Synthesis and Crystal Structure of Cobalt(II) and Copper(II) Complexes Involving L-Aamino Alcohols
LUO Mei HUANG Yong-HuaWANG Leia YIN Hao
a (Hefei University of Technology, Department of Chemical Engineering, Hefei 230009, China)
b (University of Science and Technology of China, Hefei 230009, China)
1 INTRODUCTION
Many novel Co(II) and Cu(II) complexes have been reported recently[1-5]not only owing to their rich coordination chemistry but also for their potential applications in catalytic chemistry and biochemistry, for example, the synthesis of [HNEt3]-[Cu2(CH3CO2)2-(2-(O)-5-(Me)C6H2-1,3-(HC=NC-(Ph2CO2)2], Cu2(dmap-H)2Cl4, Co(MMAMT)Cl·2H2O and Co(bpbH2)Cl2·2H2O complexes[6-9]. As the catalysts, they have shown high activities in Noror- nene polymerization, Hydroboration reaction,Henry reaction, Heck reaction and so on. Additionally, the synthesis of copper and cobalt complexes supported by imino- or amino- pyridyl alcohol ligands and α-amino amides has been documented[10-13]. En-couraged by the pioneer’s research work, in this paper, we synthesized the Co(II)and Cu(II) com- plexes derived from chiral α-amino alcohol ligands and got their crystal structures. Then,our complexes were used to catalyze the Henry reaction.
2 EXPERIMENTAL
2.1 General information
L-plenylglycinol, L-leucinol, cobalt acetate tetrahydrate and copper chloride dihydrate were pur-chased from Acros; Flash column chromatography was performed using E. Merck silica gel (60,particle size 0.02~0.03 mm);1H and13C NMR spectra were recorded on a Bruker AM-300 spectrometer. Proton chemical shifts are reported in ppm (δ) with the solvent relative to tetramethylsilane (TMS) employed as the internal standard(CDCl3, δ 7.26 ppm). The following abbreviations were used to designate chemical shift multiplicities:s = singlet, d = doublet, t = triplet, m = multiplet.Infrared spectra were recorded on a Mattson Galaxy Series FTIR 3000 spectrometer; peaks are reported in cm-1.
2.2 General procedure
2. 2. 1 Preparation of Co(II) complex I
4.1164 g (30 mmol) L-plenylglycinol and 50 mL anhydrous ethanol were dispersed in a 100 mL round-bottomed flask, then 2.4912 g (10 mmol)Co(II) acetate tetrahydrate was added into the above solution. The mixture was refluxed for 48 h,obtaining red oily solution. And the products were dealt with rotary evaporation. After recrystallization with anhydrous methanol, pink crystals were obtained. Yield: 35%. Elemental analysis: Anal.Calcd. for [Co3(C51H66N3O16)]2(OAc): C, 50.97; H,5.55; N, 6.86%. Found: C, 50.99; H, 6.31; N, 6.73%.IR (KBr, cm-1): 3379, 3222, 3113, 3036, 2924, 2844,1603, 1556, 1535, 1495, 1456, 1406, 1329, 1269,1159, 1054, 1013, 914, 847, 762, 698, 649, 541.
2. 2. 2 Preparation of Cu(II) complex II
3.8337 mL (30 mmol) L-leucinol and 30 mL anhydrous methanol were dispersed in a 100 mL round-bottomed flask. After that, 1.7054 g (10 mmol) Cu(II) chloride dihydrate was added into the above solution followed by reflux for 48 h. Blue oily solution was obtained, and the products were dealt with rotary evaporation. Recrystallization with anhydrous ethanol afforded blue crystals. Yield:37%. Elemental analysis: Anal. Calcd. for Cu2[C24H58N4O7Cl]Cl: C, 40.44; H, 8.20; N, 7.86%.Found: C, 40.02; H, 8.91; N, 7.74%. IR (KBr, cm-1):3397, 2958, 1613, 1509, 1470, 1389, 1369, 1172,1131, 1053, 991, 557.
2.3 Crystallographic data collection and structure determination
A pink crystal of compound I with approximate dimensions of 0.165mm × 0.121mm× 0.089mm was selected for data collection on a BRUKER SMART diffractometer equipped with graphitemonochromated MoKα radiation (λ = 0.71073 Å).Totally, 35582 reflections were collected in a range of 1.64<θ<25.50° by using an ω-Ф scan mode at 273(2) K, Mr= 1231.96, monoclinic, P21, a =15.022(3), b = 14.242(3), c = 28.922(6) Å, β =98.944(4)º, V = 6112(2) Å3, Z = 4, Dc= 1.339 g/cm3,the final R = 0.0860 for 21906 observed reflections with I > 2σ(I) and Rw = 0.2490 for all data. The structure was solved by full-matrix least-squares on F2using the SHELXTL program[17-19]. All non-H atoms were refined with anisotropic thermal parameters. All hydrogen atoms were located theoretically and refined with riding model position parameters and fixed isotropic thermal parameters.
