Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
MUHAMMAD Neem Ahme SHAHID Hmee KHAWAJA Ansr Ysin IFZAN Arsh IHSAN-ul -Hq SAFEENA Zfr MUHAMMAD Nwz Thir
a (Department of Chemistry, The University of Azad Jammu and Kashmir, Muzaffarabad 13100, Pakistan)
b (Department of Chemistry, Quaid-i-Azam University, Islamabad 45320, Pakistan)
c (Department of Pharmacy,Faculty of Biological Sciences,Quaid-i-Azam University, Islamabad 45320, Pakistan)
d (University of Sargodha, Department of Physics, Sargodha)
1 INTRODUCTION
Now a days research is focused on the synthesis of new and safe therapeutic agents of clinical importance. 1,2,3-triazoles are enjoying their importance day by day in organic synthesis, biology and material sciences. Significance of 1,4,5-trisubstituted 1,2,3-triazole and their derivatives is of key importance due to their lots of biological activities[1]like antibacterial, antifungal, antimalarial, antiviral[2],anticonvulsant[3], antidepressant and anticancer[4-6].Recently, many 1,4,5-trisubstituted 1,2,3-triazoles have shown novel properties[7]because of their extensive structural diversity. Currently, more structurally diverse 1,2,3-triazoles can be easily synthesized by CuAAC method using one-pot strategy. In view of the emerging biological and synthetic importance of 1,4,5-trisubstituted 1,2,3-triazoles, we have designed and synthesized some novel 1,2,3-triazoles and their derivatives using one-pot, three component reaction with high yield.
2 EXPERIMENTAL
2.1 Materials and instruments
Melting point was determined on a Stuart SMP3 melting point apparatus. IR spectrum was recorded on a Nicolet FTIR SDX spectrometer. The1H and13C NMR spectra were recorded on a JEOL JNM-ECA 300 instrument in CDCl3. TMS was used as internal standard and J values are given in Hz.HRMS was obtained on a Bruker micrOTOF-Q II spectrometer. 1-Copper(I) phenylethyne was prepared according to the procedure reported in references[8,9]. Reaction was monitored by thin layer chromatography (TLC) using F254silica gel. All chemicals were purchased from commercial suppliers and were dried and purified where necessary.
2.2 Theoretcial calculations
All the calculations were performed by DFT(B3LYP)[10,11]method by taking the crystal structure coordinates as an initial geometry with 6-311G basis set[12]using PC GAMEES/Firefly Version 8.0.0[13]together with the Gabedit graphical user interface[14].All geometric parameters were visualized with Chemcraft[15].
2.3 Synthesis
Ethoxalyl chloride (0.968 g, 0.5 mmol) was added to a suspension of 1-azidocyclohexane (0.075 g, 0.6 mmol) and 1-copper(I) phenylethyne (0.082 g, 0.5 mmol) in PhCl (2 mL). The resulting mixture was stirred at room temperature for 4 h and then passed through a column chromatography (silica gel,EtOAc in petroleum ether (60~90 °C ) to gave the product (I) as a white solid. Crystals suitable for crystallographic study were grown by slow evaporation of an ethanol:ethyl acetate solution at room temperature. m.p.: 97~99 °C, yield = 85%, IR (KBr)ν 3057, 2996, 2960, 2938, 2859, 1746, 1671, 1477,1449, 1290, 1277, 1221, 1202, 992, 975, 789, 735,705 cm-1;1H-NMR δ 7.55~7.44 (m, 5H), 4.93~4.81 (m, 1H), 3.77 (q, 2H, J = 7.20 Hz), 2.24~1.95(m, 6H), 1.82~1.76 (m, 1H), 1.57~1.26 (m, 3H),0.98 (t, 3H, J = 7.20 Hz);13C-NMR δ 177.8, 161.1,152.6, 129.9, 129.5, 129.1, 128.6, 127.2, 62.8, 60.9,33.0, 25.3, 25.0, 13.2 ppm HRMS (ESI-TOF) (m/z):calculated for C18H21N3O3, [M+H]+328.1656; found 328.1654.
