Role of GABA plasticity in stroke recovery
Role of GABA plasticity in stroke recovery
Stroke is a leading cause of long-term disability. Most stroke patients regain their function partially or fully during the fi rst 3—6 months depending on location and size of the lesion. During this functional recovery phase, cortical reorganization or plasticity occurs, and physiological responsiveness or neuronal excitability is altered in the ipsilesional and contralesional areas. However, how to drive successful plastic changes or successful stroke recovery are not fully elucidated yet.
γ-Aminobutyric acid (GABA) is the dominant inhibitory neurotransmitter in the brain and GABA plasticity is known to play an important role in stroke recovery. The role of GABAergic modulation in motor cortical plasticity is well established in rat brains, however, the role of peri-lesional inhibition in human adult brain is less well understood, yet (Bachtiar and Stagg, 2014). Some animal studies found that GABA activity in the peri-infarct cortex initially increases but decreases over time during functional recovery and motor improvement (Clarkson et al., 2010), whereas, human studies indicated that intracortical inhibition was reduced after stroke when measure with transcranial magnetic stimulation (TMS) (Liepert et al., 2000). This discrepancy between animal and human brains might be due to difference in time onset after the stroke or difference in species.
Stroke and GABA:GABAergic signaling in the motor cortex plays an important role in the development of peri-lesional or use-dependent plasticity after stroke.
Previous studies have shown that cortical excitability was reduced in the affected hemisphere when measured corticospinal and intracortical excitability using TMS (Manganotti et al., 2008). In contrast, some studies have demonstrated that short-interval intracortical inhibitions, a marker of GABA synaptic activity was decreased in both hemispheres and this was associated with functional recovery after stroke (Bute fi sch et al., 2008). Lazar et al. (2010) found that the administration of the GABA agonist, midazolam re-induced clinical de fi cits after stroke, which suggest a clinical relevance for the specifi city of GABA-sensitive pathways for stroke recovery.
In animal models of cerebral ischemia, decreased GABAAreceptor density with increased extracellular GABA concentration has been demonstrated in in vivo and in vitro studies (Schwartz-Bloom and Sah, 2001). After a stroke, tonic neuronal inhibition is increased in the peri-infarct zone in mice (Clarkson et al., 2010). This tonic inhibition has been shown to be mediated by extrasynaptic GABA receptors. A speci fi c suggested mechanism is the dysfunction in GABA reuptake from extracellular fl uid mediated by GABA transporter proteins (GATs). A reduced level of GAT function was demonstrated in the peri-infarct zone in an animal model (Clarkson et al., 2010).
We also observed that the GABA receptor availability was signi fi cantly increased in the ipsilesional cortical area (supramarginal cortex, Bradmann area (BA) 40) and contralateral cerebellum as compared to age-matched healthy controls at the initial stage of stoke (Kim et al., 2014) (Figure 1A).
Recovery after stroke and GABA:Cortical reorganization or plasticity is enduring change in morphological or functional properties, and is of major interest because it is engaged in motor learning and functional recovery after stroke. Among them, GABA plasticity plays an important role for motor improvement after stroke (Lazar et al., 2010).
A decrease in GABA-related inhibition facilitates use-dependent plasticity with the expansion of trained representations in the human motor cortex, whereas the pharmacological administration of GABA agonists, such as lorazepam, can reduce use-dependent plasticity in the human motor cortex. Studies on monkeys also demonstrated that activating GABAAreceptors in the medial motor cortex with a GABA agonist increased error rates during motor sequence production. Thus, increased GABAAreceptor binding in the motor cortex could be related to poor functional linking of the neurons for complex motor skills with less effective plasticity. In a rat stroke model, it was also demonstrated that peri-lesional chronic tonic GABAergic inhibition, mediated by extrasynaptic GABAAreceptors impaired the motor recovery (Clarkson et al., 2010).
In cases with good functional recovery, there is initially reduced cortical excitability in the ipsilesional brain areas and transfer of increased cortical exitability to the contralateral cortex, which subsequently returns to the peri-infarct and ipsilesional networks when studied with transcranial magnetic stimulation or neuroimaging study. In a rat stroke model, early application of an inverse agonist speci fi c to the benzodiazepine binding site on extrasynaptic GABAAreceptors, resulted in early and sustained motor function recovery by lowering the tonic neuronal inhibition. Genetic knockdown of GABAAreceptors containing α5 and δ subunits, found primarily in extrasynaptic GABAAreceptors, also resulted in a similar increased functional recovery (Clarkson et al., 2010).
