静电纺有机/无机杂化纳米纤维载药体系的构建及其生物医学应用
王 哲,史向阳
(东华大学化学化工与生物工程学院,上海201620)
静电纺有机/无机杂化纳米纤维载药体系的构建及其生物医学应用
王 哲,史向阳
(东华大学化学化工与生物工程学院,上海201620)
纳米纤维具有极大的比表面积、可控的多孔二级结构等一系列优良特性,使其在环境保护、能源利用、催化剂、药物载体、组织工程支架材料等领域得到了广泛应用。通过静电纺技术制备的纳米纤维主要有有机纳米纤维、无机纳米纤维、以及有机/无机杂化纳米纤维3类。结合作者课题组之前的研究成果积累,综述了各种不同的静电纺有机/无机杂化纳米纤维载药体系的构建及其生物医学应用。着重介绍了如何将药物负载在无机纳米颗粒(埃洛石、锂皂石、羟基磷灰石、介孔二氧化硅等)的表面或内部并进而和高分子混纺形成双载体纳米载药纤维的过程和相关药物缓释机理,并探讨了有机/无机杂化纳米纤维载药体系的生物医学应用,尤其是在抗菌和抗肿瘤方面的治疗应用。文章最后对该领域的研究方向和前景作了展望。
静电纺;杂化纳米纤维;药物载体;生物医学应用
1 前 言
近年来,纳米载药体系的研究和应用得到了人们广泛的重视[1-4]。在纳米载药体系中,药物分子通常均匀分散于载体基质中或者吸附于载体表面,得到的载体-药物复合体除了起到携带药物的作用外,还可以影响和控制药物的释放动力学,更有利于其药效的发挥[3]。纳米科技和药物载体结合的研究源于20世纪60~70年代,当时,英国学者Bangham将磷脂分散在水中,辅以超声波等手段处理后,通过显微镜观察发现了脂质体[5]。后来,Ryman和Gergoriadis又分别在模拟生物膜的基础上,将酶和药物等包裹在脂质体中,开启了将脂质体用于药物载体的研究[6]。此后,包括纳米颗粒[7-8]、纳米纤维[9-10]、纳米管[11-12]、纳米棒[13]在内的一系列的纳米级药物载体被相继开发出来。在这些纳米材料中,纳米纤维具有表面形貌可控、表面容易修饰[14-16]、载药操作简单、对药物药效影响较小等一系列优点[17-19],因而倍受研究者们的关注。
常用的制备纳米纤维的方法主要有拔拉法、模板合成法、热致相分离法、自组装法、乳液聚合法和静电纺丝法等[20]。其中拔拉法的不足在于其要求聚合物有较好的粘性,能承受外力的拉扯作用,且单位时间内只能生产单根纤维,产量低,应用受到了限制;模板法需要额外的模板,操作过程较复杂;自组装法操作步骤繁琐。静电纺丝技术是目前唯一能够大量地制备连续的纳米纤维的方法[21],因而受到了研究者的青睐。
自从Kenawy等人于2002年报道了将静电纺纳米纤维用作药物载体的研究后,基于静电纺纳米纤维的药物负载体系得到了长足的发展[18,19,22]。截止目前,通过静电纺技术制备药物载体的方法主要包括传统静电纺丝法、同轴静电纺和乳液静电纺等[9,22-26]。传统静电纺丝法即将药物简单地和高分子溶液混合,然后将混有药物的高分子溶液直接纺丝。在制备过程中,药物通常会均匀分散在纤维中,形成“药物-基质”结构。此结构中,药物和基质间的作用力不强,常常伴有药物突释现象的发生,且随着释放时间的延长,药物的释放速度降低,影响药效的发挥。同轴静电纺和乳液静电纺是两种改进的静电纺丝技术。采用这两种技术制备的载药纳米纤维,药物往往存在于纤维内部,形成所谓的“核-壳”结构,药物处于核层,高分子层处于壳层。在这种“核-壳”结构中,药物首先要从核层释放到壳层,再从壳层扩散到纤维外,才可以完成对药物的释放。壳层的纤维对药物起到了额外的屏障作用,因此可以控制药物释放速度[10,26]。但是,这两种静电纺丝技术仍然存在着一定的弊端。同轴静电纺需要进行大量的仪器参数和溶液参数调试;而采用乳液静电纺技术时内层的溶剂很难彻底挥发,残留的溶剂将会影响纤维的细胞相容性和药物药效[27]。因此,需要探索以静电纺为基础的更加新颖的药物载体制备方法。
大量研究表明,一些无机纳米材料如埃洛石纳米管(Halloysite Nanotubes,HNTs)[28-32]、碳纳米管(Carbon Nanotubes,CNTs)[33-40]、羟基磷灰石[41-42]、锂皂石[7,43-44]、介孔二氧化硅[45]等具有高的比表面积、高的表面活性和良好的生物相容性,对多种化学物质及生物活性大分子有较强的吸附能力,是药物的良好载体。但这些纳米材料载药后多以粉末形式存在,易发生团聚,无法实现器件化,而且大多存在比较明显的突释现象。
杂化纤维通常是指由不同类别材料组成的纳米纤维,如聚合物/无机纳米颗粒杂化纤维[46-49]、聚合物/聚合物杂化纤维[50-52]等。由于掺杂了部分功能性物质,杂化纤维通常会显示出更多优良性能,例如催化性能增强[53-55]、机械性能提高[56-57]、药物包裹能力[58]以及细胞相容性[18]增强改善等。正是因为具有上述优良性能,杂化纤维在组织工程[59-60]、催化剂[53,54,61]、传感器[62-63]和药物载体[64]等领域具有广泛的应用前景。
基于以上研究现状和问题,作者课题组及其他研究团队将载药纳米管或纳米颗粒与高分子纺丝液混合电纺,制备了一系列新型的有机/无机杂化纳米纤维双重载药体系。一方面双重载药体系有效地延长了药物的扩散历程;另一方面,负载的无机成分可有效地提高纤维的机械性能。本文主要从负载药物的生物功能方面,综述了有机/无机杂化纳米纤维载药体系负载抗生素类药物和抗肿瘤类药物方面的研究进展。
