Angioplasty and stenting for severe vertebral artery ori fi ce stenosis: effects on cerebellar function remodeling veri fi ed by blood oxygen level-dependent functional magnetic resonance imaging
Bo Liu, Zhiwei Li Peng Xie,
1 Department of Neurology, Yongchuan Hospital, Chongqing Medical University, Chongqing, China
2 Institute of Neuroscience, Chongqing Medical University, Chongqing, China
3 Chongqing Key Laboratory of Neurobiology, Chongqing Medical University, Chongqing, China
4 Department of Neurology, the First Af fi liated Hospital of Chongqing Medical University, Chongqing, China
Angioplasty and stenting for severe vertebral artery ori fi ce stenosis: effects on cerebellar function remodeling veri fi ed by blood oxygen level-dependent functional magnetic resonance imaging
Bo Liu1,2,3, Zhiwei Li1, Peng Xie1,2,3,4
1 Department of Neurology, Yongchuan Hospital, Chongqing Medical University, Chongqing, China
2 Institute of Neuroscience, Chongqing Medical University, Chongqing, China
3 Chongqing Key Laboratory of Neurobiology, Chongqing Medical University, Chongqing, China
4 Department of Neurology, the First Af fi liated Hospital of Chongqing Medical University, Chongqing, China
Vertebral artery ori fi ce stenting may improve blood supply of the posterior circulation of the brain to regions such as the cerebellum and brainstem. However, previous studies have mainly focused on recovery of cerebral blood fl ow and perfusion in the posterior circulation after interventional therapy. This study examined the effects of functional recovery of local brain tissue on cerebellar function remodeling using blood oxygen level-dependent functional magnetic resonance imaging before and after interventional therapy. A total of 40 Chinese patients with severe unilateral vertebral artery ori fi ce stenosis were enrolled in this study. Patients were equally and randomly assigned to intervention and control groups. The control group received drug treatment only. The intervention group received vertebral artery ori fi ce angioplasty and stenting + identical drug treatment to the control group. At 13 days after treatment, the Dizziness Handicap Inventory score was compared between the intervention and control groups. Cerebellar function remodeling was observed between the two groups using blood oxygen level-dependent functional magnetic resonance imaging. The improvement in dizziness handicap and cerebellar function was more obvious in the intervention group than in the control group. Interventional therapy for severe vertebral artery ori fi ce stenosis may effectively promote cerebellar function remodeling and exert neuroprotective effects.
nerve regeneration; posterior circulation ischemia; vertebrobasilar insufficiency; dizziness; Dizziness Handicap Inventory; vertebral artery stenosis; angioplasty and stenting; endovascular treatment; functional magnetic resonance imaging; cerebellar function remodeling; cerebral vessels; atheromatous plaque; neural regeneration
Funding: This study was supported by the Natural Science Foundation of Yongchuan District of Chongqing in China, No. Ycstc, 2013nc8031; the Foundation of Chongqing Municipal Health Bureau in China, No. 2010-2-250; the Foundation of Chongqing Health and Family Planning Commission in China, No. 20143001; the Soft Science Foundation of Yongchuan District of Chongqing in China, No. Ycstc, 2011BE5004.
Liu B, Li ZW, Xie P. Angioplasty and stenting for severe vertebral artery orifice stenosis: effects on cerebellar function remodeling verified by blood oxygen level-dependent functional magnetic resonance imaging. Neural Regen Res. 2014;9(23):2095-2101.
Introduction
Posterior circulation ischemia is a common ischemic cerebrovascular disease, accounting for 20% of ischemic stroke, with common symptoms including dizziness, vertigo, and gait or limb ataxia (Bulut et al., 2014; Peng et al., 2014). The most common cause of posterior circulation ischemia involves vertebral artery orifice stenosis, which is mainly induced by atheromatous plaque infiltration (Kocak et al., 2012; Chen et al., 2013). Patients with mild vertebral artery orifice stenosis do not have any symptoms, while aggravation of stenosis can cause significant vertigo. Patients with severe stenosis can exhibit posterior circulation infarction, and previous conservative treatment often cannot reverse this process (Marquardt et al., 2009; Compter et al., 2011).
In recent years, the development of percutaneous transluminal angioplasty and stenting has provided potential treatments for patients with vertebral artery ori fi ce stenosis. Suitable stent implantation to the site of stenosis can reconstruct the original form of the vertebral artery and improve blood supply to the brain (Li et al., 2014). Numerous studies have reported the therapeutic effects of vertebral artery orifi ce stenting (Antoniou et al., 2012; Edgell et al., 2013). Percutaneous transluminal angioplasty and stenting have been shown to apparently improve blood supply to the posteriorcirculation, with mitigation of patient’s clinical symptoms. However, previous studies have mainly focused on the recovery of cerebral blood fl ow and cerebral perfusion in the posterior circulation of the brain after interventional therapy, but seldom addressed the functional recovery of local brain tissue.
Blood oxygen level-dependent functional magnetic resonance imaging can provide a measure of regional brain function remodeling and restructuring (Lukas et al., 2013; Pugnaghi et al., 2014). Thus, in the present study, we examined the effects of interventional therapy on cerebellar function remodeling using blood oxygen level-dependent functional magnetic resonance imaging in patients undergoing vertebral artery orifice angioplasty and stenting for severe vertebral artery ori fi ce stenosis.
Subjects and Methods
Subjects
A total of 40 patients with severe unilateral vertebral artery ori fi ce stenosis, who were treated in the Department of Neurology, Yongchuan Hospital, Chongqing Medical University, China from January 2013 to June 2014, were enrolled in this study. Patients were equally and randomly divided into the intervention group (9 males and 11 females) and control group (10 males and 10 females). Patient’s age and course of disease are listed inTable 1.
