弥漫性特发性肺神经内分泌细胞增生合并肺原发性滑膜肉瘤1例临床分析并文献复习
邹天宇,雷 振,杨 静,卞婷婷
辽宁医学院附属第一医院,辽宁锦州 121000 1放射科;2病理科
弥漫性特发性肺神经内分泌细胞增生合并肺原发性滑膜肉瘤1例临床分析并文献复习
邹天宇1,雷 振1,杨 静2,卞婷婷1
辽宁医学院附属第一医院,辽宁锦州 1210001放射科;2病理科
目的提高对弥漫性特发性肺神经内分泌细胞增生(diffuse idiopathic pulmonary neuroendocrine cell hyperplasia,DIPNECH)及肺原发性滑膜肉瘤(primary pulmonary synovial sarcoma,PPSS)的认识,并探讨两种疾病发生的关联性。方法对本院确诊的1例DIPNECH合并PPSS的临床资料、病理学及影像学表现进行回顾性分析,并复习相关文献。结果患者为20岁男性,临床表现为胸闷、咳嗽、咯血。CT增强扫描发现:1)双肺多发斑片影;2)左肺上叶空腔伴壁结节;3)左肺下叶血肿伴肿块。术后病理诊断为弥漫性特发性肺神经内分泌细胞增生合并肺原发性单相纤维型滑膜肉瘤。随访10个月,患者无任何不适,未见复发和转移。结论DIPNECH和PPSS均是极其罕见的疾病,临床及影像学表现复杂多样且无特异性,确诊有赖于病理学检查。
弥漫性特发性肺神经内分泌细胞增生;肺原发性滑膜肉瘤;SYT-SSX基因融合
弥漫性特发性肺神经内分泌细胞增生(diffuse idiopathic pulmonary neuroendocrine cell hyperplasia,DIPNECH)是一种进程缓慢的良性病变,是类癌及微小瘤的浸润前病变[1]。本病1992年才被认识和正式命名,临床上极为罕见,英文文献仅报道100多例,国内仅报道2例[2-4]。肺原发性滑膜肉瘤(primary pulmonary synovial sarcoma,PPSS)是一种十分罕见的间叶组织源性肿瘤,占所有肺恶性肿瘤0.1% ~ 0.5%[5-6]。其组织学类型包括4型:单相纤维型、单相上皮型、双相型和低分化型。90%以上的滑膜肉瘤中存在t(X;18)(p11.2;q11.2)染色体易位,融合基因SYT-SSX及其亚型的检测具有重要临床意义[7]。复习文献发现,本文报告的DIPNECH合并PPSS是首例。现结合相关文献,回顾性分析其临床资料、影像学及病理学表现,以提高对此两种疾病的认识。
资料和方法
1 临床资料 患者男性,20岁,于2013年2月16日以“胸闷咳嗽,咯血1 d”为主诉入辽宁医学院附属第一医院胸外科。患者1 d前无明显诱因突然出现胸闷、咳嗽、咯血(约200 ml),呈鲜红色。既往体健,无吸烟史,家族史无特殊。查体:左下肺呼吸音弱,可闻及湿啰音,余无异常。2月16日急查胸部增强CT诊断:1)双肺多发斑片影;2)左肺上叶空腔伴壁结节;3)左肺下叶血肿伴肿块,肉瘤可能性大。临床初步诊断:左肺下叶肿物并咯血,左肺上叶肺大疱。
2 胸部增强CT扫描 设备为GE Light Speed VCT 64排螺旋CT。病人仰卧位,以3 ml/s的速度经肘静脉团注35%碘海醇60 ml,扫描条件为120 kV,200 mA,层厚5 mm,间距5 mm,扫描范围为胸骨切迹平面至后肋膈角下界,于吸气末行平扫及双期常规增强扫描。以512×512矩阵重建图像。
3 病理学检查 手术中切除标本均经10%中性甲醛固定,常规脱水、石蜡包埋,3 μm厚切片,HE染色,在光镜下观察。免疫组织化学检测采用SP-9000通用型SPkit,一抗包括CgA、CK、Syn、BCL-2、CD99、Vim、SMA、EMA、TTF-1、CD34、Calretinin等,均购自北京中杉金桥生物技术有限公司。具体操作步骤按说明书进行。
结 果
