Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
TENG Teng CHEN Jin CHEN Xu-Lin YU Rong-Min② LU Can-Zhong②
a (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 35002, China)
b (College of Life Science, University of Fujian Agriculture and Forestry, Fuzhou 350002, China)
c (Graduate University of Chinese Academy of Sciences, Beijing 100049, China)
1 INTRODUCTION
Transition luminescent materials are of great interest currently for their potential applications in a wide range of areas, such as chemical sensors,photocatalytic organic synthesis, sensitized solar cells and electroluminescent (EL) devices[1]. Blue luminescent compounds are the most sought-after because of the urgent demand for stable blue emitters for EL devices[2]. In a previous paper, we have reported a series of blue luminescent Cu(I)complexes based on N-linked 2-pyridyl pyrazolate ligands[3]. The complexes exhibit high photoluminescence quantum yields (PLQYs) at room temperature both in nitrogen-saturated CH2Cl2solution (up to 45%) and in neat solid (up to 87%). To further explore the chemistry of blue luminescent compounds, we expanded our research on Ag(I) complexes. Many d10metal complexes with interesting luminescence properties have been synthesized[4,5].With regard to silver complexes, most reported are polynuclear complexes that have d10-d10interactions[6]. Emissive mononuclear silver complexes,however, are much less investigated[7-12]. The Ag(I)ion has a d10electronic configuration like the Cu(I)ion and is expected to form emissive complexes.However, in contrast to Cu(I), Ag(I) ion is much larger and has a tendency to display a highly versatile and irregular coordination number and geometry, which may lead to the formation of new structural motifs and new luminescent complexes.Herein, we report our new findings on the luminescent and structural properties of a three-coordinate silver complex based on an N-linked 2-pyridyl pyrazolate ligand.
2 EXPERIMENTAL
2.1 Material and instrument
All reactions were performed under air atmosphere unless specified. Chemicals were purchased from commercial sources and used without further purification. The diimine ligand Mepzpy was prepared by literature procedure[3].1H NMR and31P NMR spectra were recorded on a Bruker Avance III 400MHz NMR spectrometer. UV-Vis absorption spectra were recorded with a Perkin-Elmer Lambda 45 UV/Vis spectrophotometer. Photoluminescence spectrum and the lifetimes of powder sample were recorded on an Edinburgh Analytical instrument FLS920 with a Picosecond Laser Diode. The powder PL quantum yield was defined as the number of photons emitted per photon absorbed by the system and measured by FluoroMax-4 equipped with an integrating sphere.
2.2 Synthesis of complex[Ag(Mepzpy)(PAr3)]BF4·H2O (1)
A mixture of Ag(CH3CN)4(BF4) (36 mg, 0.1 mmol), Mepzpy (16 mg, 0.1 mmol) and PAr3(31 mg, 0.1 mmol) in 10 mL of CH3CN was stirred at room temperature for 2 h, and then filtered. The filtrate was allowed to stand undisturbed in an uncapped test tube. After slow evaporation for three days, the crystals of the product (52 mg, 76%) were isolated by decanting the supernatant from the tube,washed with ether and dried in air.1H NMR (400 MHz, CD2Cl2): δ 8.41 (d, J = 4.0 Hz, 1H), 8.15 (m),7.87 (d, J = 8.0 Hz, 1H), 7.58~7.29 (m, 10H), 6.99(t, 3H), 6.58 (d, 2H).31P NMR: –16.24 (s).