2 有机/无机杂化纳米纤维负载抗生素类药物及抗菌性能
2010年,作者课题组Qi等[65]报道了将盐酸四环素(Tetracycline Hydrochloride,TCH)作为模型药,首先将其负载到一种天然呈纳米管状结构的铝硅酸盐矿物埃洛石(Halloysite Nanotubes,HNT)的表面或内部空腔制成TCH/HNTs,然后将载药效率最优的TCH/HNTs与聚乳酸-羟基乙酸(Polylactic-co-glycolic Acid,PLGA)混纺,制备了HNTs/PLGA双载体药物缓释系统。实验结果表明,载药纳米管TCH/HNTs的加入没有影响到PLGA纤维均匀光滑的结构,与PLGA纤维的直径相比,TCH/HNTs/PLGA纤维的直径变细(图1)。TCH/HNTs载药粉末和静电纺TCH/PLGA混纺载药纳米纤维都存在明显的初始突释现象(图2),24 h后TCH释放率分别是89.4%和83.8%,48 h后两者TCH释放率基本上都趋于一个平台。然而,静电纺TH-1/PLGA(1%TCH/HNTs/PLGA)和TH-2/PLGA(2%TCH/HNTs/PLGA)载药纳米纤维没有明显的初始突释现象(图2),24 h后,分别释放18.6%和16.3%的TCH。另外,两种载药纤维体系表现出很好的TCH持续释放效果,缓释28 d后,释放的TCH分别为65.2%和61.3%,缓释42 d后,分别有77.6%和68.5%的TCH释放。体外抑菌实验表明,从TCH/HNTs载药粉末及静电纺载药纤维中释放出来的药物保持了其生物功能。由于TCH/HNTs被成功地封装到PLGA纤维内部,使得HNTs和PLGA作为TCH的双重载体进一步延缓了TCH的释放速度。释放过程中,一部分TCH从HNTs表面解吸附,一部分TCH从HNTs内部缓慢释放,然后再通过PLGA纤维基体慢慢扩散到缓释液中。总之,静电纺TCH/HNTs/PLGA双载体纳米纤维载药体系是一个很好的药物控释器件,不仅可以延缓TCH的释放,同时又实现了载药体系的器件化。
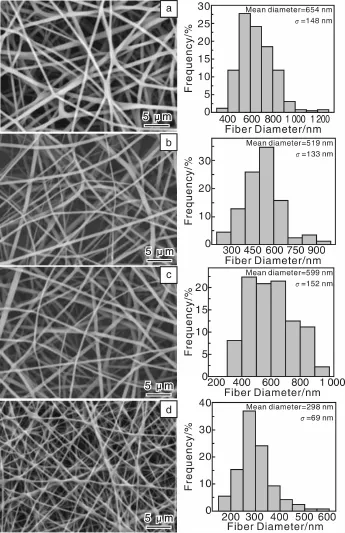
图1 静电纺PLGA纤维(a)、TH-1/PLGA(b)、TH-2/PLGA(c)及TCH/PLGA(d)纳米载药纤维的SEM照片和纤维直径分布直方图[65]Fig.1 SEM micrographs of the electrospun PLGA(a),TH-1/PLGA(b),TH-2/PLGA(c),and TCH/PLGA(d)nanofibers,and the corresponding fiber diameter distribution histograms[65]
有研究表明,合成性的纳米粘土材料锂皂石(Laponite,LAP)[7,43-44]的内部空腔可以用来高效包裹药物。按照同样的设计思路,作者课题组Wang等[66]利用静电纺丝制备出PLGA/LAP/Amoxicillin(AMX)复合纳米纤维。实验结果表明,LAP对AMX的负载效率随着AMX浓度的增加而增加,随LAP浓度的增加而降低。当LAP和AMX的浓度分别为3 mg/mL和2 mg/mL时,LAP对AMX的负载达到最优值,为9.76±0.57%。药物释放动力学研究表明,AMX从LAP/AMX粉末和PLGA/AMX纳米纤维中的释放都存在着突释现象。以LAP/AMX纳米粉末中的最为明显,在初始的1 h内AMX的释放量即接近100%。PLGA/AMX纳米纤维中AMX的释放速度相对较慢,在最初48 h内,大约40%的AMX释放出来;然后,AMX的释放速度明显降低,9 d之后释放量达到100%。相比之下,PLGA/LAP/AMX纳米纤维对AMX的释放呈现先快速释放后持续释放的特点。在最初24 h内药物的释放量约为40%;此后AMX的释放速度明显变缓,呈现缓慢释放特点,15 d后的药物释放量为55%。体外抑菌活性评价显示,PLGA/LAP/AMX载药纳米纤维在固体培养基和液体培养基中均可以有效地抑制金黄色葡萄球菌的生长,表现出良好的体外抑菌活性。这些结果对研究PLGA/LAP/AMX载药纳米纤维在药物载体、伤口敷料以及组织工程支架材料领域的应用具有重要意义。

图2 样品在37℃、pH为7.4的PBS缓冲液中孵育,TCH从TCH/HNTs载药粉末、静电纺TH-1/PLGA、TH-2/PLGA及TCH/PLGA载药纳米纤维中释放的缓释曲线[65]Fig.2 In vitro release profiles of TCH from TH-1/PLGA,TH-2/PLGA,TCH/PLGA nanofibers,and TCH/HNTs powders.The sampleswere incubated in phosphate buffered saline(PBS)buffer(pH 7.4)at37℃[65]
