Synthesis, Structure and Luminescent Property of an Europium(III) Coordination Polymer①
YE Yn-Zhu LIN Shen WU Xin-Jin
a (Department of Science Research and Training,Fujian Institute of Education, Fuzhou 350001, China)
b (College of Chemistry and Chemical Engineering,Fujian Normal University, Fuzhou 350108, China)
1 INTRODUCTION
In recent years, investigations on the design and construction of novel lanthanide organic frameworks have received rather considerable attention not only because of their intriguing topological structures but also due to their potential applications in a wide variety of fields such as luminescence[1,2], magnetism[3,4]and catalysis[5,6]. Lanthanides have higher coordination numbers and more flexible coordination geometries, so it is more difficult to control the reactions and structures of their coordination polymers[7,8]. With the fascinating coordination chemistry, interesting structure, and functional peculiarity, lanthanide coordination polymers have attracted more and more increasing interest.
It is well-known that lanthanide ions have large radius and high affinity for hard donor atoms, such as oxygen, nitrogen or hybrid oxygen-nitrogen atoms. Thus multidentate ligands with oxygen or hybrid oxygen-nitrogen atoms are good candidates to construct new lanthanide coordination polymers.H2pydc, as a chelating ligand with multiple coordination sites involving one pyridine nitrogen atom and four carboxylate oxygen atoms, has limited steric hindrance combined with weak stacking interactions. It can provide a variety of coordination modes to form novel discrete or consecutive lanthanide coordination polymers[9-11]. In addition, flexible oxalic acid (H2ox) plays an important role in the construction of novel and stable lanthanide coordination polymers, mainly because of the versatile ligating abilities of -COO-moieties with the modes of chelating, bidentate-bridging or chelating-bridging, and the enhanced affinity of lanthanide ions towards such O donors. Moreover, the sulfate ion, as a simple tetrahedral oxo anion, has versatile coordination modes, which allows it to coordinate to the lanthanide ions and serves as auxiliary supporting bridge in the construction of lanthanide coordination polymers.
Under these considerations, H2pydc, H2ox, and sulfate ion were taken as ligands to successfully synthesize a lanthanide coordination polymer formulated as [Eu3(pydc)3(SO4)(H2O)5(ox)0.5]·3H2O. Here,we report the synthesis, crystal structure and luminescent property of it featuring a 3D network through π-π stacking interactions
2 EXPERIMENTAL
2.1 General
All chemicals were obtained from commercial sources and used without further purification.Luminescent properties of 1 were recorded with a VARIAN Cary Eclipase fluorescence spectrometer.IR spectra of 1 were obtained by a Nicolet 5700 FTIR spectrometer with KBr pellets. Elemental analyses were carried out on a PE 2400II analyzer.
2.2 Synthesis of complex 1
H2pydc (1 mmol, 0.17 g), Eu2O3(0.4 mmol, 0.14 g), H2ox (2 mmol, 0.18 g), ZnSO4·7H2O (0.4 mmol,0.12 g) and distilled water (15 mL) were mixed and sealed in a 50 mL Teflon-lined stainless steel vessel.The mixture was heated under autogenous pressure at 150 ℃ for 3 days, then cooled down to room temperature to get colorless block crystals. Elemental analysis (%) for 1: calcd: C, 21.39; H, 2.04; N,3.40. Found (%): C, 21.18; H, 1.86; N, 3.26. IR (νmax,KBr, cm-1): 3448(vs(-OH)), 2580(v(-CH)), 1739(v(-C=O)), 1645(v(-C=C) + v(-C=N)), 1563(vas(-COO-)),1442(vs(-COO-)), 1368(vs(-COO-)), 1274(v(-C=C)),1134(v(-C=C)), 1081(v(-SO42-)), 779(δ(-CH)),738(δ(-CH)), 601(v(Eu-O)), 422(v(Eu-N)), 324(v(Eu-N))[9,12].