The other blue crystal of compound II with approximate dimensions of 0.26mm × 0.10mm ×0.06mm was selected for data collection on a BRUKER SMART diffractometer equipped with graphite-monochromated MoKα radiation (λ =0.71073 Ǻ). A total of 35763 reflections were collected in the range of 1.21<θ<30.61° by using an ω-Ф scan mode at 140(2) K, Mr= 718.77,orthorhombic, space group P212121, a = 6.1861(13),b = 20.838(4), c = 28.274(6) Å, V = 3644.6(13) Å3,Z = 4, Dc= 1.310 g/cm3, the final R = 0.0642 for 11106 observed reflections with I > 2σ(I) and Rw =0.1529 for all data. The structure was solved by full-matrix least-squares on F2using the SHELXTL program[17-19]. All non-H atoms were refined with anisotropic displance parameters. All hydrogen atoms were located theoretically and refined with riding medel position parameters and fixed isotropic thermal parameters.
3 RESULTS AND DISCUSSION
The synthetic routes can be summarized as follows (Schemes 1 and 2). The synthesis of complexes I and II were carried out under anhydrous ethanol or methanol, using a ratio of 3:1 of the ligand to the metal salts. After refluxing for 48 h, the crystals were both obtained after complex I was recrystallized with methanol and complex II was recrystallized with ethanol.
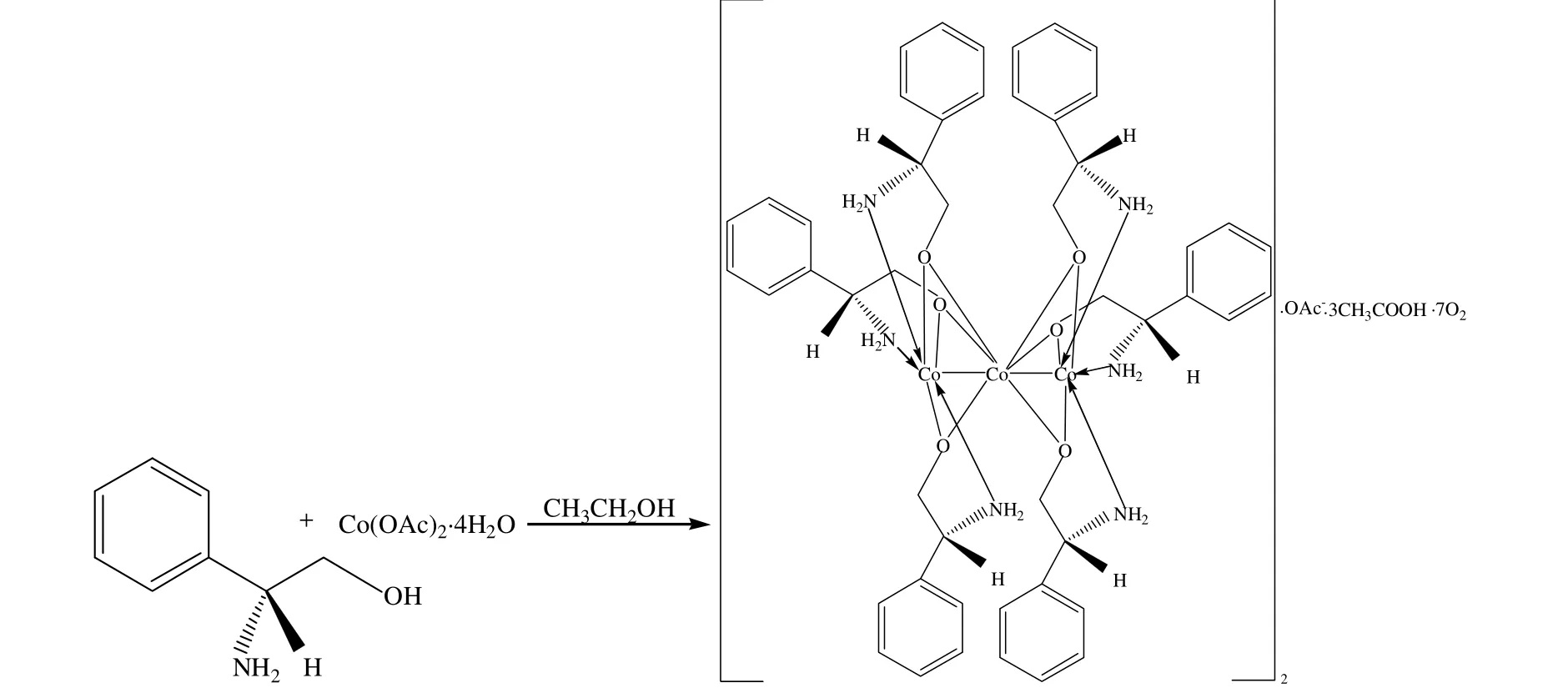
Scheme 1. Synthetic route to complex I