2.3 Structure determination X-ray crystallographic analysis
A colorless crystal of complex 1 with dimensions of 0.20mm × 0.17mm × 0.16mm was used for X-ray diffraction analysis. Diffraction data of the complex were collected on a SuperNova, Dual, Cu at zero, Atlas diffractometer equipped with graphite-monochro- mated CuKα radiation (λ =1.54184 Å). A total of 14386 reflections were collected in the range of 4.60≤θ≤76.50º by using an ω-scan mode, of which 6371 were unique with Rint= 0.0285 and 5751 were observed with I > 2σ(I).The structure was solved by direct methods with SHELXS-97 and refined by full-matrix least-squares methods with SHELXL-97 program package[13]. All of the non- hydrogen atoms were located with successive difference Fourier synthesis.Hydrogen atoms were added in idealized positions.Except the oxygen atom of water interstice molecule, the non-hydrogen atoms were refined anisotropically. The BF4-anion is disordered over two orientations in a ratio of 0.71:0.29. The final R= 0.0530, wR = 0.1493 (w = 1/[σ2(Fo2) + (0.1121P)2+ 0.6970P], where P = (Fo2+ 2Fc2)/3), S = 1.038,(Δ/σ)max= 0.002, (Δρ)max= 0.985 and (Δρ)min=–1.142 e/Å3. Selected bond lengths and bond angles are listed in Table 1.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
3 RESULTS AND DISCUSSION
Complex 1 was prepared from the reaction of Ag(CH3CN)4BF4with diimine ligand Mepzpy and tri(o-tolyl)phosphine in acetonitrile. It was characterized by several methods, including X-ray crystallography. Single-crystal analysis reveals that 1 crystallizes in the triclinic space group P1 with Z =2. The asymmetric unit contains a[Ag(Mepzpy)(PAr3)]+cation, a BF4-anion, and a H2O interstice molecule. As shown in Fig. 1, each silver atom is coordinated by two nitrogen atoms from a chelating Mepzpy diimine ligand and a phosphorus atom from a phosphine ligand. The coordinate geometry of the silver atom can be described as a distorted trigonal pyramid. The silver atom is about 0.54 Å away from the trigonal base.The N–Ag–N angle (70.1(1)º) largely differs from the usual trigonal pyramidal value because of the small bite angle of the Mepzpy ligand. The Ag–N and Ag–P bond distances are similar to those reported in the literature[7-12].
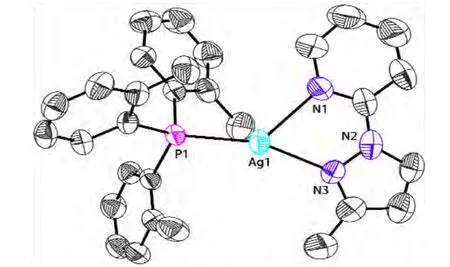
Fig. 1. ORTEP structure of the cation in complex 1.Thermal ellipsoids are draw at 50% probability.Hydrogen atoms are omitted for clarity
In the packing diagram of 1 as shown in Fig. 2, a 1-D supramolecular layer is formed by two types of intermolecular π-π stacking interactions (Table 2):one between the pyridine and pyrazole rings, and the other from the intermolecular benzene rings. The centroid-centroid distances for these π-π stacking interactions are in the range of 3.861~4.272 Å, and the perpendicular distances between the corresponding rings are from 3.424 to 3.569 Å. The relative dihedral angles range from 0 to 5.59º, all of which are below the normal range reported[14]. Thus,aromatic π-π stacking interactions are mainly responsible for the formation of 1-D supramolecular chains in 1.
Complex 1 was also characterized in1H and31P NMR spectroscopy at room temperature. The1H NMR spectrum shows a single set of signals for both Mepzpy and PAr3ligands. Analysis of the integration of1H NMR spectra revealed that the Mepzpy and PAr3ligands are present in a 1:1 ratio,which is consistent with the crystal structure. The31P NMR spectrum shows a single broad peak at–16.24 ppm. The Ag–P coupling is unresolved,presumably in consequence of the exchange equilibrium that is reasonably fast in relation to the NMR time scale. In this case, a partial decomplexation/recomplexation of the labile PAr3ligand induces an equilibrium process.

Table 2. Defined Ring and Relative Parameters of the π-π Interactions in Compound 1

Fig. 2. 1-D supramolecular chain by the two types of π-π stacking interactions in 1.All hydrogen atoms were omitted for clarity
Fig. 3 shows the absorption spectra of complex 1 as well as the free ligands in CH2Cl2at 293 K. The excitation and emission spectra of complex 1 in solid state at room temperature are shown in Fig. 4.Photophysical data are summarized in Table 3. In CH2Cl2, complex 1 exhibits multiple intense absorption peaks in the 200~350 nm region (ε > 104M-1cm-1) that can be attributed to the π-π* transition of both diimine and phosphine ligands. Complex 1 displays blue emission with the maxima at 470 nm with quantum yields of 31% and decay time of 10.4 μs. The emission spectrum is broad and unstructured,indicating that the emission process is possibly connected with a significant CT character. The calculated radiative decay rate constant of Kr= Φplτr= 2.98 × 104s-1points out that the emission is phosphorescence in origin.
