国产碘克沙醇对老年冠心病患者肾功能影响的安全性研究
赵红岩,苗志林,陶贵周,刘明新,张占修,杨 健,冯 丰,杨新斌,侯 平,陈 韦,李占全*
国产碘克沙醇对老年冠心病患者肾功能影响的安全性研究
赵红岩1,苗志林1,陶贵周2,刘明新2,张占修3,杨 健3,冯 丰4,杨新斌4,侯 平5,陈 韦5,李占全1*
(1辽宁省人民医院心内科,沈阳 110016;2辽宁医学院附属第一医院心内科,锦州 121001;3解放军第313医院心内科,葫芦岛 125001;4沈阳市第一人民医院心内科,沈阳 110041;5辽宁省中医药大学附属医院心内科,沈阳 110033)
评价国产碘克沙醇注射液对进行经皮冠状动脉介入治疗(PCI)的老年冠心病患者肾功能的影响。采用随机分组、单盲设计、阳性对照的多中心研究。于2013年7月至2014年3月在辽宁省5家医院选择60~75岁、拟行PCI的患者88例,随机分为试验组(应用江苏恒瑞医药股份有限公司生产的碘克沙醇注射液,44例)与对照组[应用通用电气药业有限公司生产的威视派克(碘克沙醇商品名),44例]。记录两组术前年龄、性别、体质量指数(BMI)及肾功能等基本临床资料,术后48h检测肾功能,从而比较两者对老年冠心病患者肾功能的影响有无差别。两组共入选88例患者,试验组与对照组均为44例。88例患者均顺利完成冠状动脉造影与PCI,成功率100%。两组年龄、BMI等基本临床资料无统计学差异(>0.05)。两组PCI前后肾功能指标相比均无统计学差异(>0.05),两组分别有1例患者术后当天出现恶心呕吐,于手术次日恢复正常;两组均未发生对比剂诱发性急性肾损伤及其他不良反应。国产碘克沙醇用于行PCI的老年冠心病患者是安全的。
碘克沙醇;经皮冠状动脉介入治疗;对比剂诱发性急性肾损伤;老年人
近年来随着我国心血管造影和经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)数量的逐年增长,含碘对比剂的应用日益广泛,其不良反应尤其是对比剂诱发性急性肾损伤(contrast-induced acute kidney injury,CI-AKI)[1,2]日益受到重视。肾小球滤过率随年龄增长而降低,故老年人应用对比剂的风险更高[3]。碘克沙醇(iodixanol)因为良好的安全性,被国内外指南[4−7]推荐选用。为评价国产碘克沙醇注射液对老年PCI患者肾功能的影响,辽宁省5家医院以通用电气药业(上海)有限公司生产的商品名为威视派克(Visipaque)的碘克沙醇为对照,进行了前瞻性的多中心单盲随机对照临床研究。现将该研究报道如下。
1 对象与方法
本研究经辽宁省人民医院伦理委员会批准,由辽宁省人民医院、辽宁医学院附属第一医院、解放军第313医院、沈阳市第一人民医院及辽宁省中医药大学附属医院的心内科,于2013年7月至2014年3月协作完成。入选患者均签署知情同意书。
1.1 研究对象入选标准
(1)60~75周岁冠心病患者88例,男女不限;(2)拟行PCI;(3)签署知情同意书。
1.2 排除标准
(1)受试者既往对碘对比剂及含碘食物有严重不良反应;(2)既往甲亢病史者;(3)有肾功能障碍,血清肌酐水平≥3mg/dl(265.2μmol/L);(4)3个月内发生过急性肾衰竭和(或)接受过血液透析治疗患者。(5)接受过肾移植手术的患者;(6)严重肝功能不全患者[丙氨酸氨基转移酶和(或)天冬氨酸氨基转移酶≥正常参考值上限2倍];(7)受试者不能中断服用盐酸二甲双胍或包含盐酸二甲双胍的药品;(8)有急性活动性出血;(9)发热,体温≥38℃;(10)严重血液系统疾病患者;(11)术前14d内接受过碘对比剂的患者;(12)预计术后7d内需要再次接受对比剂的患者;(13)有证据显示受试者临床状况不稳定,包括:急性心肌梗死(2周之内);心源性休克;充血性心力衰竭或急性肺水肿;脑卒中(3个月内);(14)既往冠状动脉造影显示为慢性完全闭塞病变、三支弥漫性病变、左主干病变等估计对比剂用量≥400ml或(和)PCI手术风险较高的患者;(15)近3个月参加过其他临床研究;(16)不具法律能力或法律能力受限者;(17)研究者认为不合适参加该临床研究的任何情况。