Using magnetic resonance spectroscopy, a more direct measure of the local concentration of GABA is possible. Blicher et al. (2014) also measured GABA content in primary motor cortex in relation to motor recovery during 2 weeks of constraint-induced movement therapy in stroke patients at 3—12 months after the onset. Before the training, stroke patients showed a significantly lower GABA activity than healthy controls. After training, patients improved signi fi cantly on motor function, and the extent of this motor improvement correlated significantly with the decreases in GABA activity.
GABA PET evidence: [18F]flumazenil ([18F]FMZ) is a specific, high-affinity neutral antagonist that binds reversibly to the benzodiazepine site of the GABAAreceptor complex, which is located on axodendritic synapses, with a wide cortical distribution closely following the local density of neu-rons. Therefore, it is a useful marker for the assessment of neuronal density and integrity.
Recently, FMZ PET imaging studies demonstrated the plasticity of GABAAreceptors. Regional FMZ binding was altered without any change in the volume of the gray matter or the affinity of the benzodiazepine site, but was affected by the functional state of GABAergic neurons (i.e., the release and uptake of the transmitter during activity). Using FMZ PET, the functional alteration of GABAergic neurons has been investigated in several neuropsychiatric disorders. Damaged cortex could be also detected by reduced FMZ binding (Heiss et al., 1998) or by decreased benzodiazepine receptor concentration. Excellent reproducibility and reliability of FMZ PET quantification has also been reported (Salmi et al., 2008). Therefore, FMZ PET study can reflect the functional alteration and neuronal density of GABA in stroke patients.
To better understand the mechanism of physiological plasticity during the recovery phase of stroke, a longitudinal observation of the changes in cerebral GABA activity was undertaken.
We examined the changes in cerebral GABA activity in patients with subcortical stroke using FMZ positron-emission tomography (PET) during the recovery and motor improvement period (Kim et al., 2014). In our study, global GABAergic activity was increased compared with the age-matched controls 1 month after stroke and returned to the control level at 3 months after stroke. This pattern of brain activation corroborates the results of other group’s study (Clarkson et al., 2010), and these fi ndings suggest that changes in the regional GABAAreceptor binding are related to motor recovery in stroke patients.
In our stroke patients, GABAergic activity decreased throughout the cerebral cortex and cerebellum, especially in the contralateral hemisphere, during the funational improvement period (Kim et al., 2014) (Figure 1B, C). This might be related to decreased intracortical inhibition or decreased transcallosal inhibition from the contralesional hemisphere to the ipsilesional hemisphere. The change in the relative inter-hemispheric GABAergic activity might re fl ect a change in the strength of inter-hemispheric interactions, which are important for the normal coordination of hand movements and stroke motor recovery. We also observed that a greater decrease in contralateral cortical and subcortical GABA availability was correlated with more improvement in motor function.
Although we observed a change in brain activation in patients with subcortical infarction, it is also expected that cortical stroke patients follows a similar longitudinal pattern of the GABAergic activity based on the report by Cramer (2008); cortical excitability is reduced initially and then increases over time, and that behavioral outcome might be correlated with the extent of this increase.
The reorganization maps could present not only in the primary motor cortex but also in the sensory, auditory, and visual cortices, and even in the white matter and basal ganglia.
Our patients also showed a decrease in GABAAreceptor binding in the temporal lobe contralateral to the lesions and a trend towards decreased receptor binding in the ipsilateral thalamus. Changes in the connectivity of motor cortex with the thalamus might be related to the cortico-basal-ganglia-thalamo-cortical loop, which is involved in processing information regarding motor control and learning motor sequences. In our study, motor recovery also correlated with changes in GABAergic activity in the thalamus. This change might reflect a reorganization of the cortico-basal ganglia-thalamo-cortical loop necessary for motor recovery.
The GABAergic activity increased in the contralateral cerebellum 1 month after stroke and persisted 3 months after stroke, but to a lesser extent. The reason for this increased GABAergic activity in the contralateral cerebellum might be to increase contralateral cortical excitability to promote functional recovery. It has been demonstrated that corticomotor excitability was associated with level of inhibition from the contralateral cerebellum (cerebello-cerebral inhibition), and the decrease in this cerebello-cerebral inhibition was associated with improvement in motor function.
Future perspectives and challenges:Measuring GABA activity could serve as a potential biomarker for post-stroke recovery. Future studies with a larger sample size correlating GABAergic activation patterns with functional recovery are needed to identify the neuroanatomical substrates that subserve functional recovery. Such researches might also guide the development of more effective rehabilitative interventions for functinal improvement after a stroke.