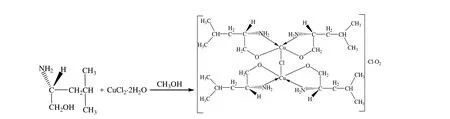
Scheme 2. Synthetic route to complex II
From Fig. 1 several interesting observations can be made about the structure. In the complex, the Co(1) ion was coordinated with oxygen atoms O(1),O(2) and O(3) as well as the L-plenylglycinol nitrogen atoms N(1), N(2) and N(3); the Co(2) ion was coordinated with oxygen atoms O(1)~O(6);and the Co(3) ion was coordinated with oxygen atoms O(4), O(5) and O(6) together with the L-plenylglycinol nitrogen atoms N(4), N(5) and N(6).The deprotonated hydroxyl groups of L-phenylglycinol bridge the Cu(II) cations to form the trinuclear complex. The bond length of Co(1)–Co(2)(2.651(2) Å) is nearly the same as that of Co(2)–Co(3) (2.657(2) Å). The uncoordinated acetate ions and seven oxygen molecules form a threedimensional hydrogen-bond network.
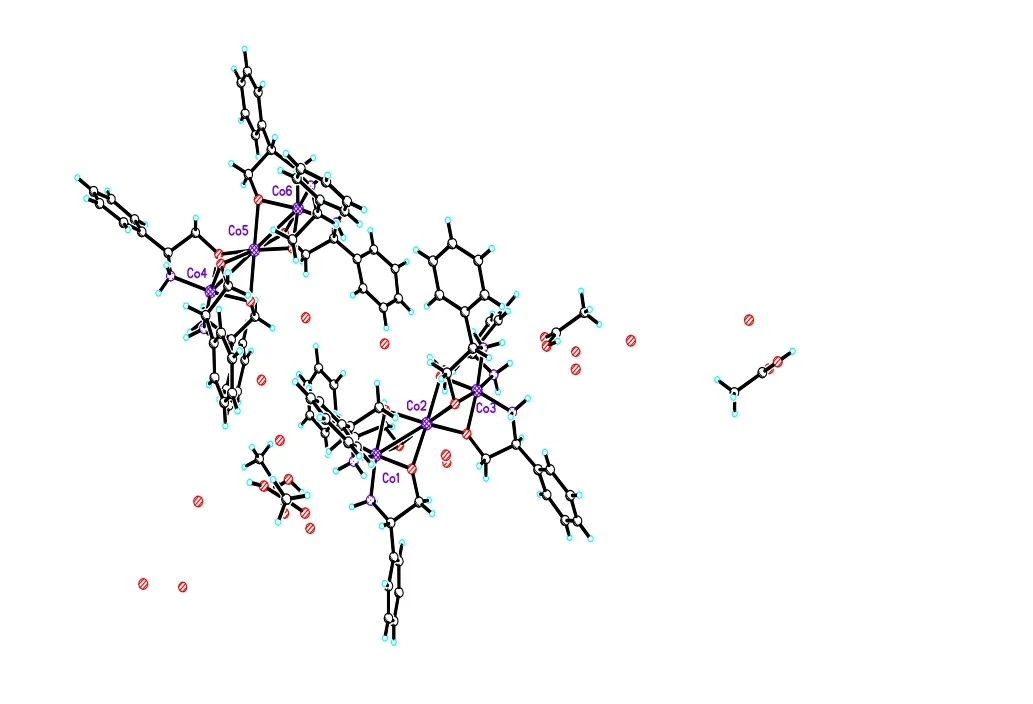
Fig. 1. Crystal structure of complex I
From Fig. 2 we can see that the structure of complex II is binuclear. Each Cu(II) cation is N,O-chelated by L-leucinol anion and a Cl-anion bridging the pair of Cu(II) cations. The uncoor-dinated one chloride ion and three oxygen atoms are rooted out. The bond Cu(2)–Cl(1) in 2.6421(14) Ǻ is slightly longer than Cu(2)–Cl(1) (2.6880(15) Ǻ).The aforementioned components were held together through hydrogen bonding interactions.