2.4 X-ray crystallography
C18H21N3O3, Mr= 327.38, crystal size 0.36mm ×0.28mm × 0.26mm, monoclinic, space group P21/n,a = 12.8167(9), b = 8.0966(6), c = 16.7079(9) Å, β =98.716(2), Z = 4, V = 1713.8(2) Å3. ρcalc= 1.269 g/cm³, μ= 0.088 mm-1and F(000) = 696. Data were collected at 296(2) K on a Bruker Kappa Apex II CCD diffractometer using MoKα radiation. 14207 intensities 1.87≤θ≤26.9°. The structure was solved by direct methods, full-matrix least-squares refinement[16]on F² and 218 parameters for 3748 unique intensities (Rint= 0.025). The structure was not only solved through direct methods but also refined by full-matrix least-squares using SHELX-97[16]. All non-hydrogen atoms were refined anisotropically.

Scheme 1. Synthesis of the title compound (I)
3 RESULTS AND DISCUSSION
3.1 Crystal structure
The molecular structure of the title compound (I)shown in Fig. 1 is closely related to that of ethyl 2-[1-(3-methylbutyl)-4-phenyl-1H-1,2,3-triazol-5-yl]-2-oxoacetate[17]with iso-pentyl group replaced by cyclohexyl group. In the molecule, the cyclohexyl group adopts the chair conformation. The dihedral angle between the mean planes of the triazole and phenyl rings is 51.03°. In the crystal, molecules related by inversion are paired into dimers via C(14)–H(14)···O(1) (2.631 Å) and C(15)···O(2)–C(16) interactions[18-20], and the latter is indicated by the short distance of 3.188 Å (Fig. 2).

Fig. 1. Molecular structure of (I). Anisotropic displacement ellipsoids are drawn at 20% probability level. H atoms are shown as spheres of arbitrary radii

Fig. 2. Dimer formed via C–H…O and C–O…C interactions (dashed lines)in the crystal structure of the compound (I).
3.2 Optimized geometry
The optimized geometry parameters, namely the selected bond lengths and bond angles, experimental and calculated by the B3LYP/6-311G level, are listed in Table 1. From the theoretical results, the expected variations of the calculated values against the experimental ones were observed. Comparing the experimental and calculated bond lengths and bond angles, it is noticed that calculated values are always higher than the experimental ones.Theoretical structure also showed identical bond lengths (both experimental and calculated) for the C–C bonds of the aryl and cyclohexyl rings.
