Syntheses and Crystal Structures of Two Coordination Polymers of Cobalt and Cadmium Assembled by 4,4΄-(Ethane-1,2-diyldioxy)dibenzoic Acid①
WANG Qing-Wei WANG Y-Nn QI Xio-Fei LI Xiu-Mei LIU Bo
a (Key Laboratory of Preparation and Applications of Environmental Friendly Materials(Jilin Normal University), Ministry of Education, Siping, Jilin 136000, China)
b (Faculty of Chemistry, Tonghua Normal University, Tonghua, Jilin 134002, China)
1 INTRODUCTION
Recently, the design and synthesis of coordination compounds have attracted much attention due to their diversity structures[1]and potential applications[2~7]. A successful strategy for preparing coordination compounds is the assembly reaction between a transition metal ion and two types of organic ligands, one acting as a terminal ligand and the other as a bridging ligand. In this aspect, the chelting ligand phen and bridging ligand bib have been widely used in the construction of metal-organic coordination polymers[8]. In addition, as a versatile ligand, 4,4΄-(ethane-1,2-diyldioxy)dibenzoic acid(H2eoba) has been hardly studied to construct coordination compounds containing transition metals.
The hydrothermal technique is well suited to the preparation of crystals of synthetic minerals, new inorganic materials, and organometallic coordination polymers. Of particular interest to us is the construction of transition metal polymers with new structural features by utilizing hydrothermal synthesis.
To explore the combination effects of this neutral ligand (phen, bib) and anionic ligands, we synthesize coordination polymers of Co(II) and Cd(II) containing these ligands. Herein, we report the preparation and crystal structure of the title compounds[Co0.5(Heoba)(bib)0.5]2n(1) and [Cd(eoba)(phen)]n(2), which executively exhibit a 2D and a 3D network structures.
2 EXPERIMENTAL
2.1 General procedures
All materials were commercially purchased and used without further purification. Infrared spectra(KBr pellets) were taken on a Perkin-Elmer 2400LS II spectrometer and elemental analyses for C, H and N were performed on a Perkin-Elmer 240C analyzer.The TG studies were performed on a Perkin-Elmer TGA7 analyzer. The fluorescent studies were carried out on a computer-controlled JY Fluoro-Max-3 spectrometer at room temperature.
2.2 Synthesis
[Co0.5(Heoba)(bib)0.5]2n(1). A mixture of Co(OAc)2·4H2O (0.2 mmol, 0.049 g), H2eoba (0.2 mmol, 0.060 g), bib (0.2 mmol, 0.038 g) and H2O(18 mL) was sealed in a 30 mL Teflon-lined autoclave under autogenous pressure at 150 ℃ for five days. After cooling to room temperature, pink block crystals were collected by filtration and washed with distilled water in 36% yield. Anal. Calcd. (%) for C42H40CoN4O12: C, 59.23; H, 4.73; N, 6.58. Found(%): C, 59.01; H, 4.58; N, 6.41. IR (KBr, cm-1):3444w, 3140w, 2941w, 1602s, 1568m, 1509w,1419w, 1393m, 1300w, 1247s, 1173m, 1141w,1107w, 1063w, 1014w, 937w, 855w, 815w, 786m,701w, 661w, 630w, 534w, 505w.
[Cd(eoba)(phen)]n(2) A mixture of Cd(OAc)2·2H2O (0.2 mmol, 0.053 g), H2eoba (0.4 mmol, 0.12 g), phen (0.2 mmol, 0.036 g) and H2O (18 mL) was sealed in a 30 mL Teflon-lined autoclave under autogenous pressure at 150 ℃ for seven days. After cooling to room temperature, yellow block crystals were collected by filtration and washed with distilled water in 42% yield (based on Cd). Anal. Calcd. (%)for C28H20CdN2O6: C, 56.72; H, 3.40; N, 4.73.Found (%): C, 56.47; H, 3.19; N, 4.26. IR (KBr,cm-1): 3437w, 2929w, 1602s, 1531s, 1406s, 1299w,1243s, 1173m, 1143w, 1107w, 1064w, 958w, 862w,847w, 785m, 729w, 702w, 716m, 666w, 527w.
2.3 Structure determination
Single crystal diffraction data of 1 and 2 were respectively collected on a Bruker SMART APEXCCD diffractometer equipped with a graphite-monochromatic MoKα (λ = 0.71073 Å) radiation at room temperature. The structure was solved by direct methods with SHELXS-97 program[9]and refined by full-matrix least-squares techniques on F2with SHELXL-97[10]. All non-hydrogen atoms were refined anisotropically and the hydrogen atoms of organic ligands were generated geometrically. The selected bond parameters are given in Table 1.