Important mechanisms for mediating reorganization in the cerebral cortex involve the unmasking of existing, but latent, horizontal connections and modulation of GABAergic inhibition and synaptic efficacy. This approach could be a novel strategy to promote motor recovery after stroke. Interventions that lead to the reduction of GABAergic inhibition are means of facilitating plastic changes in the motor cortex. This understanding of cortical reorganization may enable us to design rational strategies to enhance bene fi cial plastic changes in stroke patients, for example, promotion of repetitive behavioral training or avoiding inhibitory drive such as GABAergic drugs or applying neuromodulation techniques such as transcranial magnetic stimulation and transcranial direct current stimulation in an appropriate way.
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (2009-0076321) to Paik NJ.
Nam-Jong Paik, EunJoo Yang
Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
Bachtiar V, Stagg CJ (2014) The role of inhibition in human motor cortical plasticity. Neuroscience 278C:93-104.
Blicher JU, Near J, Naess-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Ostergaard L, Ho YC (2014) GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil Neural Repair pii: 1545968314543652.
Bute fi sch CM, Wessling M, Netz J, Seitz RJ, Homberg V (2008) Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22:4-21.
Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST (2010) Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468:305-309.
Cramer SC (2008) Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 63:272-287.
Heiss WD, Grond M, Thiel A, Ghaemi M, Sobesky J, Rudolf J, Bauer B, Wienhard K (1998) Permanent cortical damage detected by fl umazenil positron emission tomography in acute stroke. Stroke 29:454-461.
Kim YK, Yang EJ, Cho K, Lim JY, Paik NJ (2014) Functional recovery after ischemic stroke is associated with reduced GABAergic inhibition in the cerebral cortex: a GABA PET study. Neurorehabil Neural Repair 28:576-583.
Lazar RM, Berman MF, Festa JR, Geller AE, Matejovsky TG, Marshall RS (2010) GABAergic but not anti-cholinergic agents re-induce clinical de fi cits after stroke. J Neurol Sci 292:72-76.
Liepert J, Hamzei F, Weiller C (2000) Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 23:1761-1763.
Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A (2008) Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair 22:396-403.
Salmi E, Aalto S, Hirvonen J, Langsjo JW, Maksimow AT, Oikonen V, Metsahonkala L, Virkkala J, Nagren K, Scheinin H (2008) Measurement of GABAA receptor binding in vivo with [11C]flumazenil: a test-retest study in healthy subjects. Neuroimage 41:260-269.