1 影像学表现 1)双肺内可见多发斑片影及磨玻璃密度影;2)左肺上叶前段见一囊状透光病灶,其外侧壁局限增厚并见一小结节影突入囊状病灶内;3)左肺下叶内见巨大占位性病变,大小约10.5 cm×7.4 cm×6.5 cm,其内近肺门侧见一卵圆形肿块影,大小约3.2 cm×3.0 cm×1.7 cm,平扫CT值约26.8 HU,动脉期CT值约63.2 HU,静脉期CT值约75.6 HU,呈明显强化改变,肿瘤内部见低密度无明显强化,周边血肿亦始终无明显强化。见图1,图2A ~ F。
2 治疗 给予止血、止咳等对症治疗,考虑到大量咯血及肿瘤的存在,决定行开胸探查术。2013年2月17日患者在全麻下行左肺上叶前段(SⅢ)肺大疱切除术及左肺下叶全切术。标本送病理检查。术后给予抗炎、祛痰、止痛等对症治疗,患者一般情况良好,病情稳定,于2013年3月1日出院。每3个月复查1次,目前无转移及复发。术中所见:左肺上叶空腔外侧壁见大小约0.5 cm×0.5 cm灰白色小结节。左肺下叶明显充血水肿,呈黑褐色,其内触及巨大占位,质地柔软,大小约10 cm×8 cm×5 cm。
3 病理学检查所见 肉眼观:1)左肺上叶肺大疱外侧壁见大小约0.5 cm×0.5 cm灰白色小结节,界限清楚,质地较硬;2)左肺下叶占位切除后,剖开标本,近肺门处见大小约3 cm×3 cm×1 cm肿物,边界清楚,切面呈黄白色鱼肉状,质地细腻;周围出血的肺组织呈黑褐色(图2G)。镜检:1)左肺上叶细支气管上皮细胞基底部见弥漫分布增殖的神经内分泌细胞,排列呈巢状(图3A);2)左肺下叶肿瘤细胞呈圆形、卵圆形及梭形,束状排列,胞质少、核深染(图4A)。免疫组织化学表型:1)左肺上叶病变免疫组织化学示CgA、CK、Syn阳性(图3B ~ D)。2)左肺下叶肿瘤细胞免疫组织化学示BCL-2、CD99、CK、Vim阳性,SMA、EMA弱阳性,TTF-1、CgA、CD34、Calretinin阴性(图4B ~F)。经病理科室讨论以及文献复习诊断为弥漫性特发性肺神经内分泌细胞增生合并肺原发性单相纤维型滑膜肉瘤。
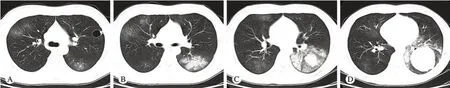
图 1 胸部CT平扫轴位肺窗 A: 左肺上叶囊状透光病灶,其外侧壁局限增厚并见小结节突入囊状病灶内; A ~ D: 双肺内多发斑片影及磨玻璃影Fig. 1 Axial lung w indow of plan chest CT scan A: show ing a cystic pervious lesion in superior lobe of left lung whose external wall thickened, and a small nodule p rojected into it; A-D: show ing multiple patchy shadows and ground glass opacity in bilateral lungs
讨 论
根据目前WHO的分类标准,DIPNECH为浸润前病变,具有下列特征:散在的单个细胞、小结节(神经内分泌小体)的弥漫性增生,或肺神经内分泌细胞的线性增生,是类癌及微小瘤的浸润前病变[8]。DIPNECH可发生于任何年龄,以中老年女性居多,年龄为22 ~ 79岁(中位年龄58岁),男女患病人数比约1∶4,且绝大部分为非吸烟者[9-10]。本例为20岁男性患者,无吸烟史,实属罕见。大多患者没有明显临床症状,有症状者主要表现为干咳、气短及呼吸困难[1,9-10]。
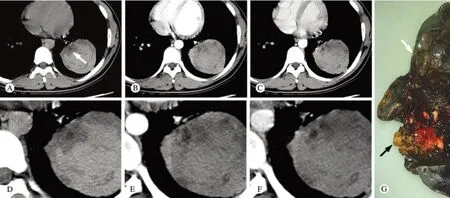