Fig. 2. Crystal structure of complex II
Tables 1 list the bond lengths and bond angles for complexes I and II, and Table 2 shows their hydrogen bond lengths and bond angles.
4 APPLICATIONS
We studied the identity of complexes I and II using the asymmetric addition of CH3NO2to benzaldehyde as a model reaction (Table 3). These two complexes showed remarkable reactivity after reaction at room temperature for 6 h.
5 CONCLUSION
In conclusion, the crystal structures of Co(II) and Cu(II) complexes involving L-amino alcohols have been first reported by one-pot method. Their applications in other various organic reactions such as the cyanosilylation reaction, Allylation reaction and so forth are under investigation.
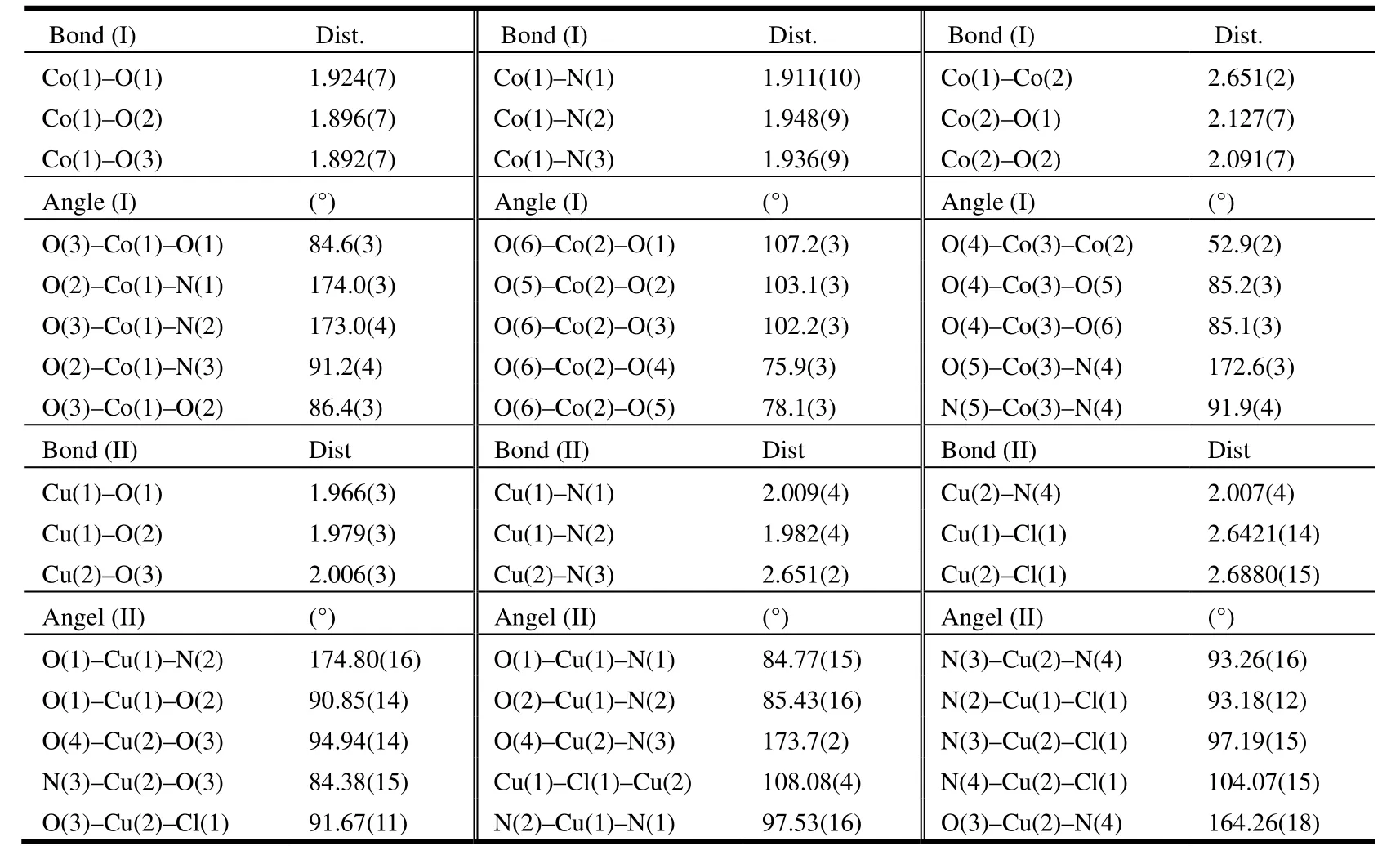
Table 1. Selected Bond Lengths (Å) and Bond Angels (°) for Complexes I and II

Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°) for Complexes I and II