Schwartz-Bloom RD, Sah R (2001) gamma-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J Neurochem 77:353-371.
Nam-Jong Paik, M.D., Ph.D.
Email: njpaik@snu.ac.kr.
10.4103/1673-5374.147920 http://www.nrronline.org/
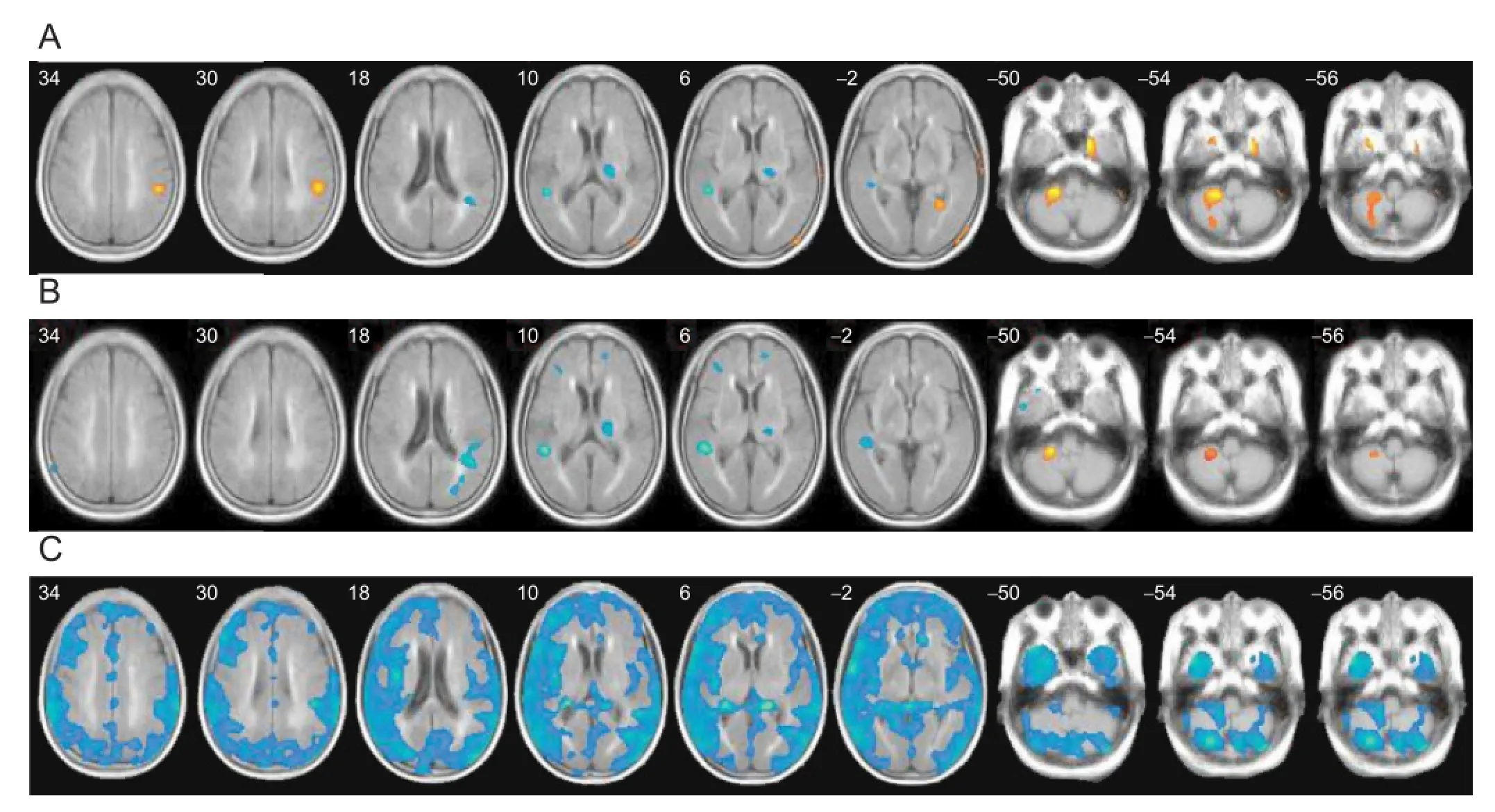
Figure 1 The GABA receptor availability in stroke patients seen with [18F]FMZ PET.
Receptor availability on [18F]FMZ PET was calculated as the regional uptake ratio, in which the regional uptake is relative to the activity of the pons, where receptor sites are negligible. Then, signi fi cant differences in receptor availability in the 1- and 3-month PET were compared with those of age-matched healthy controls using general linear models in a voxel-based manner. (A) The GABA receptor availability in stroke patients was increased in the ipsilesional cortical area (supramarginal cortex, BA 40) and contralateral cerebellum in comparison with age-matched healthy controls at 1 month after stroke. (B) The fi ndings were similar at 3 months after stroke, with a more widespread reduction in GABA receptors in the cortical and subcortical white matter in the contralateral cerebellum. The greater receptor availability in the contralateral cerebellum persisted, but to a lesser extent, and the higher perilesional receptor availability was no longer signi fi cant. (C) A comparison of the PET study between 1 month and 3 months after stroke (Δ=3 month−1 month) revealed that the GABA receptor availability generally decreased throughout the cerebral cortex and cerebellum during the functional recovery period, especially in the contralateral hemisphere. The mean uptake ratio of FMZ PET in the brain was also decreased signi fi cantly at 3 months as compared to at 1 month after stroke (Kim et al., 2014). This fi gure has been reproduced with permission. GABA: γ-Aminobutyric acid; [18F]FMZ PET: [18F] fl umazenil positron emission tomography.
Accepted: 2014-11-01
Paik NJ, Yang E. Role of GABA plasticity in stroke recovery. Neural Regen Res. 2014;9(23):2026-2028.
- 中国神经再生研究(英文版)的其它文章
- Sulindac for stroke treatment: neuroprotective mechanism and therapy
- Angioplasty and stenting for severe vertebral artery ori fi ce stenosis: effects on cerebellar function remodeling veri fi ed by blood oxygen level-dependent functional magnetic resonance imaging
- Lesion localization of global aphasia without hemiparesis by overlapping of the brain magnetic resonance images
- A novel functional electrical stimulation-control system for restoring motor function of post-stroke hemiplegic patients
- Neurotrophins and their receptors in satellite glial cells following nerve injury
- Dynamic reactive astrocytes after focal ischemia