纳米羟基磷灰石(n-HA)是一种很好的无机药物载体材料,它具有高的比表面积、高的表面活性和良好的生物相容性,并对多种化学物质及生物活性大分子有较强的吸附能力[41-42]。同时,具有纳米棒状结构和高的机械强度,可以作为纤维增强体。作者课题组Zheng等[67]设计并制备了基于n-HA和PLGA的复合纳米纤维载药体系(图3),并系统地研究了AMX和n-HA在水中的最优载药浓度配比和该复合纳米纤维载药体系的纳米纤维直径、孔隙率、表面亲水性、机械性能、模型药AMX的释放动力学、细胞毒性、血液相容性以及其对金黄色葡萄球菌的抗菌活性。结果表明,AMX和n-HA两者浓度分别为2 mg/mL和1 mg/mL时,AMX可达到最优负载百分率(20.45%)。通过静电纺丝法,n-HA纳米粉末、AMX/n-HA载药粉末及AMX被成功地负载到PLGA纤维内部,且对PLGA纤维的形貌、孔隙率和接触角均没有明显的影响。静电纺n-HA/PLGA、AMX/n-HA/PLGA和AMX/PLGA纳米纤维的直径明显降低,而n-HA/PLGA和AMX/n-HA/PLGA纳米纤维的断裂强度和杨氏模量相对PLGA纳米纤维均有所增加。缓释实验结果表明,AMX/n-HA载药粉末和AMX/PLGA载药纤维都存在明显的初始突释现象,而AMX/n-HA/PLGA双载体纳米纤维载药体系则避免了突释现象,并具有很好的持续释放效果。体外抑菌实验表明,静电纺AMX/n-HA/PLGA载药纤维毡对金黄色葡萄球菌表现出即时、长效和对载药量梯度依赖的抑菌活性(图4)。体外细胞毒性试验及溶血性试验也表明AMX/n-HA/PLGA载药纤维具有良好的生物相容性和血液相容性。总之,AMX/n-HA/PLGA载药纤维具有更好的尺寸稳定性、机械耐受性、即时长效和载药量梯度依赖的抑菌活性及很好的生物相容性和血液相容性,可预计其在伤口包覆、术后防粘连和防感染方面将有很好的应用前景。

图3 AMX/n-HA/PLGA载药纳米纤维的制备过程示意图(a)及AMX的释放机理图(b)[67]Fig.3 Schematic illustration of the encapsulation(a)and release pathways(b)of AMX within n-HA-doped PLGA nanofibers[67]

图4 在PBS缓冲液中释放4 d和9 d的AMX/n-HA/PLGA和AMX/PLGA纳米纤维毡(所有样品的AMX初始浓度是60 mg/m L,相对于5 m L的细菌悬浮液)在液体培养基中与金黄色葡萄球菌共孵育24 h得出的抑菌活性的定量分析结果(a),并与空白对照组比较;在PBS缓冲液中释放4 d(b)和9 d(c)的PLGA(1)、n-HA/PLGA(2)、AMX/n-HA/PLGA(3)和AMX/PLGA(4)纳米纤维毡在琼脂平板上孵育金黄色葡萄球菌6 h,12 h,18 h,24 h得出的抑菌活性的定性分析结果[67]Fig.4 Inhibition of bacterial(S.aureus)growth using AMX-loaded nanofibers(originalunreleased AMX contentwas60 mg/m L relative to the 5 mL bacterial suspension)after 4 or 9 days release after24 h incubation,untreated bacterial solution was set as control;Growth inhibition of bacteria(S.aureus)on agar plate at the incubation time of6 h,12 h,18 h,and 24 h using AMX-loaded nanofibers after 4(b)or 9(c)days release:spots 1~4 represents PLGA,n-HA/PLGA,AMX/n-HA/PLGA,and AMX/PLGA nanofibers,respectively[67]
除此之外,基于高聚物/磁性纳米粒子或介孔二氧化硅纳米颗粒/抗生素药物的有机/无机杂化纳米纤维载药体系也相继被报道。Haroosh等[68]制备并表征了PLAPCL/磁性纳米粒子纳米纤维,并且用其来负载抗生素类药物TCH,药物释放动力学结果完全符合“Ritger-Peppas and Zeng models”。Hu等[69]用介孔二氧化硅(Mesoporous Silica,MMS)负载布洛芬(Ibuprofen,IBU),然后与PLLA混纺制备得到PLLA-MMS-IBU复合纳米纤维。试验结果表明,布洛芬前12 h的突释率是PLLAIBU的1/8,并且药物释放时间大大延长。动物试验结果表明,使用PLLA-MMS-IBU在8周时间里发炎率最低,伤口能够很好地愈合。因此该长效释药膜应用于伤口包覆能够起到抗炎和预防粘连的作用。
3 有机/无机杂化纳米纤维负载抗肿瘤类药物及性能
阿霉素(DOX)是目前广泛使用的抗癌药物之一[70-72]。自由的DOX在临床使用时存在毒副作用大等问题[73]。静电纺纳米纤维由于可器件化[74],且可通过调节纤维形貌、孔隙率和组成有效控制药物释放特性[75],在作为抗癌药物载体应用方面日益受到关注。另外,静电纺纳米纤维作为抗癌药物载体,可以获得持续释放药物的效果,具有很好的应用前景。同时,静电纺纳米纤维具有高比表面积、高孔隙率和表面易功能化等特点,作为肿瘤组织部位的支架载体材料具有很多优势[76-78]。