2.3 Structure determination
A suitable colorless block crystal with dimensions of 0.21mm × 0.18mm × 0.15mm was mounted on a glass fiber for data collection which was performed at 296(2) K on a Bruker Smart APEX II CCD diffractometer equipped with a graphite-monochromatized MoKα radiation (λ = 0.71073 Å) by using a φ-ω scan mode. Lorentz, polarization and absorption corrections were applied. The structure was determined by direct methods using the SHELXL 97 programs[13,14]. In the range of 1.70≤θ≤25.00°, a total of 8782 reflections were collected, of which 6105 were unique with Rint= 0.0223. All nonhydrogen atoms were located by direct methods and subsequent difference Fourier syntheses. The hydrogen atoms of water molecules were not located due to the slight disorders. The hydrogen atoms bound to C atoms were located by geometrical calculations,and their positions and thermal parameters were fixed during the structure refinement. All nonhydrogen atoms were refined by full-matrix leastsquares techniques for 4872 observed reflections with I > 2σ(I) to the final R = 0.0351, wR = 0.0949(w = 1/[σ2(Fo2) + (0.0590P)2+ 1.0042P], where P =(Fo2+ 2Fc2)/3), S = 1.045 and (Δ/σ)max= 0.002. The highest and lowest residual peaks in the final difference Fourier map are 1.409 and –0.967 e/Å3,respectively. The selected bond lengths are given in Table 1.
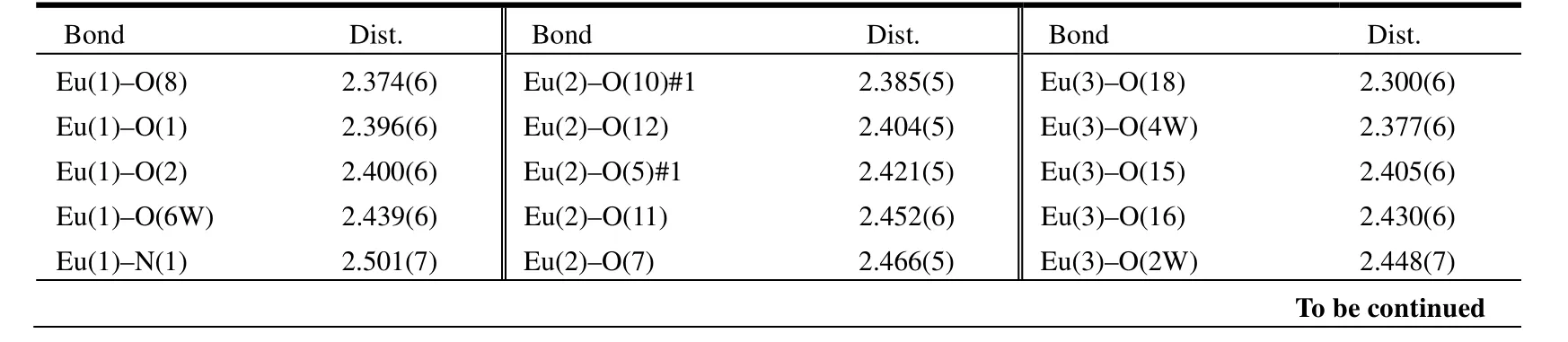
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
Symmetry transformation: #1: –x +2, –y+2, –z +1
3 RESULTS AND DISCUSSION
3.1 Crystal structure of 1