Inclusion criteria
(1) Patients or their families signed the informed consent; (2) age of 50—79 years, irrespective of gender; (3) disease onset had the symptoms of posterior circulation ischemia, such as dizziness, vertigo, or unsteady gait, with no signs of paralysis of limbs before and after the disease; (4) clinical diagnosis: posterior circulation ischemia, but no posterior circulation infarction detected by CT or MRI; (5) CT angiography, magnetic resonance angiography or digital subtraction angiography revealed severe unilateral vertebral artery ori fi ce stenosis, with a stenosis rate of ≥ 70%. No stenosis was found in the contralateral vertebral artery and basilar artery. Left or right vertebral artery stenosis is shown inFigure 1AandFigure 2A. (6) Agreed to receive blood oxygen level-dependent functional magnetic resonance imaging.
Exclusion criteria
(1) Combined with cardiovascular diseases (acute myocardial infarction, angina, and paroxysmal tachycardia); (2) combined with severe primary liver and kidney diseases; (3) hyperthyroidism; (4) cerebral hemorrhage within 2 weeks; (5) other bleeding tendency or massive blood loss recently; (6) mental disorders; (7) disturbance of intelligence, such as dementia; (8) donors thought the patient who should not be enrolled; (9) we found during the study that the patient did not comply with the study protocol, the treatment was discontinued for some reason, or we could not evaluate the therapeutic effects; (10) refused to receive blood oxygen level-dependent functional magnetic resonance imaging, or researchers believed that the patient needed to withdraw from the study.
Grouping principles
(1) After fully understanding the study design and aims, the patients and families agree to enter this clinical study, and they were randomly grouped; (2) the patients entered the control group during preliminary screening; patients were unwilling to accept the interventional treatment for personal reasons, and were enrolled in the control group; patients were willing to accept the interventional treatment and quit the control group; (3) the patients entered the intervention group during preliminary screening, were willing to accept the interventional treatment, and were enrolled in the intervention group. The patients who were unwilling to accept the interventional treatment quit the experiment.
Treatments
Subjects in the control group were administered drugs for 2 weeks immediately on the day of hospitalization. Medicine contained 600 mg gastrodin injection (Harbin Shengtai Biological Pharmaceutical Co., Ltd., Harbin, Heilongjiang Province, China) in 250 mL physiological saline by intravenous drip, once a day, 500 mg citicoline injection (Changchun Tiancheng Pharmaceutical Co., Ltd., Changchun, Jilin Province, China) in 100 mL physiological saline by intravenous drip, once a day, 100 mg aspirin enteric-coated tablets (Jiangsu Pingguang Pharmaceutical Co., Ltd., Xuzhou, Jiangsu Province, China) orally, once a day, and 75 mg clopidogrel (Hangzhou Sainuofei Pharmaceutical Co., Ltd., Hangzhou, Zhejiang Province, China) orally, once a day.
Subjects in the intervention group received these medicines and underwent vertebral artery ori fi ce angioplasty and stenting at 3—5 days after hospitalization under local anesthesia. Before the surgery, subjects were required to take oral aspirin or clopidogrel for more than 3 days. For the surgical methods, a 6F vascular sheath was inserted into the femoral artery. A 6F guiding catheter was inserted into the subclavian artery (near the narrow vertebral artery orifice) under the guide of an 0.89-mm super-slippery guidewire. A 0.35-mm micro guidewire through the head end of the narrow blood vessels was placed in the V3 segment of the vertebral artery. Under the guide of a micro guidewire, a Boston balloon expandable stent (Boston Scienti fi c, Castle Rock, CO, USA; made by platinum chromium alloy) was placed at the lesion site for expansion until stenosis was < 30%. Postoperative conventional treatment is shown inFigure 1AandFigure 2A–C.
Observation index
Each patient was observed for 2 weeks. The patients who hospitalized for more than 2 weeks were no longer studied after this time. The patients lost to follow up were excluded, and were supplemented by new subjects (two cases in the intervention group and three cases in the control group). Clinical ef fi cacy of each patient was evaluated using Dizziness Handicap Inventory (DHI) at 1 and 14 days after hospitalization. DHI is an internationally recognized method ofassessing the severity of vertigo. This scale contains 25 questions, with a score range of 0—100; score 0 represents normal. A high score indicates a severe degree of vertigo (Alsanosi, 2012; Mutlu and Serbetcioglu, 2013; Georgieva-Zhostova et al., 2014).