By comparing the theoretical and experimental data, several observations can be made. The experimental N(1)–N(2) and N(2)–N(3) bond lengths are 1.330 and 1.323 Å which are calculated as 1.360 and 1.368 Å respectively. The N(3)–C(8) bond length is 1.355 Å and calculated as 1.375 Å which is close to the experimental value and smaller than the normal C–N single bond length of about 1.47 Å[21,22]. From the calculated results it was concluded that theoretical and experimental geometric parameters were in good agreement. The X-ray structure of the title compound was compared with its optimized counterpart by superimposing experimental structure over the theoretical (Fig. 3); and minor conformational discrepancies were observed.

Table 1. Bond Lengths (Å) and Bond Angles (º) for the Title Compound (I)Determined by X-ray Diffraction and B3LYP Calculations using the 6-311G Basis Set
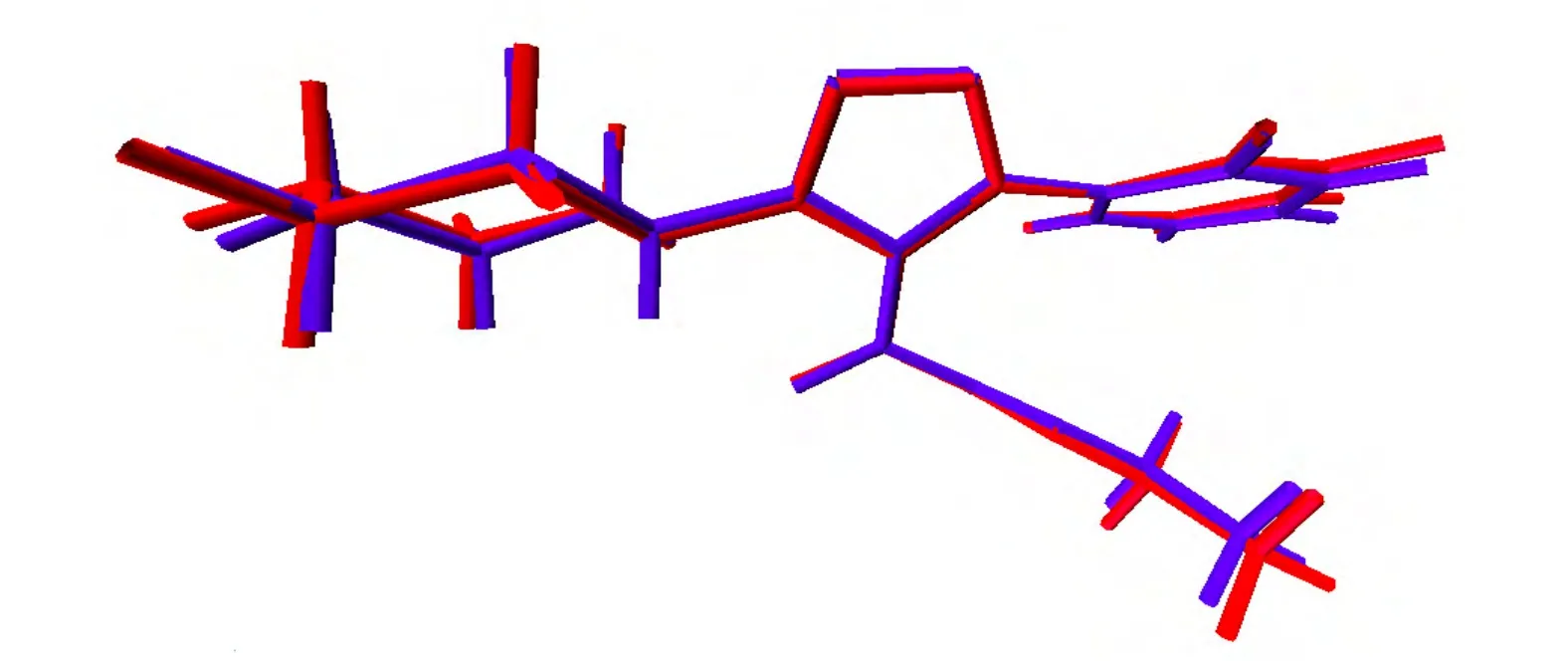
Fig. 3. Atom-by-atom superimposition of the structure calculated (red)by B3LYP/6-311G on the X-ray structure (blue) of the title compound (I)
4 ANTIMICROBIAL ACTIVITY
In this study the antibacterial activity of the compound was observed using disc diffusion method[23,24]against various pathogenic bacteria as S. typhimurium, M. luteus and B. bronchiseptica.Different fungal strains like Aspergilus niger, Mucor species and Aspergilus flavus were also used to check the antifungal potential of the synthesized compound. The values of antibacterial and antifungal activities of compound I are listed in Tables 2 and 3, respectively. Graphical results of the antibacterial and antifungal activities are shown in Fig. 4a and 4b.
4.1 Antibacterial assay
Bacterial inoculum was prepared in nutrient broth.It was prepared by dissolving 2 grams of nutrient broth in 100 mL of water and the pH was maintained to 7. Nutrient agar medium was used for the growth of bacterial strains that were produced by dissolving 2 grams in 100 mL of water. A filter paper disc of 6 mm in size was prepared from whatman no. 1 filter paper. Media, filter paper discs along with other apparatus required in this assay were autoclaved for sterilization. After autoclaving, the whole experiment was carried out in microbiology safety cabinet.Solidified plate of nutrient agar was labeled and respective bacterial strain was streaked. Then solution of the compound in DMSO was absorbed on disc which was placed on respective place in petri plate. This petri plate was incubated for 24 hours at 28 °C. Zones of inhibition were measured in mm after 24 hours. Cefixime-USP was used as the standard drug and DMSO as the negative control.