The single-crystal X-ray diffraction analysis reveals that the title complex crystallizes in the triclinic system, space group P1. The asymmetrical unit of 1 consists of three Eu(III) atoms, five pydc2–ligands, one ox2-ligand, two sulfate ligands, five coordinated water molecules and three free water molecules (Fig. 1). The complex exhibits a threenuclear structure. The Eu(1) atom is nine-coordinated by four carboxyl O atoms (O(4), O(5), O(6),O(7)) of two pydc2–, one N atom (N(1)) and two O atoms (O(1), O(2)) of one pydc2–, one O atom (O(8))of one sulfate ion and one coordinated water molecule (O(6W)). The coordination polyhedron around Eu(1) is best described by a distorted tricapped trigonal prismatic geometry where five oxygen atoms (O(1), O(5), O(8), O(2) and O(6)) of the pydc2–groups and one O(6W) oxygen atom occupy the vertices of the trigonal prism, and two oxygen atoms (O(4) and O(7)) and one nitrogen atom (N(1))of the pyridine rings occupy the equatorial plane[15].The twist angle between the two faces of the trigonal prism is 5.789°. Eu(1) is located in the plane formed by N(1) nitrogen atom and O(4), O(7) oxygen atoms of the pyridine rings (Fig. 2a). Eu(2) atom is nine-coordinated by two N atoms (N(2), N(3)) and four O atoms (O(5#1), O(7), O(12), O(14)) of two tridentate pydc2–ligands and three O atoms (O(10),O(10#1), O(11)) of two sulfate ions. The coordination polyhedron around Eu(2) can be best described by a distorted tricapped trigonal prismatic geometry where six oxygen atoms (O(5#1), O(10#1),O(14), O(11), O(12) and O(7)) of the pydc2–groups occupy the vertices of the trigonal prism, and one oxygen atom (O(10)) and two nitrogen atoms (N(2)and N(3)) of the pyridine rings occupy the equatorial plane[15]. The twist angle between the two faces of the trigonal prism is 12.926°. Eu(2) is located in the plane formed by N(2) and N(3) nitrogen atoms and O(10) oxygen atom of the pyridine rings (Fig. 2b).Eu(3) atom is