Symmetry codes: #1: x, y, z + 1; #2: x – 1, y, z; #3: x, y, z – 1; #4: –x + 1, y – 1/2, –z; #5: –x + 1, y + 1/2, –z; #6: –x + 1, y, –1/2, –z + 1;#7: x + 1, y + 1/2, –z + 1 (compound I); #1: –x + 1, y – 1/2, –z + 3/2; #2: –x + 2, y – 1/2, , –z + 3/2; #3: x + 1, y, z (compound II)
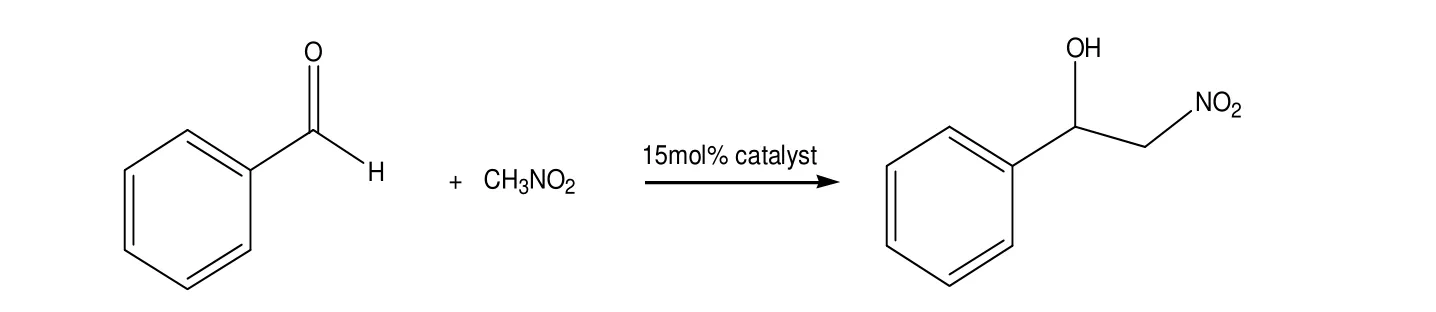
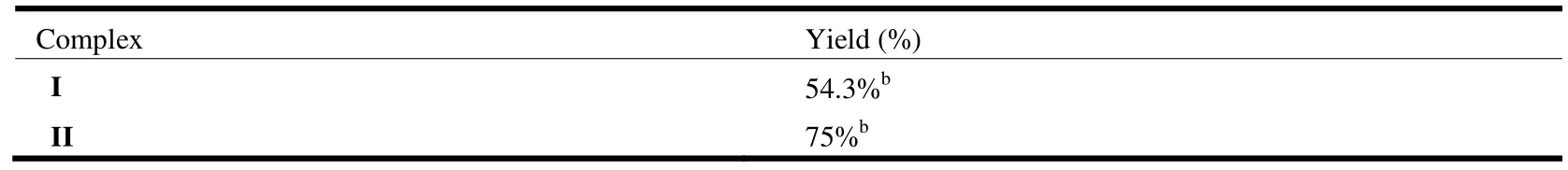
Table 3. Henry Reaction of Benzaldehyde Catalyzed by Complexes I and IIa
(1) Manuela, D.; Reimar, K.; Helmar, G.; Brunö, S. Reactions of the four diastereomeric 16-amino-17-hydroxy-3-methoxyestra-1,3,5(10)-trienes with aromatic ortho-hydroxy and heteroaromatic a-aldehydes and with 1,3-dicarbonyl compounds-molecular structures of condensation products and of copper(II) complexes. Steroids 2000, 65, 305-318.
(2) Chullikkattil, P. P.; Samar, K. D. Coordination and supramolecular aspects of the metal complexes of chiral N-salicyl-β-amino alcohol Schiff base ligands: towards understanding the roles of weak interactions in their catalytic reactions. Coordin. Chem. Rev. 2013, 257, 1699-1715.