1.3 方法
患者入选后随机分为试验组(44例)与对照组(44例)。试验组应用江苏恒瑞医药股份有限公司生产的碘克沙醇注射液,规格为100ml∶65.2g(100ml∶32g I)/瓶,批准文号为国药准字H20103675;对照组应用通用电气药业(上海)有限公司生产的威视派克,规格为32g(I)/100ml/瓶,批准文号为国药准字J20050104。常规进行冠状动脉造影与PCI,应用阿司匹林、氯吡格雷、他汀类调脂药以及手术前后生理盐水进行水化等治疗。术后随访1周。
1.4 观察指标
记录患者的年龄、性别、身高、体质量、生命体征、伴随疾病、对比剂用量、术前血清肌酐(serum creatinine,SCr)与尿素氮(blood urea nitrogen,BUN)、术后48h SCr与BUN(若升高,则术后7d复查)、术中术后有无严重心脑血管事件及对比剂引起的不良反应(包括CI-AKI等)。CI-AKI定义为48h内SCr水平升高>0.3mg/dl或7d内升高>50%。计算体质量指数(body mass index,BMI)、估测肾小球滤过率(estimated glomerular filtration rate,eGFR)以及术后eGFR的减少值。eGFR[ml/(min·1.73m2)]=175×SCr(mg/dl)-1.234×年龄-0.179×(0.79女性)[8];术后eGFR的减少值[ml/(min·1.73m2)]=术前eGFR-术后eGFR。
1.5 统计学处理
采用SPSS13.0统计软件进行统计学分析。计量资料以均数±标准差表示,两组均数比较采用检验;计数资料以例数或所占百分比表示,组间率的比较采用2检验。所有的统计检验均采用双侧检验,<0.05为差异有统计学意义。
2 结 果
2.1 基本临床资料
88例患者均顺利完成冠状动脉造影与PCI(成功率100%),随访期间均无死亡、脑卒中、急性心肌梗死、再次血运重建等严重心脑血管事件发生。两组基本临床资料比较,差异无统计学意义(>0.05;表1)。
2.2 PCI前后肾功能情况
两组PCI前后肾功能指标相比均无统计学差异(>0.05;表2),两组分别有1例患者术后当天出现恶心、呕吐,于手术次日恢复正常;两组均未发生CI-AKI及其他不良反应。
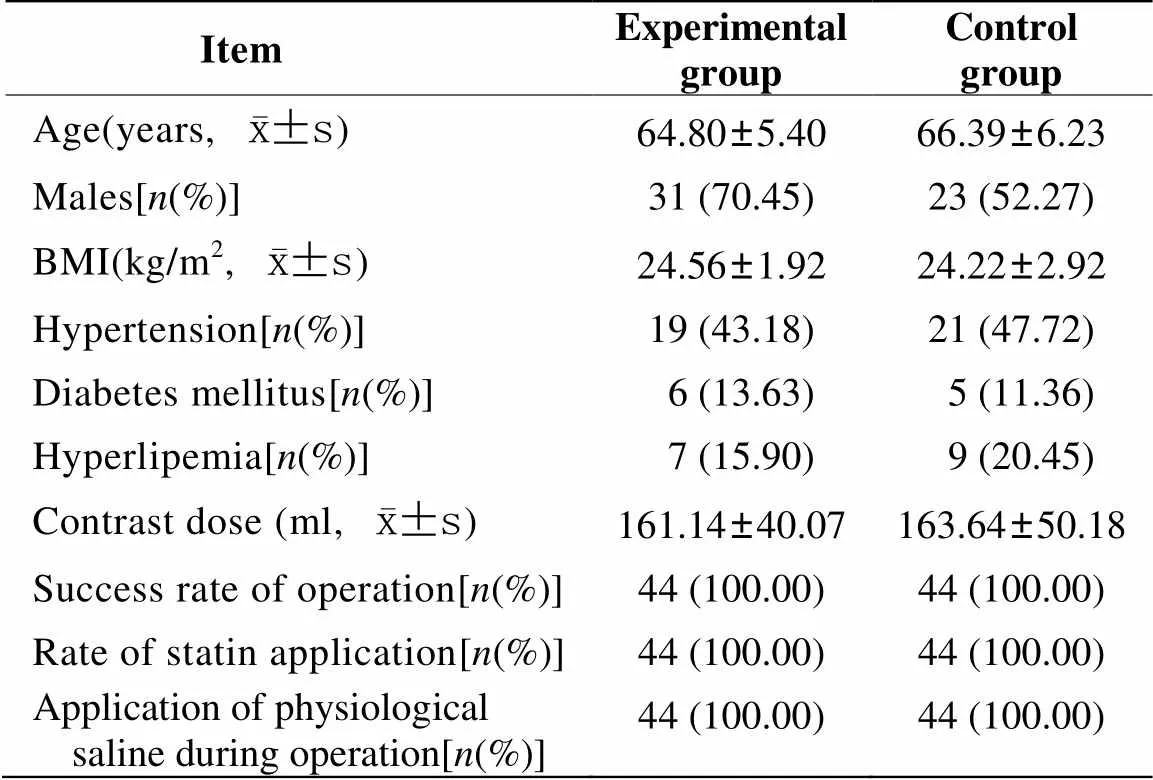
表1 两组基本临床资料表
BMI: body mass index
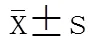
表2 两组PCI前后肾功能情况表
PCI: percutaneous coronary intervention; eGFR: estimated glomerular filtration rate; SCr: serum creatinine; BUN: blood urea nitrogen
3 讨 论
老年冠心病患者进行PCI治疗的数量逐年增多,而老年患者冠状动脉及全身动脉病理改变较中青年患者更加严重,冠状动脉多存在多支、纡曲和钙化等复杂病变,PCI术中对比剂需求量相对增多;老年患者肾小球滤过率及心脏储备功能下降,而且合并高血压病、糖尿病、心功能不全的概率增高(本研究中排除了心力衰竭,入选患者合并高血压病、糖尿病的比例仍分别超过40%与10%),肾脏对对比剂不良反应的代偿能力降低。以上因素导致老年患者发生肾功能损害的风险及其危害性增加[7]。