作者课题组Zheng等[79]在制备PLGA/n-HA/AMX复合纳米纤维的基础上,制备了PLGA/n-HA/DOX复合纳米纤维。实验结果表明,PLGA/n-HA/DOX纤维具有很好的DOX持续释放效果,同时还表现出在肿瘤滋生的酸性微环境(pH=5.0~6.0)中相对快速释放的特性(图5)。采用MTT法检测细胞活力的实验结果表明,在有效的药物浓度梯度范围内,DOX/n-HA/PLGA纤维浸渍培养基对人口腔上皮癌细胞(KB细胞)有明显的杀伤力(图6)。溶血实验也表明其具有良好的血液相容性。因此,静电纺DOX/n-HA/PLGA复合纳米纤维抗肿瘤载药体系在抗肿瘤药物控释领域具有巨大的应用潜质。
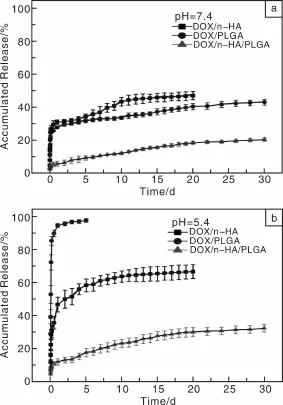
图5 DOX/n-HA载药粉末、静电纺DOX/n-HA/PLGA(1% DOX,质量分数,相对于PLGA)复合载药纳米纤维及DOX/PLGA(1%DOX,质量分数,相对于PLGA)混纺载药纳米纤维在PBS(pH=7.4,37℃)(a)和醋酸盐(pH=5.4,37℃)(b)缓冲液中DOX的缓释曲线[79]Fig.5 In vitro release profiles of DOX from DOX-n-HA particles,electrospun DOX-PLGA(1%DOX,mass fraction,relative to PLGA)and DOX-n-HA-PLGA(1%DOX,mass fraction,relative to PLGA)nanofibers.The samples were incubated in(a)PBS buffer(pH=7.4)and(b)acetate buffer solution(pH=5.4)at 37℃[79]
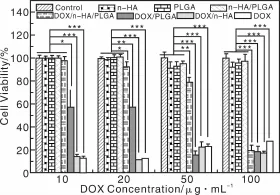
图6 KB细胞在纯培养基、n-HA粉末、静电纺PLGA、n-HA/PLGA纤维毡及纯DOX、DOX/n-HA载药粉末及DOX/PLGA和DOX/n-HA/PLGA载药纳米纤维毡的浸渍培养基中培养1 d后的MTT检测结果,用未经任何处理的KB细胞作为对照[79]Fig.6 MTT viability assay of KB cells treated with pure DOX,and the releasemedium of DOX-n-HA powder,DOX-PLGA and DOX-n-HA-PLGA composite nanofibers with different DOX contents for 24 h. Untreated KB cellswere used as control,the PLGA nanofibers,n-HA nanoparticles,and n-HA-PLGA nanofibers without DOX loading were also treated under similar conditions and compared with those materials loaded with DOX[79]
除此之外,基于高聚物/介孔二氧化硅纳米颗粒和Au纳米粒子/DOX的有机/无机复合纳米纤维载药体系也相继被报道。Qiu等[80]将DOX首先负载于介孔二氧化硅纳米颗粒(Mesoporous Silica Nanoparticles,MSNs)中,然后与Poly(L-Lactic Acid)(PLLA)混合纺丝制备得到PLLA/DOX@MSNs复合纳米纤维。试验结果表明,复合纳米纤维能够长期稳定释放DOX,并且比MSNs和PLLA单独载药有更好的杀灭Hela细胞的能力。Yan等[81]利用静电纺的方法首先制备出包含Au纳米颗粒(AuNPs)的PVA/CS纳米纤维,然后把DOX负载到复合纳米纤维上。结果表明,纳米纤维对药物有很高的封装率,通过控制交联时间,可以很好地控制药物的释放速率。Hou等[82]首先在介孔二氧化硅中封装NaYF4:Yb3+、Er3+,然后负载DOX,最后与含有吲哚美辛的Poly(ε-caprolactone)(PCL)和gelatin混合纺丝,制备得到了双载药纳米纤维,结果表明吲哚美辛释放较快,而DOX表现出持续释放的特点。
4 结 语
本文系统地介绍了有机/无机杂化纳米纤维载药体系的构建及其生物医学应用,尤其是在抗菌和抗肿瘤治疗方面的应用。综述了几种通过静电纺方法制备的有机/无机杂化纳米纤维载药体系,它们的共同优势是制备工艺简单、自身可降解、生物相容性好及机械性能高,同时实现了无机纳米粒子的器件化,对所负载药物都能够实现缓慢释放,而药物的治疗效果则不受影响。这些优点使有机/无机杂化纳米纤维载药体系在近年来得到了广泛的研究与发展。
随着纳米纤维载药体系研发的不断深入,有机/无机杂化纳米纤维载药体系的应用潜力已得到广泛认同。但将该体系进一步应用于体内试验的相关研究仍将是一项富有挑战意义的课题。总之,有机/无机杂化纳米纤维载药体系已经展示出了美好的前景,为药物缓释体系带来了很大的突破,为术后防感染和癌症治疗提供了很好的平台体系。相信经过研究者们的努力,有机/无机杂化纳米纤维载药体系将拓展用于多种不同药物的负载与控释,用于多种不同疾病的治疗。
Re ferences
[1] Hughes G A.Nanostructure-Mediated Drug Delivery[J].Nanomedicine:Nanotechnology,Biology and Medicine,2005,1(1):22-30.