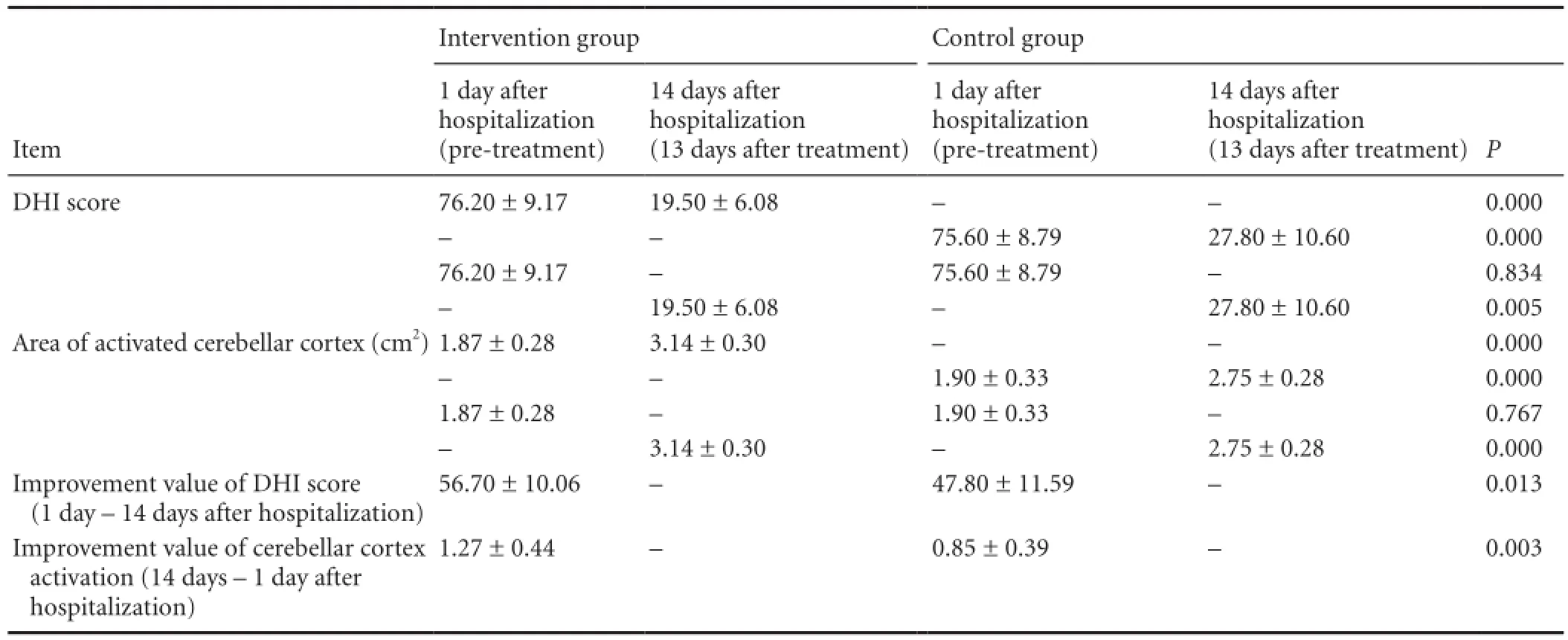
Table 2 Differences in DHI score and the area of activated cerebellar cortex before and after treatment in the intervention and control groups
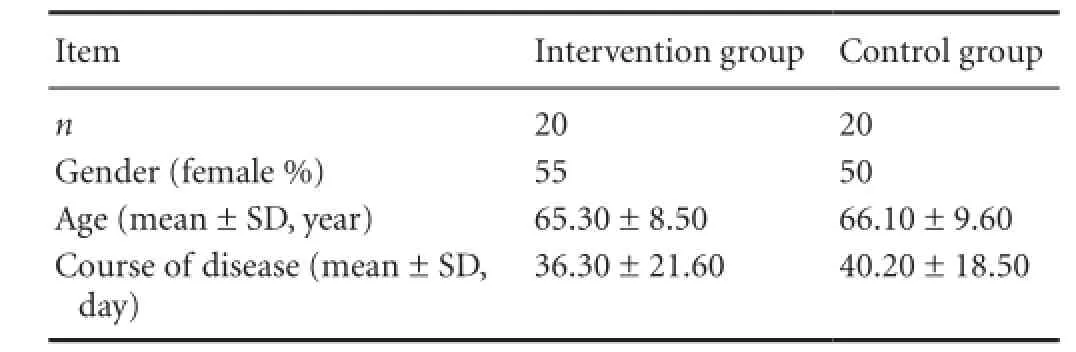
Table 1 Baseline data of patients
Blood oxygen level-dependent functional magnetic resonance imaging was conducted at 1 and 14 days of hospitalization in each patient, as previously described (Li et al., 2012; Wang et al., 2012). Imaging was performed in a block design, with rest (base-line) and stimulus (task) alternated with each other. After 12 seconds of preparation, the patients performed a fl exion and extension task with their hands (task condition), once every 3 seconds, for a total of 15 times over 30 seconds. Subjects then rested for 30 seconds. This task/ rest paradigm was repeated a total of three times. Simultaneously, blood oxygen level-dependent functional magnetic resonance imaging was conducted. Two three-dimensional images of blood oxygen level-dependent functional magnetic resonance imaging were obtained in each patient before and after treatment. Activation of the cerebellar cortex and cerebral cortex was observed from the three-dimensional images. The area of activated cerebellar cortex was measured using the MRI software, and the images were compared before and after treatment for each patient. Changes in cerebellar cortex activation in each patient were calculated by the area of activated cerebellar cortex at 14 days after hospitalization — the area of activated cerebellar cortex at 1 day after hospitalization. Differences in the improvement value of cerebellar cortex activation were compared between the two groups. The effect of remodeling of the vertebral artery orifice angioplasty and stenting on cerebellar function was also assessed.
Statistical analysis
Measurement data were expressed as the mean ± SD, and numeration data were presented by percentage. Data were analyzed using SPSS 20.0 software (SPSS, Chicago, IL, USA). The differences in age, course of disease, DHI score, the improvement value of DHI score, the area of activated cerebellar cortex, and the improvement value of cerebellar cortex activation between groups were compared using two-sample t-test. Gender composition between groups was compared using chi-square test. Alpha was set to 0.05.