eight-coordinated by two carboxyl O atoms (O(15), O(18)) of two pydc2–, two O atoms(O(16), O(17)) from one ox2-ligand and four coordinated water molecules (O(2W), O(3W),O(4W), O(5W)). The coordination polyhedron around Eu(3) can be described by a distorted bicapped trigonal prismatic geometry where three oxygen atoms (O(15), O(16) and O(18)) from the pydc2–groups and three water molecules (O(2W),O(3W), O(4W)) occupy the vertices of the trigonal prism, and one oxygen atom (O(17)) and one water molecule (O(5W)) occupy the equatorial plane[15](Fig. 2c). The average values for the bond lengths of Eu(1)–O, Eu(2)–O and Eu(3)–O are 2.474, 2.472 and 2.426 Å, respectively. The bond lengths of Eu–N are 2.501(7), 2.520(6) and 2.530(7) Å with an average value of 2.517 Å. The angles of∠O–Eu(1)–O, ∠O–Eu(2)–O and ∠O–Eu(3)–O respectively fall into the ranges of 51.38(16)~149.0(2), 53.75(17)~ 148.72(19) and 66.1(2)~149.6(2)º. Similarly, the angles of ∠N–Eu(1)–O and∠N–Eu(2)–O are within the ranges of 64.1(2)~141.5(2) and 63.43(19)~141.3(2)º, respectively. For all, the bond lengths and bond angles are within the normal ranges[9,12].
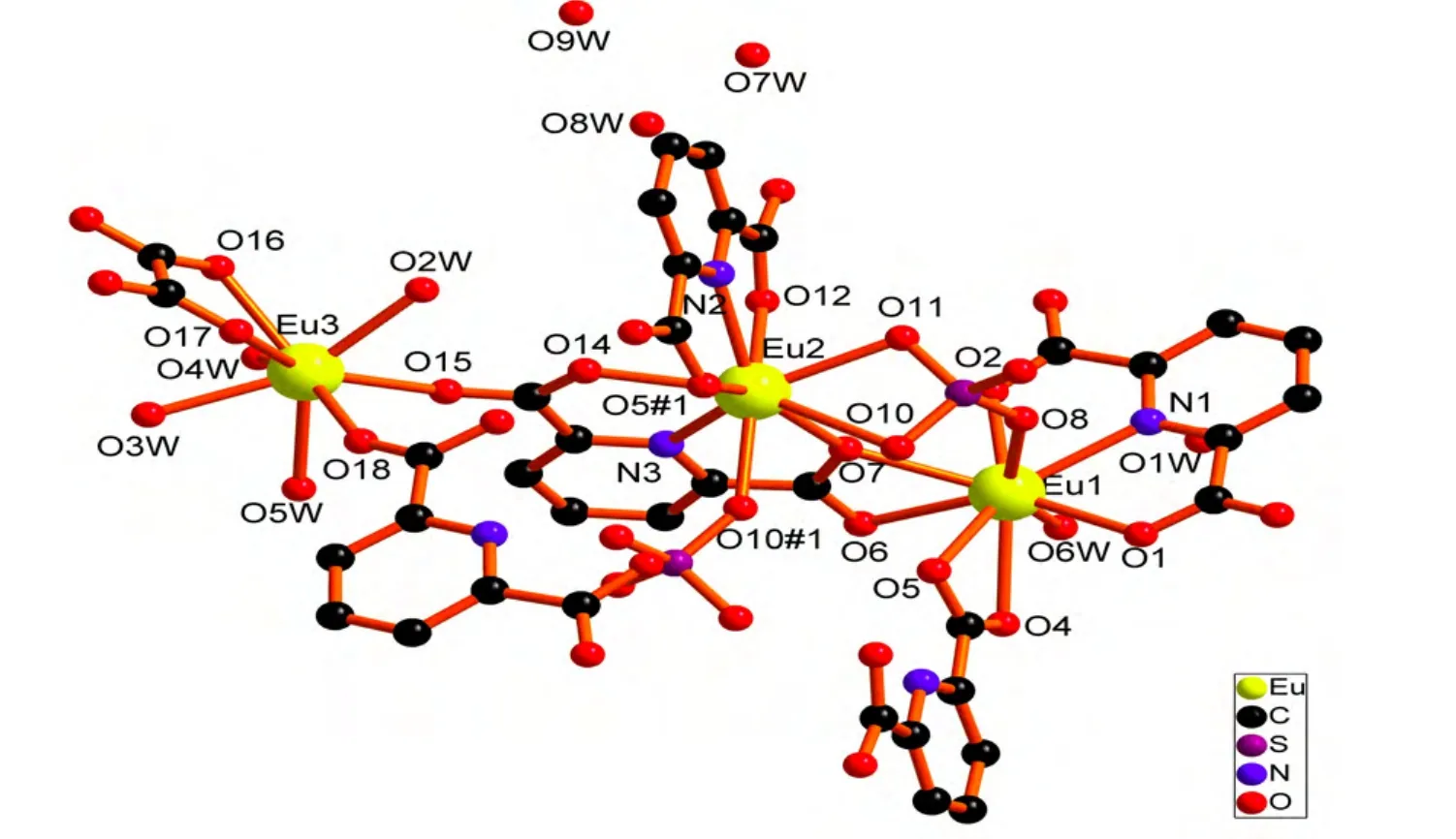
Fig. 1. Molecular structural unit of complex 1. All the H atoms are omitted for clarity. Symmetry code: #1: –x +2, –y + 2, –z + 1
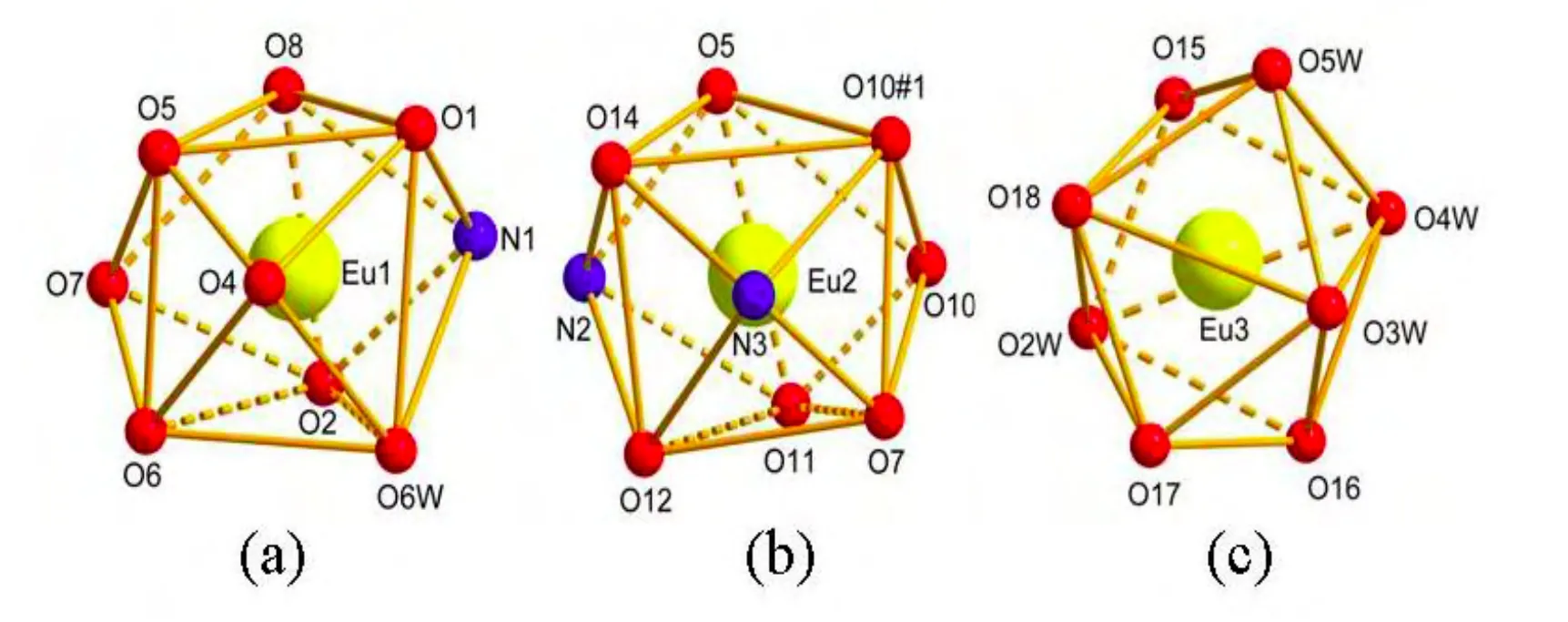
Fig. 2. View of the coordination polyhedron of (a) Eu(1), (b) Eu(2)and (c) Eu(3). Symmetry code: #1: –x + 2, –y + 2, –z + 1
In the title complex, two Eu atoms (Eu(1) and Eu(2)) are connected to each other, forming a dimeric unit A (Fig. 3a); two Eu(3) atoms are also linked together resulting in a dimmer B (Fig. 3b).These units of A and B are further connected with each other, forming a one-dimensional chain