[2] Kumari A,Yadav S K,Yadav S C.Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems[J].Colloids and Surfaces B:Biointerfaces,2010,75(1):1-18.
[3] Soppimath K S,Aminabhavi TM,Kulkarni A R,et al.Biodegradable Polymeric Nanoparticles as Drug Delivery Devices[J]. Journal of Controlled Release,2001,70(1):1-20.
[4] Vasir JK,Tambwekar K,Garg S.Bioadhesive Microspheresas a Controlled Drug Delivery System[J].International Journal of Pharmaceutics,2003,255(1):13-32.
[5] Bangham A,Standish M M,Watkins J.Diffusion of Univalent I-ons across the Lamellae of Swollen Phospholipids[J].Journal of Molecular Biology,1965,13(1):238-252.
[6] Gregoriadis G,Ryman B.Liposomes as Carriers of Enzymes or Drugs:a New Approach to the Treatmentof Storage Diseases[J]. Biochem J,1971(05).
[7] Jung H,Kim H M,Choy Y B,et al.Laponite-Based Nanohybrid for Enhanced Solubility and Controlled Release of Itraconazole[J].International Journal of Pharmaceutics,2008,349(1):283-290.
[8] Takahashi T,Yamada Y,Kataoka K,et al.Preparation of a Novel Peg-Clay Hybrid as a DDSMaterial:Dispersion Stability and Sustained Release Profiles[J].Journal of Controlled Release,2005,107(3):408-416.
[9] Wang C,Yan K W,Lin Y D,et al.Biodegradable Core/Shell Fibers by Coaxial Electrospinning:Processing,Fiber Characterization,and Its Application in Sustained Drug Release[J].Macromolecules,2010,43(15):6 389-6 397.
[10] Qi H,Hu P,Xu J,et al.Encapsulation of Drug Reservoirs in Fibers by Emulsion Electrospinning:Morphology Characterization and Preliminary Release Assessment[J].Biomacromolecules,2006,7(8):2 327-2 330.
[11] Prato M,Kostarelos K,Bianco A.Functionalized Carbon Nanotubes in Drug Design and Discovery[J].Accounts of Chemical Research,2007,41(1):60-68.
[12] Wakasugi A,Asakawa M,Kogiso M,et al.Organic Nanotubes for Drug Loading and Cellular Delivery[J].International Journal of Pharmaceutics,2011,413(1):271-278.
[13] Wu P C,Wang W S,Huang Y T,et al.Porous Iron Oxide Based Nanorods Developed as Delivery Nanocapsules[J]. Chemistry-A European Journal,2007,13(14):3 878-3 885.
[14] Greiner A,Wendorff JH.Electrospinning:a FascinatingMethod for the Preparation of Ultrathin Fibers[J].Angewandte Chemie International Edition,2007,46(30):5 670-5 703.
[15] Li D,Xia Y.Electrospinning of Nanofibers:Reinventing the Wheel?[J].Advanced Materials,2004,16(14):1 151-1 170.
[16] Wang S,Cao X,Shen M,et al.Fabrication and Morphology Control of Electrospun Poly(γ-glutamic acid)Nanofibers for Biomedical Applications[J].Colloidsand SurfacesB:Biointerfaces,2012,89:254-264.
[17] Lu X,Wang C,Wei Y.One-Dimensional Composite Nanomaterials:Synthesis by Electrospinning and Their App lications[J].Small,2009,5(21):2 349-2 370.
[18] Qi R,Guo R,Shen M,et al.Electrospun Poly(lactic-co-glycolic acid)/Halloysite Nanotube Composite Nanofibers for Drug Encapsulation and Sustained Release[J].Journal of Materials Chemistry,2010,20(47):10 622-10 629.
[19] Moreno I,González-González V,Romero-García J.Control Release of Lactate Dehydrogenase Encapsulated in Poly(vinyl alcohol)Nanofibers via Electrospinning[J].European Polymer Journal,2011,47(6):1 264-1 272.
[20] Vasita R,Katti D S.Nanofibers and Their Applications in Tissue Engineering[J].International Journal of Nanomedicine,2006,1(1):15-30.
[21] Xu X,Yang L,Xu X,et al.Ultrafine Medicated Fibers Electrospun from W/O Emulsions[J].Journal of Controlled Release,2005,108(1):33-42.
[22] Kenawy E R,Bowlin G L,Mansfield K,et al.Release of Tetracycline Hydrochloride from Electrospun Poly(ethylene-co-vinylacetate),Poly(lactic acid),and a Blend[J].Journal of Controlled Release,2002,81(1):57-64.
[23] Shen X,Yu D,Zhu L,et al.Electrospun Diclofenac Sodium Loaded Eudragit®L 100-55 Nanofibers for Colon-Targeted Drug Delivery[J].International Journal of Pharmaceutics,2011,408(1):200-207.