基础肾功能损害是预测接受碘对比剂检查的患者发生CI-AKI的最重要的标志,因此老年患者PCI术前一定要评价肾功能状态,计算eGFR而不是单纯依据血肌酐水平判断。若eGFR<60ml/(min·1.73m2),则视为CI-AKI的高危患者[9−11]。若老年患者病情需行PCI治疗,在应用他汀类调脂药物[12],尽量减少对比剂用量,及术前术后积极有效水化治疗的同时,选择循证医学证实安全的对比剂是非常重要的[13,14]。等渗对比剂碘克沙醇为非离子型二聚体,其渗透压与血浆渗透压相等[15],上市后经众多临床研究证实其安全性良好,故被国内外众多指南所推荐[4−7]。
本研究为前瞻性多中心单盲随机对照临床研究,结果显示在老年冠心病患者进行PCI时应用国产碘克沙醇注射液与威视派克均无CI-AKI等严重不良反应,其他不良反应轻微而发生率相近。因此,国产的碘克沙醇注射液与威视派克相比同样安全,可用于老年冠心病患者的PCI治疗,因其价格更加便宜,值得在临床工作中推广。但本研究样本例数偏小,更加确切的结论有待更大规模的前瞻性随机对照研究来证实。
[1] McCullough PA. Contrast-induced acute kidney injury[J]. J Am Coll Cardiol, 2008, 51(15): 1419−1428.
[2] Lakhal K, Ehrmann S, Chaari A,Acute kidney injury network definition of contrast-induced nephropathy in the critically ill: incidence and outcome[J]. J Crit Care, 2011, 26(6): 593−599
[3] Mehran R, Aymong ED, Nikolsky E,A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation[J]. J Am Coll Cardiol, 2004, 44(7): 1393−1399.
[4] Levine GN, Bates ER, Blankenship JC,2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions[J]. Circulation, 2011, 124(23): e574−e651.
[5] Wright RS, Anderson JL, Adams CD,. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/ Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines[J]. Circulation, 2011, 123(18): 2022−2060.
[6] Chinese Society of Cardiology Percutaneous Coronary Intervention Group, Editorial Board of Chinese Journal of Cardiovascular Diseases. Guideline of Percutaneous Coronary Intervention in China[J]. Chin J Crit Care Med (Electron Ed), 2012, 5(3): 169−179. [中华医学会心血管病学分会介入心脏病学组, 《中华心血管病杂志》编辑委员会. 中国经皮冠状动脉介入治疗指南2012(简本)[J]. 中华危重症医学杂志(电子版), 2012, 5(3): 169−179.]