Fig. 4a. Graphical representation of antibacterial activities of compound I

Fig. 4b. Graphical representation of antifungal activities of compound I
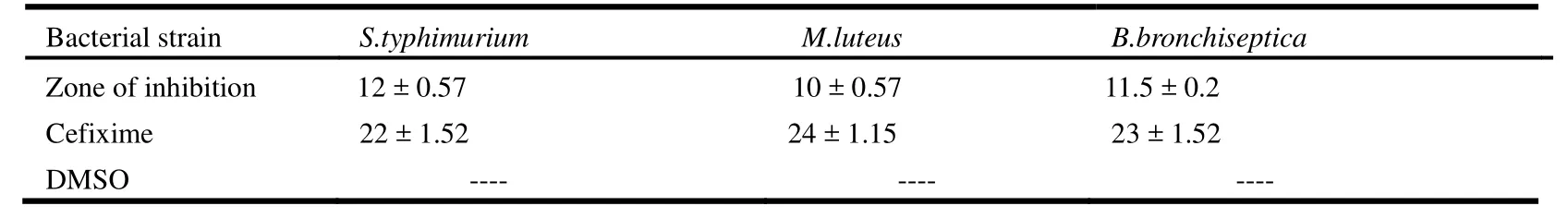
Table 2. Antibacterial Activity Data of the Title Compound (I)
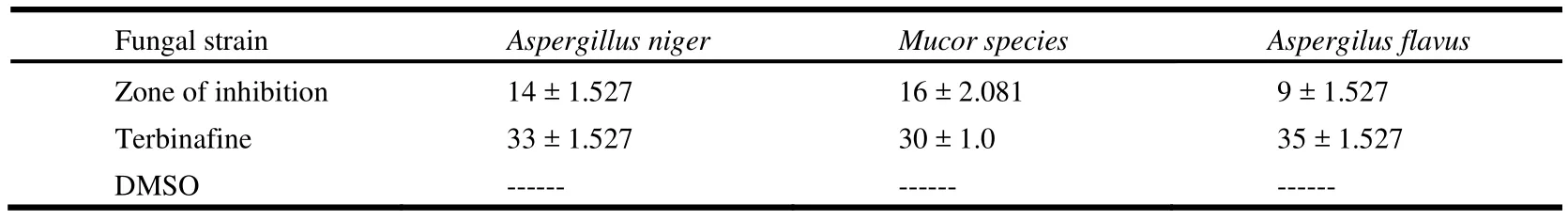
Table 3. Antifungal Activity Data of the Title Compound (I)
4.2 Antifungal assay
Nutrient agar medium was used for the growth of bacterial strains produced by dissolving 2 grams in 100 mL of water. Fungal cultures were grown on SDA medium at pH = 6.5. Sabouraud dextrose agar(SDA) was prepared by dissolving 6.5 grams in 100 mL of distilled water and the pH value was adjusted to 6.5. Whatman no. 1 filter paper was used for the preparation of circular discs of 6 mm size. All apparatus required in the assay were autoclaved for sterilization. An experiment was carried out in microbiological safety cabinet. SDA media were poured on petri plate and left for solidification. The filter paper disc was used for the absorption of sample on disc. Contamination was prevented by wrapping the petri plate with parafilm. After that,the plate was incubated for 24 hours at 28 . On next day, the inhibition zones were measured. Terbinafine was used as the standard drug and DMSO as the negative control.
5 CONCLUSION
In this study, a novel 1,4,5-trisubstituted triazole was synthesized and mainly characterized by X-ray diffraction. The experimental and theoretical structure parameters of the title compound I were compared and showed that the experimental X-ray structure is close to its optimized counterpart. Actimicrobial activity results showed that the synthesized compound displayed good antibacterial and anti-fungal activities against three different bacterial and fungal strains.
(1) Wu, P. Crystal structure of 6-ferrocenyl-3-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole. J. Struct. Chem. 2013, 54, 983-985.
(2) Chen, X. M.; Li, Z. J.; Ren, Z. X.; Huang, Z. T. Synthesis of glucosylated 1,2,3-triazole derivatives. Carbohyd. Res. 1999, 315, 262-267.
(3) Kadaba, P. K. Triazolines 14. 1, 2, 3-triazolines and triazoles. A new class of anticonvulsants. Drug design and structure-activity relationships. J. Med.Chem. 1988, 31, 196-203.
(4) Kumar, S.; Khan, S. A.; Alam, O.; Azim, R.; Khurana, A.; Shaquiquzzaman, M.; Siddiqui, N.; Ahsan, W. Synthesis of tetrazolo[1,5-a]quinoxaline based azetidinones & thiazolidinones as potent antibacterial & antifungal agents. B Korean Chem. Soc. 2011, 32, 2260-2266.