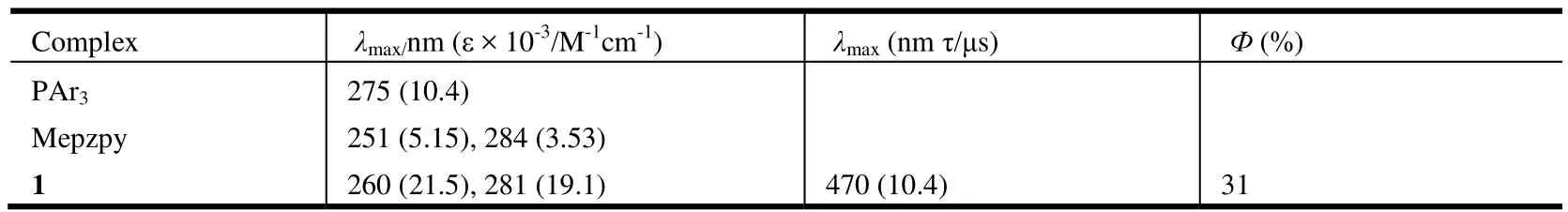
Table 3. Photophyscial Data of 1
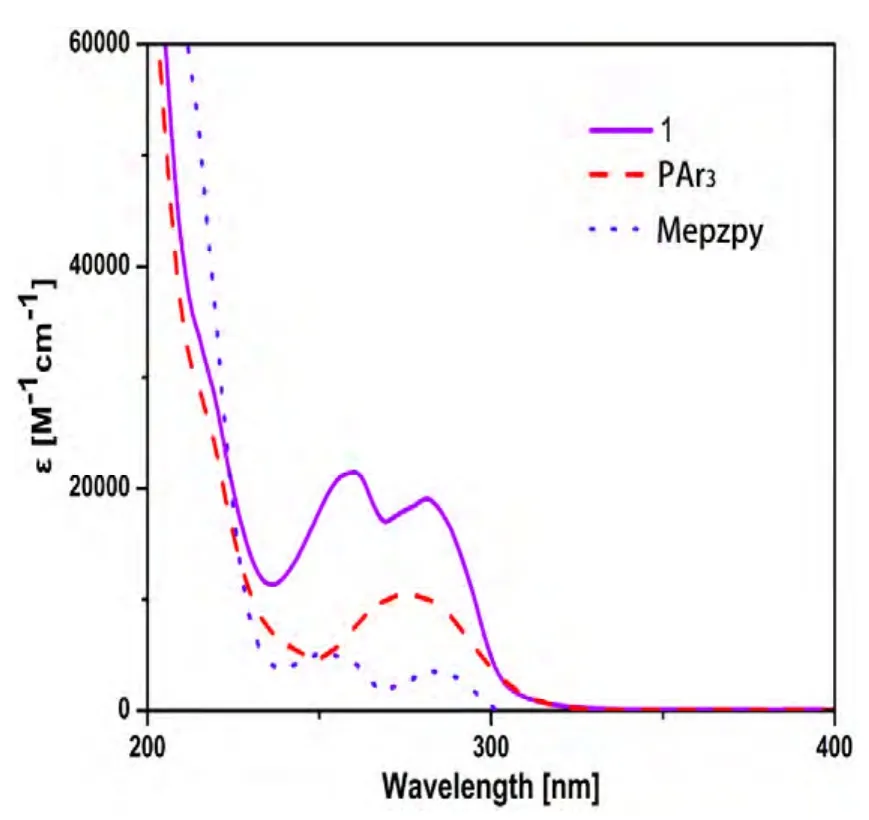
Fig. 3. Absorption spectra of PAr3,Mepzpy and complex 1 in CH2Cl2

Fig. 4. Excitation and emission spectra of complex 1 in the solid state
(1) Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal-organic complexes for optoelectronic applications. Chem.Soc. Rev. 2014, DOI: 10.1039/c3cs60449g.
