.
[4]Li F,Gong Q H,Wu Q,Lu Y F.,Icariin isolated from Epimedium brevicornum Maxim attenuates learning and memory deficits induced by D-galactose in rats[J].Pharmacology,Biochemistry and Behavior,2010,96,301-305.
[5]Zhang W J,Chen H X,Wang Z H.,Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai[J].J Food Sci Technol,2013,50(6):1122-1129.
[6]Song J,Shu L,Zhang Z H,et al.Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer[J].Chemico-Biological Interactions,2012,199,9-17.
[7]Kang H K,Choi Y H,Kwon H,Lee S B,et al.Estrogenic/antiestrogenic activities of a Epimedium koreanum extract and its major components:in vitro and in vivo studies[J].Food and Chemical Toxicology,2012,50,2751-2759.
[8]Du Q Z,Cui H G.,A new flavone glycoside from the fruits of Luffa cylindrica[J].Fitoterapia,2007,78,609-610.
[9]Lee M H,Son Y K,Han Y N.,Tissue Factor Inhibitory Flavonoids from the Fruits of Chaenomeles sinensis[J].Arch Pharm Res,2002,25(6):842-850.
[10]董刘宏,太志刚,杨亚滨等.白刺花花的化学成分研究[J].华西药学杂志,2010,42(8):1490-1493.
[11]王盈盈,梁鑫,钟惠民.云贵腺药珍珠菜化学成分研究[J].中草药,2012,43(7):1280-1284.
[12]Pan Y X,Dou H,Zhang X X,et al.Flavonol and monoterpene glycosides from Ligularia macrophylla[J].Chin J MAP,2005,22(3):175-178.
[13]张忠立,左月明,徐璐等.三白草黄酮类化学成分的研究[J].中草药,2011,42(8):1490-1493.