Results
Clinical ef fi cacy of stenting for severe vertebral artery ori fi ce stenosis
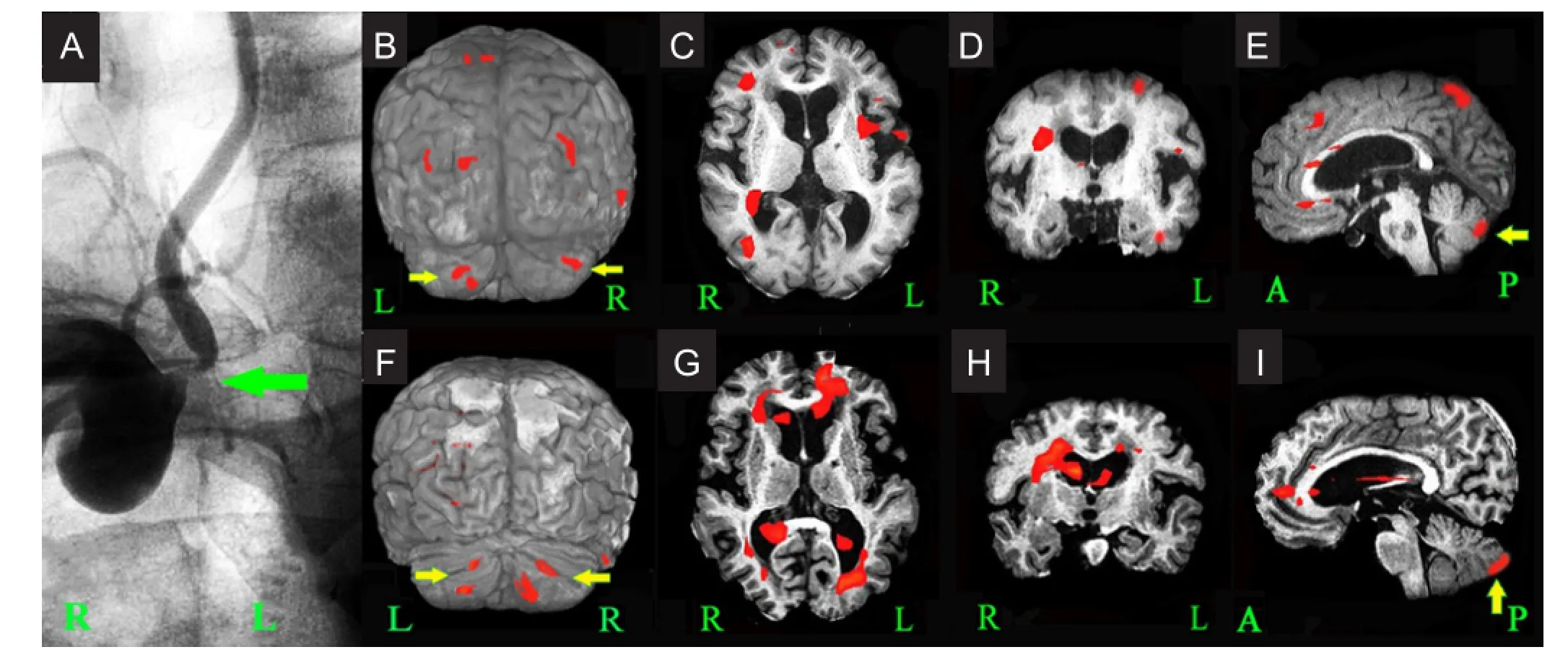
Figure 1 Cerebral angiography images and functional MRI images before and after drug treatment (1 and 14 days after hospitalization) in a case of severe right vertebral artery ori fi ce stenosis of the control group.
No signi fi cant differences in gender composition ratio, age, or course of disease were found between the intervention and control groups (Table 1; P < 0.05). All patients experienced symptoms of posterior circulation ischemia such as varying degrees of dizziness and vertigo at 1 day after hospitalization. No signi fi cant differences in average DHI score were detectable between the intervention and control groups (Table 2). Signi fi cant differences in average DHI score in the intervention and control groups were detected at 13 days after treatment (14 days after hospitalization) compared with pre-treatment (P < 0.05), and there were signi fi cant differences between the two groups at this time (P < 0.05;Table 2). The mean improvement value of DHI score was signi fi cantly greater in the intervention group than in the control group (P < 0.05;Table 2).
Imaging evaluation of stenting for severe vertebral artery ori fi ce stenosis
Blood oxygen level-dependent functional magnetic resonance imaging results during patient hand movement at before (1 day after hospitalization) and after treatment (14 days after hospitalization) demonstrated evidence of motor cortex activation in 4 and 6 regions of the bilateral cerebral hemispheres and the bilateral cerebellar cortex activation. Simultaneously, the cortices of other parts of the bilateral frontal lobe, bilateral parietal lobe, temporal lobe, occipital lobe, and insula were activated to different degrees (Figures 1and2).
The measurement results of the surface area of activated cerebellar cortex using the MRI software before and after treatment in each patient are shown inTable 2. At 1 day after hospitalization, the cerebellar cortex was activated in both groups, while no signi fi cant differences in the mean area of the activated cerebellar cortex were detected between the intervention and control groups. The mean area of activated cerebellar cortex in the two groups was significantly larger at 13 days of treatment compared with pre-treatment (P <0.05). Significant differences in the mean area of activated cerebellar cortex were observed between the two groups after treatment (P < 0.05;Table 2). The mean of improvement value of activated cerebellar cortex was signi fi cantly greater in the intervention group than that in the control group (P < 0.05;Table 2).
Discussion
Posterior circulation ischemia can have marked effects on patients, including dizziness and vertigo, limb or head and facial numbness, limb weakness, headache, vomiting, diplopia, transient loss of consciousness, visual disturbances, unsteady gait or falling, or even death (Ito et al., 2010; Wang et al., 2011; Choi et al., 2012). Posterior circulation ischemia is an ischemic cerebrovascular disease induced by severe vertebral artery ori fi ce stenosis. In the acute stage, treatment is typically provided using the organized stroke care model, involving antiplatelet and anticoagulant drug therapy, supplemented with neuroprotective agents and symptomatic drugs, while secondary thrombosis is treated by ultra-early thrombolytic therapy (Amole et al., 2012). For convales-cent treatment, anti-platelet aggregation, plaque-stabilizing drugs, and controlling the risk factors for hypertension, diabetes, and hyperlipemia are commonly used (Amole et al., 2012; Koch et al., 2014). However, as vertebral artery stenosis is often caused by hard plaque infiltration, the drug treatment can reduce the clinical symptoms acutely, but is unable to fundamentally improve the narrow anatomical structures (Kim et al., 2013).