[7] Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiovascular Diseases. Clinical application of iodinated contrast in angiocardiopathy expert consensus[J]. Chin J Cardiol, 2013, 41(2): 94−98. [中华医学会心血管病学分会, 《中华心血管病杂志》编辑委员会. 含碘对比剂在心血管疾病中临床应用的专家共识(2012)[J]. 中华心血管病杂志, 2013, 41(2): 94−98.]
[8] Ma YC, Zuo L, Chen JH,Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease[J]. J Am Soc Nephrol, 2006, 17(10): 2937−2944.
[9] Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion[J]. Clin J Am Soc Nephrol, 2008, 3(1): 273−280.
[10] Mehran R, Aymong ED, Nikolsky E,. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation[J]. J Am Coll Cardiol, 2004, 44(7): 1393−1399.
[11] Kini AS, Sarkar K, Rafael OC,. Serum creatinine ratio: a novel predictor of mortality after percutaneous coronary intervention in patients with normal and abnormal renal function[J]. Catheter Cardiovasc Interv, 2009, 74(1): 49−55.
[12] Patti G, Nusca A, Chello M,Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention[J]. Am J Cardiol, 2008, 101(3): 279−285.
[13] Dong M, Jiao Z, Liu T,. Effect of administration route on the renal safety of contrast agents: a meta-analysis of randomized controlled trials[J]. J Nephrol, 2012, 25(3): 290−301.
[14] Effects of intra-arterial and intravenous iso-osmolar contrast medium (iodixanol) on the risk of contrast-induced acute kidney injury: a meta-analysis[J]. Cardiorenal Med, 2011, 1(4): 220−234.
[15] Aspelin P, Benin MF, Jakobsen J,Classification and Terminology[A]//Thomsen HS. Contrast Media Safety Issues and ESUR Guidelines[M]. Berlin: Springer, 2006: 1−4.
(编辑: 李菁竹)
Nephrotoxic effects of domestic iodixanol injection in old patients with coronary heart disease
ZHAO Hong-Yan1, MIAO Zhi-Lin1, TAO Gui-Zhou2, LIU Ming-Xin2; ZHANG Zhan-Xiu3, YANG Jian3, FENG Feng4, YANG Xin-Bin4; HOU Ping5, CHEN Wei5, LI Zhan-Quan1*
(1Department of Cardiology, Liaoning Provincial People’s Hospital, Shenyang 110016, China;2Department of Cardiology, the First Affiliated Hospital, Liaoning Medical College, Jinzhou 121001, China;3Department of Cardiology, Chinese PLA Hospital No.313, Huludao 125001, China;4Department of Cardiology, Shenyang First People’s Hospital, Shenyang 110041, China;5Department of Cardiology, Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110033, China)
To evaluate the nephrotoxic effects of domestic iodixanol injection in the elderly patients with coronary heart disease (CHD).A multicenter, single blind, randomized controlled clinic trial was prospectively carried out in 5 hospitals in Liaoning Province from July 2013 to March 2014. Eighty-eight patients of 60 to 75 years old with CHD undergoing percutaneous coronary intervention (PCI) were divided into 2 groups: experimental group (domestic iodixanol injection group,=44) and control group [Visipaque (trade name of iodixanol) group,=44]. The clinical information including age, gender, body mass index (BMI), serum creatinine (SCr) and blood urea nitrogen (BUN) was recorded before PCI. Their SCr and BUN were determined in 48 h after PCI for renal function in the patients of the 2 groups.All of them underwent PCI smoothly, with a successful rate of 100%. There was no significant difference in the age, gender, BMI, SCr and BUN between the 2 groups before PCI (>0.05). No difference was found in the renal function in the 2 groups before and after PCI (>0.05). One patient of each group had nausea and vomiting respectively just in the day after operation, and recovered on the next day. None of patient had contrast-induced acute kidney injury or other adverse reactions.Domestic iodixanol injection is safe for old patients with CHD underwent PCI.
iodixanol; percutaneous coronary intervention; contrast-induced acute kidney injury; aged
R654.33; R541.4; R592
A
10.3724/SP.J.1264.2014.000160
2014−05−18;
2014−07−07
李占全, E-mail: lzqlr@medmail.com.cn