[24] Yoo H S,Kim T G,Park T G.Surface-Functionalized Electrospun Nanofibers for Tissue Engineering and Drug Delivery[J]. Advanced Drug Delivery Reviews,2009,61(12):1 033-1 042.
[25] Bölgen N,Vargel I,Korkusuz P,et al.In Vivo Performance of Antibiotic Embedded Electrospun PCL Membranes for Prevention of Abdominal Adhesions[J].Journal of BiomedicalMaterials Research Part B:Applied Biomaterials,2007,81(2):530-543.
[26] Xu X,Chen X,Ma P,et al.The Release Behavior of Doxorubicin Hydrochloride from Medicated Fibers Prepared by Emulsion-Electrospinning[J].European Journal of Pharmaceutics and Biopharmaceutics,2008,70(1):165-170.
[27] Moghe A,Gupta B.Co-Axial Electrospinning for Nanofiber Structures:Preparation and Applications[J].Polymer Reviews,2008,48(2):353-377.
[28] Price R,Gaber B G,Lvov Y.In-Vitro Release Characteristics of Tetracycline HCl,Khellin and Nicotinamide Adenine Dineculeotide from Halloysite;a Cylindrical Mineral[J].Journal of Microencapsulation,2001,18(6):713-722.
[29] Levis S,Deasy P.Characterisation ofHalloysite for Use asaMicrotubular Drug Delivery System[J].International Journal of Pharmaceutics,2002,243(1):125-134.
[30] Lvov Y M,Shchukin D G,Mohwald H,et al.Halloysite Clay Nanotubes for Controlled Release of Protective Agents[J].ACS Nano,2008,2(5):814-820.
[31] Kelly H,Deasy P,Ziaka E,et al.Formulation and Preliminary in Vivo Dog Studies of a Novel Drug Delivery System for the Treatmentof Periodontitis[J].International Journal of Pharmaceutics,2004,274(1):167-183.
[32] Abdullayev E,Price R,Shchukin D,etal.Halloysite Tubes as Nanocontainers for Anticorrosion Coatingwith Benzotriazole[J]. ACSApplied Materials&Interfaces,2009,1(7):1 437-1 443.
[33] Liu Z,Tabakman S,Welsher K,et al.Carbon Nanotubes in Biology and Medicine:In Vitro and In Vivo Detection,Imaging and Drug Delivery[J].Nano Research,2009,2(2):85-120.
[34] Portney N G,Ozkan M.Nano-Oncology:Drug Delivery,Imaging,and Sensing[J].Analytical and Bioanalytical Chemistry,2006,384(3):620-630.
[35] Ali-Boucetta H,Al-Jamal K T,McCarthy D,et al.Multiwalled Carbon Nanotube-Doxorubicin Supramolecular Comp lexes for Cancer Therapeutics[J].Chemical Communications,2008,(4):459-461.
[36] Liu Z,Sun X,Nakayama-Ratchford N,et al.Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery[J].ACSNano,2007,1(1):50-56.
[37] Bianco A,Kostarelos K,Prato M.Applications of Carbon Nanotubes in Drug Delivery[J].Current Opinion in Chemical Biology,2005,9(6):674-679.
[38] Kam,NW S,DaiH.Carbon Nanotubes as Intracellular Protein Transporters:Generality and Biological Functionality[J].Journal of the American Chemical Society,2005,127(16):6 021-6 026.
[39] Shi Kam NW,Jessop TC,Wender P A,et al.Nanotube Molecular Transporters:Internalization of Carbon Nanotube-Protein Conjugates into Mammalian Cells[J].Journal of the American Chemical Society,2004,126(22):6 850-6 851.
[40] Feazell R P,Nakayama-Ratchford N,Dai H,et al.Soluble Single-Walled Carbon Nanotubes as Longboat Delivery Systems for Platinum(IV)Anticancer Drug Design[J].Journal of the American Chemical Society,2007,129(27):8 438-8 439.
[41] Zhang J,Wang Q,Wang A.In Situ Generation of Sodium Alginate/Hydroxyapatite Nanocomposite Beads as Drug-Controlled Release Matrices[J].Acta Biomaterialia,2010,6(2):445-454.
[42] Mizushima Y,Ikoma T,Tanaka J,et al.Injectable Porous Hydroxyapatite Microparticles as a New Carrier for Protein and Lipophilic Drugs[J].Journal of Controlled Release,2006,110(2):260-265.
[43] Jung H,Kim H M,Choy Y B,et al.Itraconazole-Laponite:Kinetics and Mechanism of Drug Release[J].Applied Clay Science,2008,40(1):99-107.
[44] Viseras C,Cerezo P,Sanchez R,et al.Current Challenges in Clay Minerals for Drug Delivery[J].Applied Clay Science,2010,48(3):291-295.
[45] Song B,Wu C,Chang J.Controllable Delivery of Hydrophilic and Hydrophobic Drugs from Electrospun Poly(lactic-co-glycolic acid)/Mesoporous Silica Nanoparticles Composite Mats[J]. Journal of BiomedicalMaterialsResearch Part B:Applied Biomaterials,2012,100(8):2 178-2 186.
[46] Patel P A,Eckart J,Advincula M C,etal.Rapid Synthesis of Polymer-Silica Hybrid Nanofibers by Biomimetic Mineralization[J].Polymer,2009,50(5):1 214-1 222.
[47] Sun D,Yang J,Wang X.Bacterial Cellulose/TiO2Hybrid Nanofibers Prepared by the Surface HydrolysisMethod with Molecular Precision[J].Nanoscale,2010,2(2):287-292.