(3) Getova, V. T.; Bontchev, R. P.; Mehandjiev, D. R.; Skumryev, V.; Bontchev, P. R. Copper(II) complexes with 4-amino-α-(t-butylaminomethyl)-3,5-dichlorobenzyl alcohol hydrochloride(Clenbuterol). Crystal structures of the binuclear and mononuclear Cu(II)complexes with clenbuterol. Polyhedron 2005, 24, 1983-1990.
(4) Donald, A. H.; Vickie, M. K.; Peter, J. S. Transition-metal complexes with polyamino alcohols. 1. Structures of a dinuclear chromium(III) complex and a trinuclear cobalt complex with N-(3-aminopropyl)diethanolamine, a bifurcated quadridentate tripod ligand. Inorg. Chem. 1986, 25, 4884-4889.
(5) Lu, H. J.; Tao, J. R.; Jess, E. J.; Lukasz, W.; Zhang, X. P. Cobalt(II)-catalyzed intramolecular C–H amination with phosphoryl azides: formation of 6-and 7-membered cyclophosphoramidates. Org. Lett. 2010, 6, 1248-1251.
(6) Abdessamad, A.; Carl, R.; Noelia, M. S. B.; Mark, R. J. E.; David, L. H. Bimetallic copper(II) and zinc(II) complexes of acyclic Schiff base ligandsderived from amino acids. Inorg. Chim. Acta 2011, 365, 96-102.
(7) Zheng, J. C.; Rousseau, R. J.; Wang, S. N. Homonuclear copper complexes with multidentate amino alcohol ligands. Synthesis and characterization of a dicopper zwitterion, Cu2(1,3-bis(d imethylamin0)-2-propanol)2Cl4and a tricopper compound, Cu3(1,3-bis(dimethylamino)-2-propanolato)2C14.Inorg. Chem. 1992, 31, 106-110.
(8) Maravalli, P. B.; Goudar, T. R. Thermal and spectral studies of 3-N-methyl-morpholino-4-amino-5-mercapto-1,2,4-triazole and 3-N-methyl-morpholino-4-amino-5-mercapto-1,2,4-triazolecomplexes of cobalt(II), nickel(II) and copper(II). Thermochim. Acta 1999, 325, 35-41.
(9) Bordoloi, A.; Hwang, Y. K.; Hwang, J. S.; Halligudi, S. B. Mesoporous silica immobilized cobalt complex: an efficient catalyst for epoxides ring opening by aromatic amines under ambient conditions. Catal. Commun. 2009, 10, 1398-1403.
(10) Wang, S. N.; Pang, Z.; Zheng, J. C.; Wagner, M. J. Homonuclear copper(II) complexes with multidentate amino alcohol ligands. Synthesis and characterization of a hexanuclear copper compound with a propeller structure: Cu6(dmap)3Cl6(O)(OH) (bdmap =1,3-bis(dimethylamino)-2-propanolato). Inorg. Chem. 1993, 32, 5975-5980.
(11) Petri, S.; Enrique, C.; Antonio, J. M.; Reijo, S. Structural diversity due to amino alcohol ligands leading to rare μ4- hydroxo-bridged tetranuclear and“bicapped cubane” cores in copper(II) complexes: a theoretical and experimental magnetostructural study. Inorg. Chem. 2013, 52, 11096-11109.
(12) Jacques, A.; Barry, R. S.; Constantinos, G. S.; Christine, J. C.; Jorge, F. Synthesis of new phosphino amino alcohol ligands via ortho-alkyllithiation reactions. Versatile coordination behavior toward copper(I) and palladium(II). Organometallics 1998, 17, 839-845.
(13) Chullikkattil, P. P.; Samar, K. D. Coordination and supramolecular aspects of the metal complexes of chiral N-salicyl-β-amino alcohol Schiff base ligands: towards understanding the roles of weak interactions in their catalytic reactions. Coordin. Chem. Rev. 2013, 257, 1699-1715.
(14) Sheldrick, G. M. SHELXS-97, Program for X-ray Crystal Structure. Solution. Univisity of Gottingen, Germany 1997.
(15) Sheldrick, G. M. SHELXS-97, Program for X-ray Crystal Structure Refinement. University of Gottingen, Germany 1997.
(16) Stout, G. H.; Jensen, L. H. X-ray Structure Determination: A Practical Guide. New York, MacMillan 1968.
- 结构化学的其它文章
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis, Structure and Luminescent Property of an Europium(III) Coordination Polymer①
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①