Surgical treatment for severe vertebral artery ori fi ce stenosis is not ideal. Wehman et al. (2004) reported an endarterectomy on the vertebral artery origin through the vertebral artery wall or the subclavian artery. There are also a few cases of successful posterior circulation extracranial-intracranial bypass grafting, although the rate of relative complications is high, and this technique is not widely used clinically. Endovascular treatment has also been used for stenosis. Higashida et al. (1993a, b) con fi rmed that the incidence of postoperative neurological dysfunction was 8.8% in 34 vertebral artery origin stenosis patients undergoing percutaneous transluminal angioplasty, and the incidence of restenosis was 8.8% 5 months later. Bruckmann et al. (1986) veri fi ed that the longterm restenosis rate of percutaneous transluminal angioplasty without stenting reached 15—30%. Therefore, balloon angioplasty alone is no longer recommended because of its ease of recoil and high restenosis rate. Nevertheless, the combination of balloon angioplasty with stenting is becoming increasing popular. Stenting itself can reduce the rate of restenosis after percutaneous transluminal angioplasty, and numerous studies have examined the clinical therapeutic effects of combination treatment (Mohammadian et al., 2013; Sakamoto et al., 2013). In the present study, the improvement value of the DHI score and cerebellar cortex activation were higher in the intervention group than in the control group, indicating the recovery of ischemic cerebellar function. Thus, combination balloon angioplasty and stenting is an appropriate treatment method for severe vertebral artery ori fi ce stenosis.
Blood oxygen level-dependent functional magnetic resonance imaging directly re fl ects the activation of the human brain, and has been extensively used in clinical studies of brain remodeling (Chao et al., 2014; Galazzo et al., 2014; Zhang et al., 2014). However, these studies have generally focused on the cerebral hemispheres, rather than the cerebellum. Posterior circulation ischemia can directly affect cerebellar function. Blood oxygen level-dependent functional magnetic resonance imaging has been shown to effectively detect the activated domain of the cerebellar cortex, which can be used to directly assess cerebellar reorganization and remodeling. Our results demonstrated that interventional therapy effectively promoted cerebellar function remodeling in patients with severe vertebral artery ori fi ce stenosis.
The bilateral internal carotid arteries and the vertebral artery supply blood to intracranial brain tissues. The internal carotids account for 80—90% the total blood supply of the brain, while the vertebral artery accounts for 10—20%. When the unilateral or bilateral vertebral artery blood flow is reduced by stenosis or occlusion, clinical evidence of ischemia would not be observed if the posterior communicating artery or other collateral arteries are open. Nevertheless, in many normal subjects, the posterior communicating artery was reported to be not opened or exhibit hypoplasia (Morris and Choi, 1996; Burgess et al., 1999). Thus, these subjects would be highly sensitive to posterior circulation ischemia. When vertebral artery stenosis accounts for more than 80% of the lumen cross-sectional area, cerebral blood fl ow is reduced in the posterior circulation. When cerebral blood fl ow is below a critical level (18—20 mL/100 g per minute), cerebral ischemia would occur (Abe et al., 2014; Chi et al., 2014). The cases in the present study exhibit these conditions, and interventional therapy is considered an effective treatment method.
In chronic cerebral ischemia, a range of pathophysiological changes occur in the brain, including energy metabolism dysfunction, decreased glucose decreased use, abnormal protein synthesis, neurotransmitter changes, cholinergic receptor deletion, white matter damage, and neuronal defects (Macdonald and Stoodley, 1998; Villringer and Dirnagl, 1999). These changes ultimately lead to functional decline in brain tissue, thus reducing the areas of activation in the cortex. The methods described in the present study are currently used for treatment of brain injury induced by chronic cerebral ischemia (Nagahiro et al., 1998). For example, cilostazol inhibits glial cell changes in brain cells, osthole reduces oxidation in brain tissue, succinylcholine regulates neurotransmitter disorders, and anticoagulant and antiplatelet drugs improve cerebral ischemia. Additionally, glutamate antagonists, glutamate release inhibitors, free radical scavengers, and cell membrane stabilizers can be neuroprotective. Traditional Chinese medicine preparations for promoting blood circulation and removing blood stasis are also used (Behravan et al., 2014; Chen et al., 2014; Sun et al., 2014; Tang et al., 2014). Further, stem cells and vascular endothelial growth factor have therapeutic effects on chronic cerebral ischemia (Gu et al., 2014; Song et al., 2014; Yang et al., 2014). However, these treatment methods are conservative and passive, and although they can improve metabolism and clinical symptoms of ischemic brain tissue acutely, they do not remove the underlying causes of cerebral ischemia. Thus, the symptoms of chronic cerebral ischemia may recur.
Percutaneous transluminal angioplasty and stenting for cerebrovascular stenosis can rebuild normal vascular morphology, remove the actual cause of chronic cerebral ischemia, and provide therapeutic bene fi ts. Although ischemia/ reperfusion injury may appear in distal brain tissue in the early stage after percutaneous transluminal angioplasty and stenting, ischemic brain tissue can adapt to the sufficient blood fl ow within several days. Local micro-circulation and metabolism gradually return to normal, followed by improved brain function indicated by enlarged areas of activation in the cortex (Han et al., 2013; Jiang et al., 2014). Thus, interventional therapy can effectively promote cerebellar function remodeling in patients with severe vertebral artery ori fi ce stenosis.