[48] Wei K,Ohta T,Kim B S,et al.Development of Electrospun Metallic Hybrid Nanofibers via Metallization[J].Polymers for Advanced Technologies,2010,21(10):746-751.
[49] Yan X,Liu G,HaeusslerM,etal.Water-Dispersible Polymer/Pd/Ni Hybrid Magnetic Nanofibers[J].Chemistry of Materials,2005,17(24):6 053-6 059.
[50] Du J,Hsieh Y L.Cellulose/Chitosan Hybrid Nanofibers from Electrospinning of Their Ester Derivatives[J].Cellulose,2009,16(2):247-260.
[51] Gu M,Wang K,LiW,et al.Preparation and Characterization of PVA/PU Blend Nanofiber Mats by Dual-Jet Electrospinning[J].Fibers and Polymers,2011,12(1):65-72.
[52] Kim K O,Seo Y A,Kim B S,et al.Transition Behaviors and Hybrid Nanofibers of Poly(vinylalcohol)and Polyethylene Glycol-POSS Telechelic Blends[J].Colloid and Polymer Science,2011,289(8):863-870.
[53] Xiao S,Ma H,Shen M,et al.Excellent Copper(II)Removal Using Zero-Valent Iron Nanoparticle-Immobilized Hybrid Electrospun Polymer Nanofibrous Mats[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2011,381(1):48-54.
[54] Xiao S,Shen M,Guo R,et al.Fabrication ofMultiwalled Carbon Nanotube-Reinforced Electrospun Polymer Nanofibers Containing Zero-Valent Iron Nanoparticles for Environmental Applications[J].Journal of Materials Chemistry,2010,20(27):5 700-5 708.
[55] Xiao S,Wu S,Shen M,et al.Polyelectrolyte Multilayer-Assisted Immobilization of Zero-Valent Iron Nanoparticles onto Polymer Nanofibers for Potential Environmental Applications[J].ACSApplied Materials&Interfaces,2009,1(12):2 848-2 855.
[56] Lee J,Tae G,Kim Y H,et al.The Effect of Gelatin Incorporation into Electrospun Poly(l-lactide-co-ε-caprolactone)Fibers on Mechanical Properties and Cytocompatibility[J].Biomaterials,2008,29(12):1 872-1 879.
[57] Weisenberger M,Grulke E,Jacques D,et al.Enhanced Mechanical Properties of Polyacrylonitrile/Multiwall Carbon Nanotube Composite Fibers[J].Journal of Nanoscience and Nanotechnology,2003,3(6):535-539.
[58] Yun J,Im JS,Lee Y S,etal.Electro-Responsive Transdermal Drug Delivery Behavior of PVA/PAA/MWCNT Nanofibers[J]. European Polymer Journal,2011,47(10):1 893-1 902.
[59] Wang S,Castro R,An X,et al.Electrospun Laponite-Doped Poly(lactic-co-glycolic acid)Nanofibers for Osteogenic Differentiation of Human Mesenchymal Stem Cells[J].Journal of Materials Chemistry,2012,22(44):23 357-23 367.
[60] Ignatova M,Manolova N,Markova N,et al.Electrospun Non-Woven Nanofibrous Hybrid Mats Based on Chitosan and PLA for Wound-Dressing Applications[J].Macromolecular Bioscience,2009,9(1):102-111.
[61] Fang X,Ma H,Xiao S,et al.Facile Immobilization of Gold Nanoparticles into Electrospun Polyethyleneimine/Polyvinyl Alcohol Nanofibers for Catalytic Applications[J].Journal of Materials Chemistry,2011,21(12):4 493-4 501.
[62] Wang X,Drew C,Lee S H,et al.Electrospun Nanofibrous Membranes for Highly Sensitive Optical Sensors[J].Nano Letters,2002,2(11):1 273-1 275.
[63] Aussawasathien D,Dong JH,Dai L.Electrospun Polymer Nanofiber Sensors[J].Synthetic Metals,2005,154(1):37-40.
[64] Ashammakhi N,Wimpenny I,Nikkola L,et al.Electrospinning:Methods and Developmentof Biodegradable Nanofibres for Drug Release[J].Journal of Biomedical Nanotechnology,2009,5(1):1-19.
[65] Qi R,Guo R,Zheng F,et al.Controlled Release and Antibacterial Activity of Antibiotic-Loaded Electrospun Halloysite/Poly(lactic-co-glycolic acid)Homposite Nanofibers[J].Colloids and Surfaces B:Biointerfaces,2013,110:148-155.
[66] Wang S,Zheng F,Huang Y,et al.Encapsulation of Amoxicillin within Laponite-Doped Poly(lactic-co-glycolic acid)Nanofibers:Preparation,Characterization,and Antibacterial Activity[J].ACSApplied Materials&Interfaces,2012,4(11):6 393-6 401.
[67] Zheng F,Wang S,Wen S,etal.Characterization and Antibacterial Activity of Amoxicillin-Loaded Electrospun Nano-Hydroxyapatite/Poly(lactic-co-glycolic acid)Composite Nanofibers[J].Biomaterials,2013,34(4):1 402-1 412.
[68] Haroosh H J,Dong Y,Ingram G D.Synthesis,Morphological Structures,and Material Characterization of Electrospun PLA:PCL/Magnetic Nanoparticle Composites for Drug Delivery[J]. Journal of Polymer Science Part B:Polymer Physics,2013,51(22):1 607-1 617.