(5) Hein, J. E.; Fokin, V. V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev.2010, 39, 1302-1315.
(6) Tornoe, C. W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057-3064.
(7) Wang, B.; Ahmed, M. N.; Zhang, J.; Chen, W.; Wang, X.; Hu, Y. Easy preparation of 1,4,5-trisubstituted 5-(2-alkoxy-1,2-dioxoethyl)-1,2,3-triazoles by chemoselective trapping of copper(I)-carbon bond with alkoxalyl chloride. Tetrahedron Lett. 2013, 54, 6097-6100.
(8) Owsley, D. C.; Castro, C. E. Substitution of aryl halides with copper(I) acetylides: 2-phenylfuro[3,2-b]pyridine. In Organic Syntheses; John Wiley &Sons, Inc. 2003.
(9) Wu, Y. M.; Deng, J.; Li, Y.; Chen, Q. Y. Regiospecific synthesis of 1,4,5-trisubstituted-1,2,3-triazole via one-pot reaction promoted by copper(I) salt.Synthesis 2005, 1314-1318.
(10) Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648-5652.
(11) Lee, C.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785-789.
(12) Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J.Chem. Phys. 2008, 72, 650-654.
(13) Granovsky, A. Firefly. Version 8.0. 0 2013.
(14) Allouche, A. R. Gabedit—a graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174-182.
(15) Zhurko, G.; Zhurko, D. ChemCraft: Tool for treatment of chemical data. Lite Version Build 8 2005.
(16) Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 2007, 64, 112-122.
(17) Ahmed, M. N.; Yasin, K. A.; Tahir, M. N.; Hafeez, M.; Aziz, S. Ethyl 2-[1-(3-methylbutyl)-4-phenyl-1H-1,2,3-triazol-5-yl]-2-oxoacetate. Acta Crystallogr. E 2013, 69, o1768.
(18) Naseer, M. M.; Hameed, S. Layer-by-layer assembly of supramolecular hexagonal blocks driven by CH-π and π-π interactions. Crystengcomm. 2012,14, 4247-4250.
(19) Saeed, A.; Arshad, I.; Flörke, U. One-pot synthesis and crystal structure of methyl 5-hydroxy-1-phenyl-1H-pyrazole-3-carboxylate. Crystals 2012, 2,1248-1252.
(20) Abbas, A.; Nazir, H.; Naseer, M. M.; Bolte, M.; Hussain, S.; Hafeez, N.; Hasan, A. Synthesis, spectral characterization, self-assembly and biological studies of N-acyl-2-pyrazolines bearing long alkoxy side chains. Spectrochim. Acta A 2014, 120, 176-184.
(21) Saeed, A.; Arshad, I.; Flörke, U. Synthesis, crystal structure, and DFT study of N΄-(2,4-dinitrophenyl)-2-fluorobenzohydrazide. J. Chem. 2013, 2013,Article ID 648121, 5 pages, doi:10.1155/2013/648121.
(22) Ifzan, A.; Shumaila, A.; Asghar, A.; Shahid, H.; Kong, M. L.; Naseer, M. M. Conformational ssomerism in a conformaional polymorph of 2,5-dibenzylidenecyclopentanone: crystallographic strucure and quantum chemical computations. European Chemical Bulletin 2014, 3, 587-592.
(23) Yang, G. S.; Liu, C. B.; Li, H. N.; Chen, Y.; Hang, D. H.; Wen, H. L. Syntheses, crystal structures and antibacterial activities of two heterocyclic Schiff base compounds. Chin. J. Struct. Chem. 2014, 33, 528-534.
(24) Wang, X. H.; Lin, Q.; Yin, X. Q.; You, C. H.; Yang, J. X. Synthesis, bioactivity and crystal structure analysis of novel benzo[d]isothiazol-3(2H)-ones.Chin. J. Struct. Chem. 2012, 31, 1170-1174.
- 结构化学的其它文章
- Syntheses, Crystal Structures, and Magnetic Properties of the Cobalt(II) and Nickel(II) Coordination Polymers Constructed from 5-Halonicotinate and 2,2΄-Biimidazole①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①
- A New Inorganic-organic Hybrid Based on Biisoquinoline and Hexachloridostannate: Structure, Photoluminescence,Electrochemical Behavior and Theoretical Study
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①