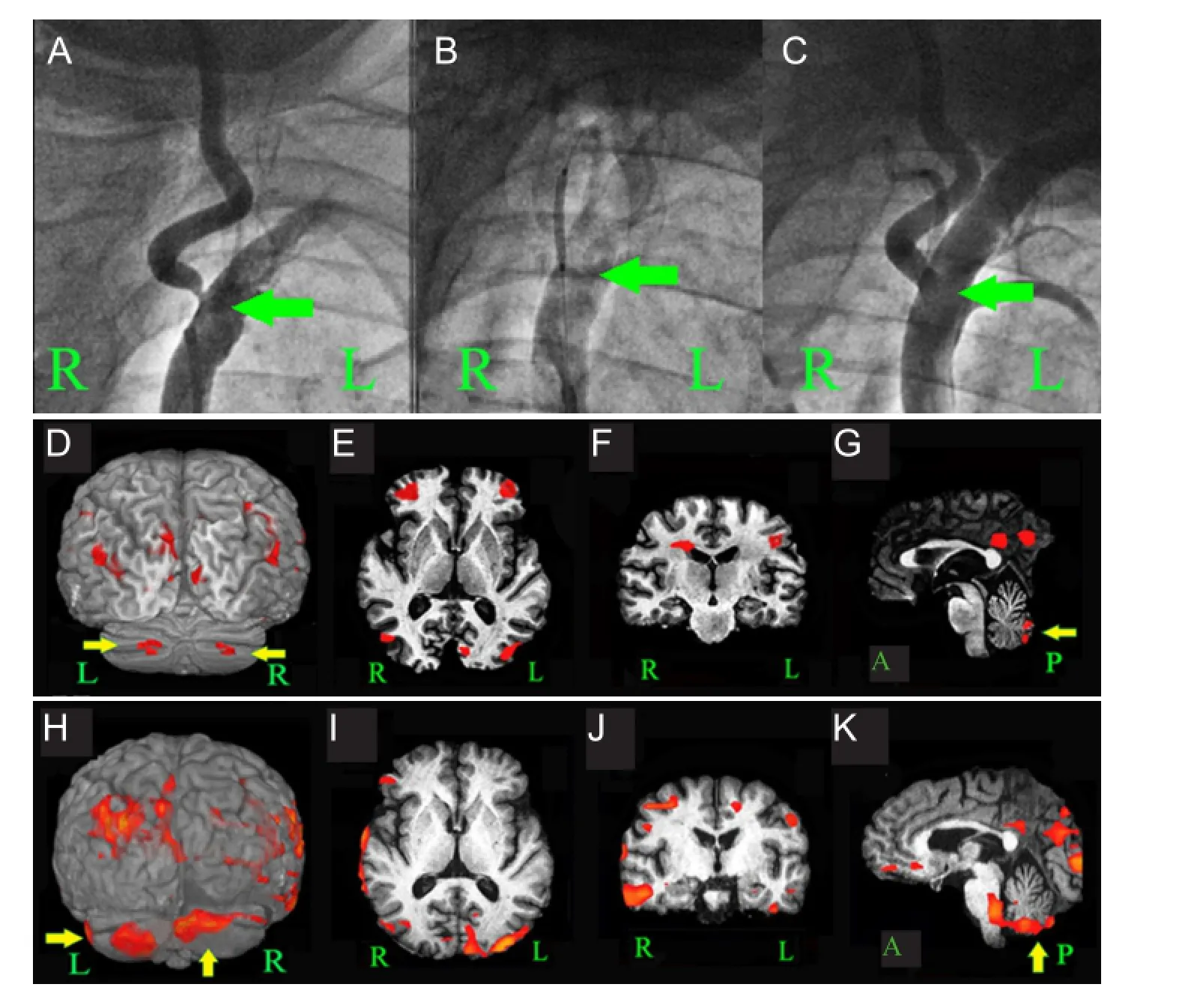
Figure 2 Cerebral angiography images before and after stenting and functional MRI images before and after interventional therapy (1 and 14 days after hospitalization) in a case of severe left vertebral artery ori fi ce stenosis of the intervention group.
In summary, both drugs and interventional methods can provide therapeutic effects in severe vertebral artery ori fi ce stenosis patients with posterior circulation ischemia. However, compared with drug treatment, interventional therapy can eliminate the fundamental aspects of the disease, and promote the remodeling of ischemic brain tissue.
Author contributions:Liu B provided data. Li ZW was responsible for statistical analysis. Xie P was the article validator, fund manager and research mentor. All authors were responsible for writing the manuscript and approved the final version of the manuscript.
Con fl icts of interest:None declared.
Abe T, Suzuki M, Sasabe J, Takahashi S, Unekawa M, Mashima K, Iizumi T, Hamase K, Konno R, Aiso S, Suzuki N (2014) Cellular origin and regulation of D- and L-serine in in vitro and in vivo models of cerebral ischemia. J Cereb Blood Flow Metab doi:10.1038/jcbfm.2014.164.
Alsanosi AA (2012) Adaptation of the dizziness handicap inventory for use in the Arab population. Neurosciences (Riyadh) 17:139-144.
Amole AO, Akdol MS, Wood CE, Keyrouz SG, Erdem E (2012) Endovascular management of symptomatic vertebral artery origin stenosis in the presence of an acute thrombus. J Neurointerv Surg 4:e11.
Antoniou GA, Murray D, Georgiadis GS, Antoniou SA, Schiro A, Serracino-Inglott F, Smyth JV (2012) Percutaneous transluminal angioplasty and stenting in patients with proximal vertebral artery stenosis. J Vasc Surg 55:1167-1177.