图 2 胸部CT平扫、增强扫描轴位纵隔窗及左肺下叶大体标本 A ~ F: 左肺下叶血肿内近肺门侧卵圆形肿物(白箭),增强扫描明显强化,肿瘤内部见低密度无强化囊变区,周边血肿无明显强; G: 左肺下叶近肺门处见类圆形黄白色肿物(黑箭),周围出血的肺组织呈黑褐色(白箭)Fig. 2 Axial mediastinum w indow of plan, enhanced chest CT scan and gross specimen of inferior lobe of left lung A-F: An oval mass showed in hilum side of the hematoma in in ferior lobe of left lung (white arrow), and it enhanced obviously. The cystic area in the tumor and the hematoma around it were non-enhanced; G: A yellow-white mass showed in hilum side of the inferior lobe of left lung (black arrow), and the hem orrhagic lung around it was dark brown (white arrow)
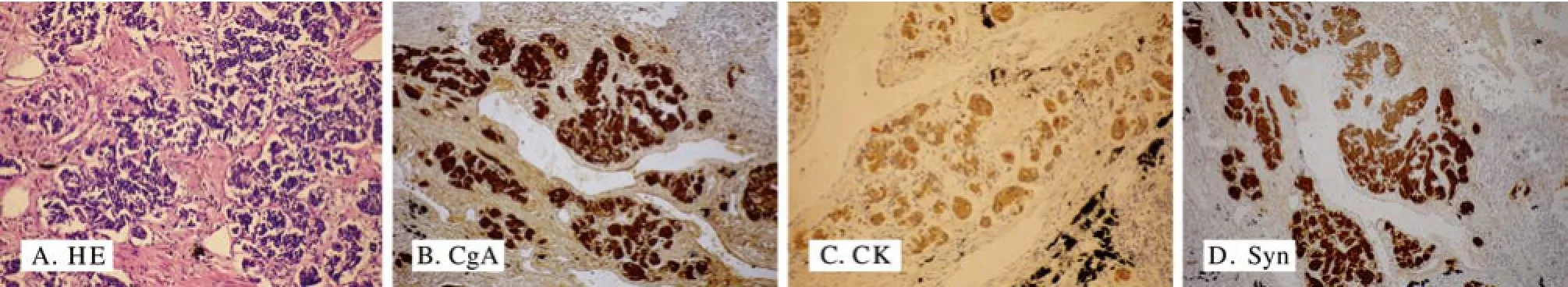
图 3 左肺上叶小结节HE染色病理切片及免疫组织化学染色(×200) A: 细支气管上皮细胞基底部巢状分布增殖的神经内分泌细胞; B ~D: CgA、CK、Syn阳性表达于细胞质Fig. 3 HE stain and immunohistochem istry of the small nodule in superior lobe of left lung (×200) A: Proliferous PNCs form ing a nest at the base of the bronchiolar epithelium; B-D: Positive expression of CgA, CK and Syn distributed in cytoplasm

图 4 左肺下叶肿物HE染色病理切片及免疫组织化学染色(×200) A: 圆形或卵圆形梭形细胞呈束状排列,胞质少、核深染; B ~F: BCL-2、CK、Vim阳性表达于细胞质,CD99阳性表达于细胞质及细胞膜,CD34阴性Fig. 4 HE stain and imm unohistochem istry of the tum or in in ferior lobe of left lung (×200) A showed round and oval spind le cell arranging in bund les, with less cytop lasm and hyperchrom atic nuclei; B-F showed BCL-2, CK and Vim were positive in cytop lasm, CD99 were positive in cytop lasm and cell membrane, CD34 were negative
影像学表现无特异性,高分辨率CT(high resolution CT,HRCT)表现可有:双肺斑片影、磨玻璃密度影、马赛克灌注、闭塞性细支气管炎、支气管壁增厚、支气管扩张、结节影等,本例表现为:1)双肺斑片影及磨玻璃密度影,与文献报道相符;2)左肺空腔伴壁结节,复习文献未见类似报道,其形成机制尚不明确。DIPNECH与其他肺间质性病变的HRCT表现极为相似,鉴别诊断存在困难,最终诊断需要病理学检查。
本病的处理措施主要是长期随访观察或者类固醇激素的吸入治疗、肺叶的切除或肺移植。本病进展缓慢,大部分患者预后良好[1,9]。
PPSS是一种极其罕见的间叶源性恶性肿瘤,可显示不同程度上皮分化,占所有肺恶性肿瘤的0.1% ~ 0.5%,好发于青壮年,平均年龄38岁,发病率的性别差异存在争议[5-6,11-14]。患者主诉主要有胸痛(24% ~ 80%)、咳嗽(8% ~ 33%)、咯血(20% ~25%)[11,15-16]。本例病人以“胸闷咳嗽,咯血”为主诉,具有典型的PPSS的临床特点。