structure (Fig. 3c). Then such chains are extended into a two-dimensional layered structure (Fig. 4). In addition, the structure shows π-π interactions with the shortest distance of 3.353 Å among the parallel pyridyl rings of pyridine-2,6-dicarboxylate ligands.By means of the π···π stacking interactions in the layers, a three-dimensional supramolecular structure comes into being.
3.2 Luminescent property
Taking into account the excellent luminescent property of Eu(III) ions in the visible region, we investigated the luminescent property of 1 in the solid state at room temperature. 1 exhibits the characteristic red europium emission upon 394 nm excitation. As shown in Fig. 5, transitions from the excited5D0state to different J-levels of the lower7F state were observed in the emission spectrum (J =1~4). The red-emitting characteristic from the transitions5D0–7Fn(n = 1~4) is centered at 592,616, 651 and 692 nm, respectively. The narrow-band emission at 616 nm is characteristic of the hypersensitive5D0–7F2transition of Eu(III)[7].
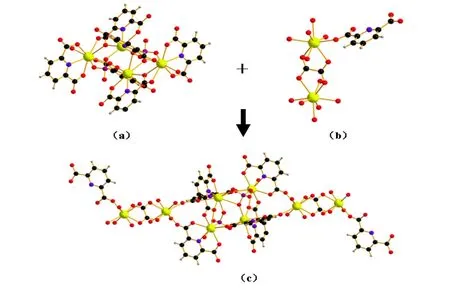
Fig. 3. Dimeric unit A formed by the connecting of two Eu(1) and two Eu(2) atoms (a), the dimeric unit B formed by the connecting of two Eu(3) atoms (b) and one-dimensional chain structure in the structure of 1 (c)
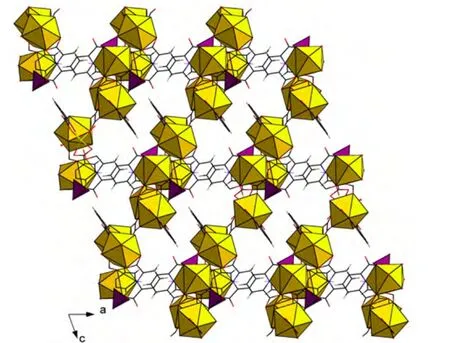
Fig. 4. Two-dimensional layered structure in 1 viewed down the b-axis

Fig. 5. Room-temperature solid-state photoluminescence spectra of complex 1
(1) Trivedi, E. R.; Eliseeva, S. V.; Jankolovits, J.; Olmstead, M. M.; Petoud, S.; Pecoraro, V. L. Highly emitting near-infrared lanthanide “encapsulated sandwich” metallacrown complexes with excitation shifted toward lower energy. J. Am. Chem. Soc. 2014, 136, 1526–1534.