Behravan E, Razavi BM, Hosseinzadeh H (2014) Review of plants and their constituents in the therapy of cerebral ischemia. Phytother Res 28:1265-1274.
Boscolo Galazzo I, Storti SF, Formaggio E, Pizzini FB, Fiaschi A, Beltramello A, Bertoldo A, Manganotti P (2014) Investigation of brain hemodynamic changes induced by active and passive movements: A combined arterial spin labeling-BOLD fMRI study. J Magn Reson Imaging 40:937-948.
Bruckmann H, Ringelstein EB, Buchner H, Zeumer H (1986) Percutaneous transluminal angioplasty of the vertebral artery. A therapeutic alternative to operative reconstruction of proximal vertebral artery stenoses. J Neurol 233:336-339.
Bulut HT, Yildirim A, Ekmekci B, Eskut N, Gunbey HP (2014) False-negative diffusion-weighted imaging in acute stroke and its frequency in anterior and posterior circulation ischemia. J Comput Assist Tomogr 38:627-633.
Burgess RE, Yu Y, Christoforidis GA, Bourekas EC, Chakeres DW, Spigos D, Kangarlu A, Abduljalil AM, Robitaille PM (1999) Human leptomeningeal and cortical vascular anatomy of the cerebral cortex at 8 Tesla. J Comput Assist Tomogr 23:850-856.
Chao TH, Chen JH, Yen CT (2014) Repeated BOLD-fMRI imaging of deep brain stimulation responses in rats. PLoS One 9:e97305.
Chen LF, Tian YF, Lin CH, Huang LY, Niu KC, Lin MT (2014) Repetitive hyperbaric oxygen therapy provides better effects on brain in fl ammation and oxidative damage in rats with focal cerebral ischemia. J Formos Med Assoc 113:620-628.
Chen WH, Chui C, Yin HL (2013) Zoster sine herpete, vertebral artery stenosis, and ischemic stroke. J Stroke Cerebrovasc Dis 22:e234-237.
Chi OZ, Grayson J, Barsoum S, Liu X, Dinani A, Weiss HR (2014) Effects of Dexmedetomidine on microregional O balance during reperfusion after focal cerebral ischemia. J Stroke Cerebrovasc Dis doi: 10.1016/j.jstrokecerebrovasdis.2014.08.004.
Choi JM, Hong HJ, Chang SK, Oh SH (2012) Cerebellar infarction originating from vertebral artery stenosis caused by a hypertrophied uncovertebral joint. J Stroke Cerebrovasc Dis 21:e907-909.
Compter A, van der Worp HB, Algra A, Kappelle LJ, Second Manifestations of AdSG (2011) Prevalence and prognosis of asymptomatic vertebral artery origin stenosis in patients with clinically manifest arterial disease. Stroke 42:2795-2800.
Edgell RC, Zaidat OO, Gupta R, Abou-Chebl A, Linfante I, Xavier A, Nogueira R, Alshekhlee A, Kalia J, Etezadi V, Aghaebrahim N, Jovin T (2013) Multicenter study of safety in stenting for symptomatic vertebral artery origin stenosis: results from the Society of Vascular and Interventional Neurology Research Consortium. J Neuroimaging 23:170-174.
Georgieva-Zhostova S, Kolev OI, Stambolieva K (2014) Translation, cross-cultural adaptation and validation of the Bulgarian version of the Dizziness Handicap Inventory. Qual Life Res 23:2103-2107.
Gu N, Rao C, Tian Y, Di Z, Liu Z, Chang M, Lei H (2014) Anti-in fl ammatory and antiapoptotic effects of mesenchymal stem cells transplantation in rat brain with cerebral ischemia. J Stroke Cerebrovasc Dis doi:10.1016/j.jstrokecerebrovasdis.2014.05.032.
Han YS, Xu Y, Han YZ, Xu L, Liu XG, Liu ZB, Wang P (2013) Protective effect of electroacupuncture intervention on neurovascular unit in rats with focal cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu 38:173-180.
Higashida RT, Tsai FY, Halbach VV, Dowd CF, Hieshima GB (1993a) Cerebral percutaneous transluminal angioplasty. Heart Dis Stroke 2:497-502.
Higashida RT, Tsai FY, Halbach VV, Dowd CF, Smith T, Fraser K, Hieshima GB (1993b) Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J Neurosurg 78:192-198.
Ito Y, Matsumaru Y, Suzuki K, Matsumura A (2010) Impaired cognitive function due to cerebellar infarction and improvement after stent-assisted angioplasty for intracranial vertebral artery stenosis-case report. Neurol Med Chir (Tokyo) 50:135-138.
Jiang W, Liu Q, Yuan X (2014) Combined intervention of preconditioning and postconditioning against cerebral ischemia/reperfusion injury. Zhong Nan Da Xue Xue Bao Yi Xue Ban 39:30-35.
Kim YJ, Lee JH, Choi JW, Roh HG, Chun YI, Lee JS, Kim HY (2013) Long-term outcome of vertebral artery origin stenosis in patients with acute ischemic stroke. BMC Neurol 13:171.
Kocak B, Korkmazer B, Islak C, Kocer N, Kizilkilic O (2012) Endovascular treatment of extracranial vertebral artery stenosis. World J Radiol 4:391-400.
Koch S, Bustillo AJ, Campo B, Campo N, Campo-Bustillo I, McClendon MS, Katsnelson M, Romano JG (2014) Prevalence of vertebral artery origin stenosis and occlusion in outpatient extracranial ultrasonography. J Vasc Interv Neurol 7:29-33.