PPSS影像学表现复杂且不特异,主要表现为肺内边界清楚的肿块,直径2 ~ 15 cm,不均匀强化,分叶不明显,内部可出现出血、囊变、坏死或钙化;侵犯胸膜则出现胸腔积液[17]。本例表现为直径约3 cm肿物,增强CT示明显不均匀强化,内部出现囊变坏死区,与文献报道相符。CT扫描对肿瘤内部钙化灶有较高的特异性和敏感性,但对肿瘤内部出血、囊变、坏死的区分比较困难,而MRI检查对出血的分期以及囊变坏死有较高的特异性及敏感性,如果术前即行MRI平扫及增强检查,即可对肿瘤内部成分进行定性分析,从而提高该病诊断的准确率。目前,国内外关于MRI对PPSS诊断的相关报道较少,有待进一步研究探索。
组织学4种类型中以单相纤维型最为常见,其镜下特点为:肿瘤组织主要由梭形细胞构成,排列紧密或交织呈束状;瘤细胞间含少量或无明显的胶原纤维;增生的梭形细胞周围可见大小不一、扩张的裂隙;核分裂象多见。免疫组织化学染色特点为:Vim、CK、EMA、BCL-2、CD99大多阳性,而CD34、S-100、CD68通常阴性。本例具有典型的单相纤维型PPSS HE及免疫组织化学染色特点。
90%以上的滑膜肉瘤中可检测到t(X;18) (p11.2;q11.2)染色体易位及SYT-SSX1、SYTSSX2融合基因[7]。常用的检测方法有RT-PCR及荧光原位杂交。
本病的处理措施目前是手术切除及术后化疗,5年生存率为50%[18]。
DIPNECH是类癌及微小瘤的浸润前病变,这一理论被广泛接受,直到2008年Warth等[19]首次报告了1例个案,该患者DIPNECH病变NSE阳性表达,且血清中NSE水平升高,说明其肺腺癌与DIPNECH有关,这使我们对DIPNECH有了更进一步的认识。而本研究中,增强CT示DIPNECH病变存在于双肺,DIPNECH的存在是否和PPSS的发生有相关性,尚需研究探索。
1 Walker CM, Vummidi D, Benditt JO,et al. What is DIPNECH?[J]. Clin Imaging, 2012, 36(5): 647-649.
2 Aguayo SM, Miller YE, Waldron JA,et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease[J]. N Engl J Med, 1992, 327(18): 1285-1288.
3 刘计宽,王志刚,梁克,等.弥漫性特发性肺神经内分泌细胞增生伴微小瘤形成一例[J].中国胸心血管外科临床杂志,2012,19(2):188.
4 林清华,郑智勇,姚丽青.弥漫性特发性肺神经内分泌细胞增生伴微小瘤形成1例并文献复习[J].临床与实验病理学杂志,2009,25(3):325-326.
5 Zamarrón C, Abdulkader I, Alvarez UC,et al. Primary synovial sarcoma of the lung[J]. Intern Med, 2006, 45(10):679-683.
6 Dennison S, Weppler E, Giacoppe G. Primary pulmonary synovial sarcoma: a case report and review of current diagnostic and therapeutic standards[J]. Oncologist, 2004, 9(3): 339-342.
7 Dos Santos NR, De Bruijn DR, Van Kessel AG. Molecu lar mechanisms underlying human synovial sarcoma development[J]. Genes Chromosomes Cancer, 2001, 30(1):1-14.