[69] Hu C,Liu S,Zhang Y,et al.Long-Term Drug Release from Electrospun Fibers for In Vivo Inflammation Prevention in the Prevention of Peritendinous Adhesions[J].Acta Biomaterialia,2013,9(7):7 381-7 388.
[70] Capranico G,Zunino F,Kohn KW,et al.Sequence-Selective Topoisomerase II Inhibition by Anthracycline Derivatives in SV40 DNA:Relationship with DNA Binding Affinity and Cytotoxicity[J].Biochemistry,1990,29(2):562-569.
[71] Binaschi M,Bigioni M,Cipollone A,et al.Anthracyclines:Selected New Developments[J].Current Medicinal Chemistry-Anti-Cancer Agents,2001,1(2):113-130.
[72] Das G,Nicastri A,Coluccio M L,et al.FT-IR,Raman,RRS Measurements and DFTCalculation for Doxorubicin[J].Microscopy Research and Technique,2010,73(10):991-995.
[73] Kunieda K,Seki T,Nakatani S,et al.Implantation Treatment Method of Slow Release Anticancer Doxorubicin Containing Hydroxyapatite(DOX-HAP)Complex.A Basic Study of a New Treatment for Hepatic Cancer[J].British Journal of Cancer,1993,67(4):668-673.
[74] Ignatova M G,Manolova N E,Toshkova R A,et al.Electrospun Nanofibrous Mats Containing Quaternized Chitosan and Polylactide with In Vitro Antitumor Activity against HeLa Cells[J].Biomacromolecules,2010,11(6):1 633-1 645.
[75] Wang S,Zhao Y,Shen M,et al.Electrospun Hybrid Nanofibers Doped with Nanoparticles or Nanotubes for Biomedical App lications[J].Therapeutic Delivery,2012,3(10):1 155-1 169.
[76] Liu F,Guo R,Shen M,et al.Effect of Processing Variables on the Morphology of Electrospun Poly[(lactic acid)-co-(glycolic acid)]Nanofibers[J].Macromolecular Materialsand Engineering,2009,294(10):666-672.
[77] Kim K,Luu Y K,Chang C,etal.Incorporation and Controlled Release of a Hydrophilic Antibiotic Using Poly(lactide-co-glycolide)-Based Electrospun Nanofibrous Scaffolds[J].Journal of Controlled Release,2004,98(1):47-56.
[78] Wang S,Wen S,Shen M,et al.Aminopropyltriethoxysilane-Mediated Surface Functionalization of Hydroxyapatite Nanoparticles:Synthesis,Characterization,and in Vitro Toxicity Assay[J].International Journal of Nanomedicine,2010,6:3 449-3 459.
[79] Zheng F,Wang S,Shen M,et al.Antitumor Efficacy of Doxorubicin-Loaded Electrospun Nano-Hydroxyapatite-Poly(lacticco-glycolic acid)Composite Nanofibers[J].Polymer Chemistry,2013,4(4):933-941.
[80] Qiu K,He C,Feng W,et al.Doxorubicin-Loaded Electrospun Poly(L-lactic acid)/Mesoporous Silica Nanoparticles Composite Nanofibers for Potential Postsurgical Cancer Treatment[J]. Journal of Materials Chemistry B,2013,1(36):4 601-4 611.
[81] Yan E,Fan S,LiX,et al.Electrospun Polyvinyl Alcohol/Chitosan Composite Nanofibers Involving Au Nanoparticles and Their In Vitro Release Properties[J].Materials Scienceand Engineering:C,2013,33(1):461-465.
[82] Hou Z,Li X,LiC,etal.Electrospun Upconversion Composite Fibers as Dual Drugs Delivery System with Individual Release Properties[J].Langmuir,2013,29(30):9 473-9 482.
Construction of Drug-Loaded Electrospun Organic/Inorganic Hybrid Nanofibers for Biomedical Applications
WANG Zhe,SHIXiangyang
(College of Chem istry,Chemical Engineering and Biotechnology,Donghua University,Shanghai201620,China)
The distinctive features of nanofibers(such as huge surface area to volume ratio,controllable porous secondary structure,etc.)enable them to be widely used in the fields of environmental protection,energy,catalysts,drug delivery,and tissue engineering scaffolds.Nanofibers prepared by electrospinning technology mainly include organic nanofibers,inorganic nanofibers,and inorganic/organic hybrid nanofibers.In this review,based on our previouswork associated with electrospun hybrid nanofiber systems,the construction of various drug-loaded electrospun organic/inorganic hybrid nanofiber systems for biomedicalapplications has been reviewed.In particular,this review mainly introduces processing of loading drugmolecules onto orwithin various inorganic nanoparticles(e.g.,halloysitenanotubes,laponitenanodisks,nanohydroxyapatite,mesoporous silica,etc.)and subsequent electrospinning with polymers to form electrospun hybrid nanofiber-based double drug carrier systems,and the associated mechanisms related to the sustained release of the encapsulated drugmolecules.The biomedical applications of the drug-loaded hybrid electrospun nanofibers,especially antibacterial and antitumor therapy applications have also been reviewed.The review ends with a brief outlook of the future directions and prospects of this research area.
electrospinning;hybrid nanofibers;drug carriers;biomedical app lications
TB383
A
1674-3962(2014)11-0661-08
2014-04-03
上海高校特聘教授(东方学者)支持计划
王 哲,男,1989年生,硕士研究生
史向阳,男,1970年生,教授,博士生导师,Email:xshi@dhu.edu.cn
10.7502/j.issn.1674-3962.2014.11.03