Li Z, Zhu Y, Childress AR, Detre JA, Wang Z (2012) Relations between BOLD fMRI-derived resting brain activity and cerebral blood fl ow. PLoS One 7:e44556.
Li Z, Zhang Y, Hong B, Deng B, Xu Y, Zhao W, Liu J, Huang Q (2014) Stenting of symptomatic vertebral artery ostium stenosis with selfexpanding stents. J Clin Neurosci 21:274-277.
Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM (2013) Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage 78:176-185.
Macdonald RL, Stoodley M (1998) Pathophysiology of cerebral ischemia. Neurol Med Chir (Tokyo) 38:1-11.
Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM (2009) Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain 132:982-988.
Mohammadian R, Shari fi pour E, Mansourizadeh R, Sohrabi B, Nayebi AR, Haririan S, Farhoudi M, Charsouei S, Najmi S (2013) Angioplasty and stenting of symptomatic vertebral artery stenosis. Clinical and angiographic follow-up of 206 cases from Northwest Iran. Neuroradiol J 26:454-463.
Morris PP, Choi IS (1996) Cerebral vascular anatomy. Neuroimaging Clin N Am 6:541-560.
Mutlu B, Serbetcioglu B (2013) Discussion of the dizziness handicap inventory. J Vestib Res 23:271-277.
Nagahiro S, Uno M, Sato K, Goto S, Morioka M, Ushio Y (1998) Pathophysiology and treatment of cerebral ischemia. J Med Invest 45:57-70.
Peng C, Wang X, He C, Ma Y, Duan S, Guan X, Wang D, Tang Y (2014) The value of the ucN13-P15 interpeak latency predicted acute posterior circulation ischemia and the chronic outcome. J Clin Neurophysiol 31:462-468.
Pugnaghi M, Carmichael DW, Vaudano AE, Chaudhary UJ, Benuzzi F, Di Bonaventura C, Giallonardo AT, Rodionov R, Walker MC, Duncan JS, Meletti S, Lemieux L (2014) Generalized spike and waves: effect of discharge duration on brain networks as revealed by BOLD fMRI. Brain Topogr 27:123-137.
Sakamoto S, Kiura Y, Kajihara Y, Mukada K, Kurisu K (2013) Endovascular stenting of symptomatic innominate artery stenosis under distal balloon protection of the internal carotid and vertebral artery for cerebral protection: a technical case report. Acta Neurochir (Wien) 155:277-280.
Song S, Park JT, Na JY, Park MS, Lee JK, Lee MC, Kim HS (2014) Early expressions of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor increase the neuronal plasticity of activated endogenous neural stem cells after focal cerebral ischemia. Neural Regen Res 9:912-918.
Sun L, Wolferts G, Veltkamp R (2014) Oxygen therapy does not increase production and damage induced by reactive oxygen species in focal cerebral ischemia. Neurosci Lett 577:1-5.
Tang YH, Vital S, Russell J, Seifert H, Senchenkova E, Granger DN (2014) Transient ischemia elicits a sustained enhancement of thrombus development in the cerebral microvasculature: Effects of anti-thrombotic therapy. Exp Neurol 261C:417-423.
Villringer A, Dirnagl U (1999) Pathophysiology of cerebral ischemia. Z Arztl Fortbild Qualitatssich 93:164-168.
Wang CM, Tsai WL, Lo YL, Chen JY, Wong AM (2011) Unilateral right occipital condyle to C2level spinal cord infarction associated with ipsilateral vertebral artery stenosis and contralateral vertebral artery dissection: a case report. J Spinal Cord Med 34:118-121.
Wang L, Chen D, Olson J, Ali S, Fan T, Mao H (2012) Re-examine tumor-induced alterations in hemodynamic responses of BOLD fMRI: implications in presurgical brain mapping. Acta Radiol 53:802-811.
Wehman JC, Hanel RA, Guidot CA, Guterman LR, Hopkins LN (2004) Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J Interv Cardiol 17:219-232.
Yang C, Liu H, Liu D (2014) Mutant hypoxiainducible factor 1alpha modi fi ed bone marrow mesenchymal stem cells ameliorate cerebral ischemia. Int J Mol Med 34:1622-1628.
Zhang J, Wang Z, Xu S, Chen Y, Chen K, Liu L, Wang Y, Guo R, Zhang Z (2014) The effects of CCRC on cognition and brain activity in aMCI patients: a pilot placebo controlled BOLD fMRI study. Curr Alzheimer Res 11:484-493.
Copyedited by Dean J, Maxwell R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.147937
Peng Xie, M.D., Department of Neurology, Yongchuan Hospital, Chongqing Medical University, Chongqing 402160, China; Institute of Neuroscience, Chongqing Medical University, Chongqing 400016, China; Chongqing Key Laboratory of Neurobiology, Chongqing Medical University, Chongqing 400016, China; Department of Neurology, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China, xiepeng@cqmu.edu.cn.
http://www.nrronline.org/
Accepted: 2014-11-01
- 中国神经再生研究(英文版)的其它文章
- A more consistent intraluminal rhesus monkey model of ischemic stroke
- Human bone marrow mesenchymal stem cell transplantation attenuates axonal injury in stroke rats
- Pathogenesis of glaucoma: how to prevent ganglion cell from axonal destruction?
- Puerarin protects brain tissue against cerebral ischemia/reperfusion injury by inhibiting the in fl ammatory response
- Pretreatment with scutellaria baicalensis stem-leaf total fl avonoid protects against cerebral ischemia/ reperfusion injury in hippocampal neurons
- Overexpression of C-terminal fragment of glutamate receptor 6 prevents neuronal injury in kainate-induced seizure via disassembly of GluR6-PSD95-MLK3 signaling module