8 Travis WD, Brambilla E, MüllerHermelink HK,et al. Tumours of the lung,pleura,thymus and heart[M]. Lyons: IARC Press,2004: 76-77.
9 Nassar AA, Jaroszewski DE, Helmers RA,et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a systematic overview[J]. Am J Respir Crit Care Med, 2011, 184(1): 8-16.
10 Ge Y, Eltorky MA, Ernst RD,et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia[J]. Ann Diagn Pathol, 2007, 11(2): 122-126.
11 Gladish GW, Sabloff BM, Munden RF,et al. Primary thoracic sarcomas[J]. Radiographics, 2002, 22(3): 621-637.
12 Mirzoyan M, M uslim ani A, Setrak ian S,et al. Prim ary pleuropulmonary synovial sarcoma[J]. Clin Lung Cancer, 2008, 9(5): 257-261.
13 Hartel PH, Fanburg-Smith JC, Frazier AA,et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series[J]. Mod Pathol, 2007,20(7): 760-769.
14 Suster S, Moran CA. Primary synovial sarcomas of the mediastinum:a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases[J]. Am J Surg Pathol, 2005, 29(5): 569-578.
15 Essary LR, Vargas SO, Fletcher CD. Primary p leuropulmonary synovial sarcoma: reappraisal of a recently described anatomic subset[J]. Cancer, 2002, 94(2): 459-469.
16 Gaertner E, Zeren EH, Fleming MV,et al. Biphasic synovial sarcomas arising in the pleural cavity. A clinicopathologic study of five cases[J]. Am J Surg Pathol, 1996, 20(1):36-45.
17 Polverosi R, Muzzio PC, Panunzio A,et al. Synovial sarcoma: CT imaging of a rare primary malignant tumour of the thorax[J]. Radiol Med, 2011, 116(6): 868-875.
18 Skytting B, Meis-Kindblom JM, Larsson O,et al. Synovial sarcoma--identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases[J]. Acta Orthop Scand, 1999, 70(6): 543-554.
19 Warth A, Herpel E, Schmähl A,et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) in association with an adenocarcinoma: a case report[J]. J Med Case Rep, 2008, 2:21.
Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia combined with primary pulmonary synovial sarcoma: A clinical analysis of 1 case and literature review
ZOU Tian-yu1, LEI Zhen1, YANG Jing2, BIAN Ting-ting1
1Department of Radiology;2Department of Pathology First Aff liated Hospital of Liaoning Medical University, Jinzhou 121000, Liaoning Province, China
Corresponding author: LEI Zhen. Email: leizhen2004@163.com
ObjectiveTo improve the understanding of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) and primary pulmonary synovial sarcoma (PPSS), and explore the possible correlation between them.MethodsClinical manifestations, pathological change and imaging findings of a patient with DIPNECH and PPSS adm itted to our hospital were retrospectively analyzed with related literature.ResultsThe 20 years old male patient's clinical manifestation were chest congestion, cough and hemoptysis. Contrast enhanced chest CT revealed that patchy shadows appeared in bilateral lungs, cyst with nodule appeared in superior lobe of left lung and a mass in hematoma showed in inferior lobe of left lung. The postoperative pathology examination proved to be diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) with primary pulmonary monophasic fbrous synovial sarcoma (PPSS). The patient was followed up for 10 months during which he felt no discom fort and showed no recurrence or metastasis.ConclusionBoth DIPNECH and PPSS are extremely rare diseases with complicated and nonspecif c clinical manifestations and imaging fndings. Pathology is essential for conf rmation of the diagnosis.
diffuse idiopathic pulmonary neuroendocrine cell hyperplasia; primary pulmonary synovial sarcoma; SYT-SSX gene fusion
R 734.2
A
2095-5227(2014)08-0823-05
10.3969/j.issn.2095-5227.2014.08.013
2014-04-16 15:37
http://www.cnki.net/kcms/detail/11.3275.R.20140416.1537.003.html
2013-12-24
邹天宇,男,在读硕士。研究方向:CT断层解剖与影像诊断。Email: 353662111@qq.com
雷振,男,博士,教授,主任医师,副主任。Email: lei zhen2004@163.com