(2) Fu, H.; Chou, P. T.; Chi, Y. “Feeling blue? The blue phosphorescence materials for OLEDs: advance, obstacle and perspective” Materials Today 2011,14, 472–479.
(3) Chen, X. L.; Yu, R.; Zhang, Q. K.; Zhou, L. J.; Wu, X. Y.; Zhang, Q.; Lu, C. Z. Rational design of strongly blue-emitting cuprous complexes with thermally activated delayed fluorescence and application in solution-processed OLEDsChem. Mater. 2013, 25, 3910–3920.
(4) Che, C. M.; Lai, S. W. Structural and spectroscopic evidence for weak metal-metal interactions and metal-substrate exciplex formations in d10metal complexes. Coord. Chem. Rev. 2005, 249, 1296-1309.
(5) Yam, V. W. W.; Wong, K. M. C. Luminescent metal complexes of d6, d8and d10transition metal centres. Chem. Commun. 2011, 47, 11579-11592.
(6) Sculfort, S.; Braunstein, P. Intramolecular d10-d10interactions in heterometallic clusters of the transition metals. Chem. Soc. Rev. 2011, 40,2741-2760.
(7) Kaeser, A.; Delavaux-Nico, B.; Duhayon, C.; Duhayon, J. E. Heteroleptic silver(I) complexes prepared from phenanthroline and bis-phosphine ligands. Inorg Chem. 2013, 52, 14343–54.
(8) Osawa, M.; Kawata, I.; Ishii, R.; Igawa, S.; Hashimoto, M.; Hoshino, M. Application of neutral d10coinage metal complexes with an anionic bidentate ligand in delayed fluorescence-type organic light-emitting diodes. J. Mater. Chem. C 2013, 1, 4375–4383.
(9) Osawa, M.; Hoshino, M. Photochemistry and photophysics of the tetrahedral silver(I) complex with diphosphine ligands: [Ag(dppb)2]PF6(dppb =1,2-bis[diphenylphosphino]benzene). Chem. Commun. 2008, 6384-6386.
(10) Hsu, C. W.; Lin, C. C.; Chunag, M. W.; Chi, Y.; Lee, G. H.; Chou, P. T.; Chang, C. H.; Chen, P. Y. Systematic investigation of the metal-structure-photophysics relationship of emissive d10-complexes of group 11 elements: the prospect of application in organic light emitting devices. J. Am. Chem. Soc. 2011, 133, 12085–12099.
(11) Igawa, S.; Hashimoto, A.; Kawata, I.; Hoshino, M.; Osawa, M. Photoluminescence properties, molecular structures, and theoretical study of heteroleptic silver(I) complexes containing diphosphine ligands. Inorg. Chem. 2012, 51, 5805–5813.
(12) Matsumoto, K.; Shindo, T.; Mukasa, N.; Tsukuda, T.; Tsubomura, T. Luminescence mononuclear Ag(I)-bis(diphosphine) complexes: correlation between the photophysics and the structures of mononuclear Ag(I)-bis(diphosphine) complexes. Inorg. Chem. 2010, 49, 805–814.
(13) Sheldrick, G. M. SHELXS-97 and SHELXL-97. Program for X-ray Crystal Structures Solution and Refinement. University of Göttingen: Göttingen,Germany 1997.
(14) Xiao, S.; Ge, S. S.; Wang, X. G. ; Liu, Q. X. Dinuclear silver(I) complex based on 1-picolyl-3-propylbenzimidazolium salt: crystal structure and weak interactions. Chin. J. Struct. Chem. 2012, 31, 167–172.
- 结构化学的其它文章
- Syntheses, Crystal Structures, and Magnetic Properties of the Cobalt(II) and Nickel(II) Coordination Polymers Constructed from 5-Halonicotinate and 2,2΄-Biimidazole①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①
- A New Inorganic-organic Hybrid Based on Biisoquinoline and Hexachloridostannate: Structure, Photoluminescence,Electrochemical Behavior and Theoretical Study
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①