(2) Tang, Q.; Liu, S. X.; Liu, Y. W.; He, D. F.; Miao, J.; Wang, X. Q.; Ji, Y. J.; Zheng, Z. P. Color tuning and white light emission via in situ doping of luminescent lanthanide metal-organic frameworks. Inorg. Chem. 2014, 53, 289–293.
(3) Wei, X. H.; Yang, L. Y.; Liao, S. Y.; Zhang, M.; Tian, J. L.; Du, P. Y.; Gu, W.; Liu, X. A series of rare earth complexes with novel non-interpenetrating 3D networks: synthesis, structures, magnetic and optical properties. Dalton Trans. 2014, 43, 5793–5800.
(4) Biswas, S.; Jena, H. S.; Goswami, S.; Sanda, S.; Konar, S. Synthesis and characterization of two lanthanide (Gd3+and Dy3+)-based three-dimensional metal organic frameworks with squashed metallomacrocycle type building blocks and their magnetic, sorption, and fluorescence properties study. Cryst. Growth Des. 2014, 14, 1287–1295.
(5) Yang, S.; Nie, K.; Zhang, Y.; Xue, M. Q.; Yao, Y. M.; Shen, Q. New [ONOO]-type amine bis(phenolate)ytterbium(II) and -(III) complexes: synthesis, structure, and catalysis for highly heteroselective polymerization of rac-lactide. Inorg. Chem. 2014, 5, 105–115.
(6) Li, L.; Wu, C. J.; Liu, D. T.; Li, S. H.; Cui, D. M. Binuclear rare-earth-metal alkyl complexes ligated by phenylene-bridged β-diketiminate ligands:synthesis, characterization, and catalysis toward isoprene polymerization. Organometallics 2013, 32, 3203–3209.
(7) Tang, R. R.; Gu, G. L.; Zhao, Q. Synthesis of Eu(III) and Tb(III) complexes with novel pyridine dicarboxamide derivatives and their luminescence properties. Spectrochim. Acta A 2008, 71, 371–376.
(8) Feng, W.; Zhang, Y.; Lü, X.; Hui, Y.; Shi, G.; Zou, D.; Song, J.; Fan, D.; Wong, W. K.; Richard, A. J. Near-infrared (NIR) luminescent homoleptic lanthanide Salen complexes Ln4(Salen)4(Ln = Nd, Yb or Er). Cryst. Eng. Comm. 2012, 14, 3456–3463.
(9) Xu, J.; Su, W. P.; Hong, M. C. A series of lanthanide secondary building units based metal-organic frameworks constructed by organic pyridine-2,6-dicarboxylate and inorganic sulfate. Cryst. Growth Des. 2011, 11, 337–346.
(10) Yang, L. R.; Song, S.; Shao, C. Y.; Zhang, W.; Zhang, H. M.; Bu, Z. W.; Ren, T. G. Synthesis, structure and luminescent properties of 3D lanthanide(La(III), Ce(III)) coordination polymers possessing 1D nanosized cavities based on pyridine-2,6-dicarboxylic acid. Synth. Met. 2011, 161,1500–1508.
(11) Zhu, T. Y.; Ikarashi, K.; Ishigaki, T.; Uematsu, K.; Toda, K.; Okawa, H.; Sato, M. Structure and luminescence of sodium and lanthanide(III)coordination polymers with pyridine-2,6-dicarboxylic acid. Inorg. Chim. Acta 2009, 362, 3407–3414.
(12) Çolak, A. T.; Yeşilel, O. Z.; Büyükgüngőr, O. Synthesis, structural characterization of zinc(II)-pyridine-2,5-dicarboxylate complexes and self-assembled 1D water cluster in a supramolecular architecture. Polyhedron 2010, 29, 2127–2133.
(13) Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution. University of Göttingen, Göttingen (Germany) 1997.
(14) Sheldrick, G. M. SHELXL-97, Program for the Refinement of Crystal Structures. University of Göttingen, Göttingen (Germany) 1997.
(15) Mei, C. Z; Wang, H. R.; Xiong, H. L.; Meng, R. J.; Shan, W. W.; Li, H. H. Hydrothermal synthesis and crystal structure of new 3D coordination polymer of nickel(II) achieved from 5-iodo-isophthalic acid ligand. Chin. J. Struct. Chem. 2014, 33, 563–568.
- 结构化学的其它文章
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis and Crystal Structure of Cobalt(II) and Copper(II) Complexes Involving L-Aamino Alcohols
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①