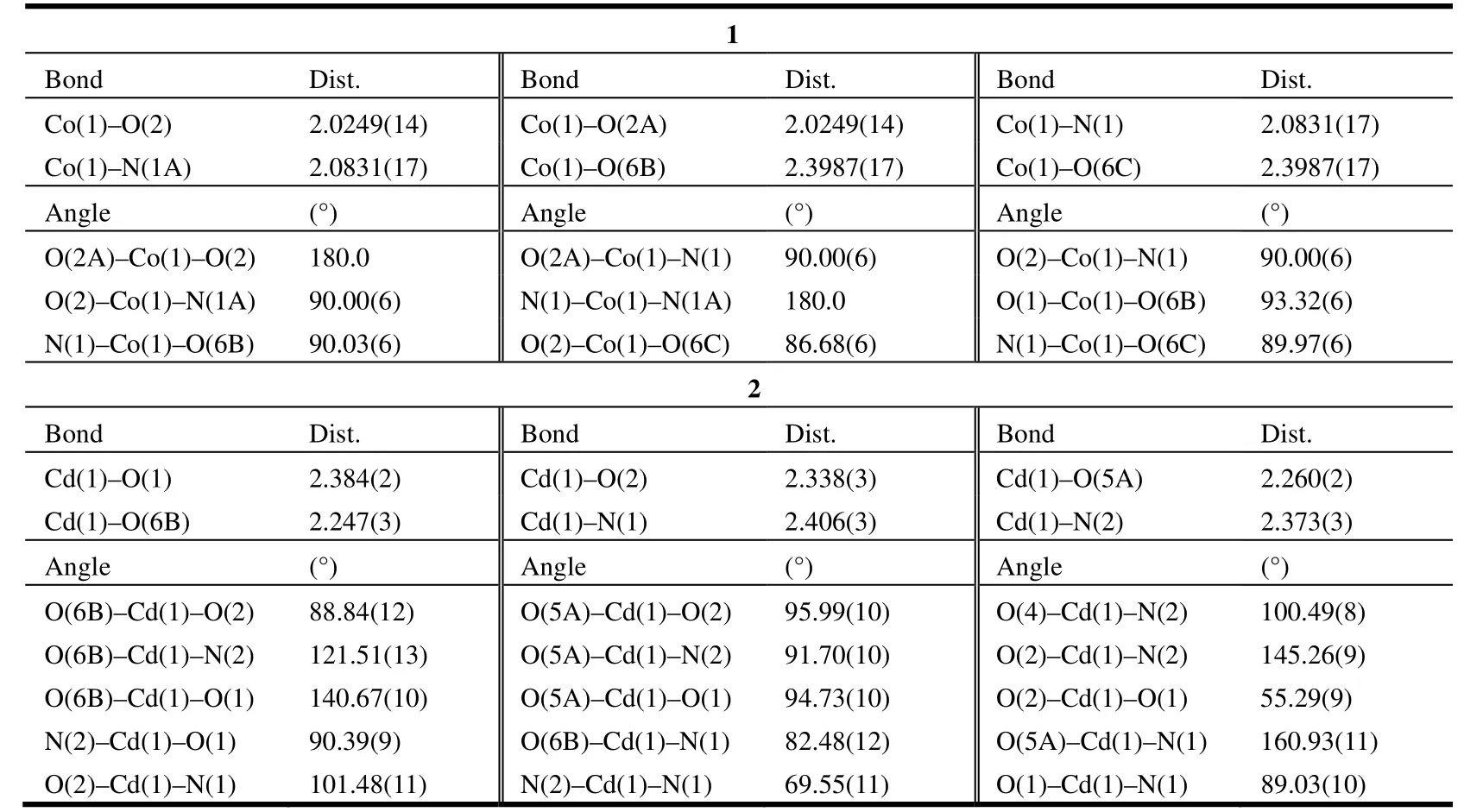
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Compounds 1 and 2
Crystal data for 1: C42H40CoN4O12, monoclinic,space group P21/n, Mr= 851.71, a = 8.9878(17), b =19.037(3), c = 11.902(2) Å, β = 111.144(3)º, V =1899.3(6) Å3, Z = 2, F(000) = 886, μ(MoKα) = 0.525 mm-1, Dc= 1.489 g/cm3, 10254 reflections measured,3732 unique (Rint= 0.0260), 2756 observed reflections with I > 2σ(I), R = 0.0395, wR = 0.0924 and S = 1.012.
Crystal data for 2: C28H20CdN2O6, orthorhombic,space group Fdd2, Mr= 592.86, a = 22.0122(9), b =44.1020(17), c = 9.8888(4) Å, V = 9599.9(7) Å3, Z =16, F(000) = 4768, μ(MoKα) = 0.958 mm-1, Dc=1.641 g/cm3, 12984 reflections measured, 4582 unique (Rint= 0.0270), 3986 observed reflections with I > 2σ(I), R = 0.0278, wR = 0.0491 and S =1.016.
3 RESULTS AND DISCUSSION
3.1 IR spectrum
Complex 1: The COO–is coordinated with its asymmetric and symmetric stretching appearing at 1602 cm–1(v(OCO)assym) and 1393 cm–1(v(OCO)sym)[11], respectively. The Δν (ν(OCO)assym–ν(OCO)sym) is 209 cm–1(> 200), showing the presence of monodentate linkage of carboxylates in the dianions. Thus the carboxylates coordinate to the metal as monodentate ligands via the carboxylate groups[12].
Complex 2: The COO–is coordinated with its asymmetric and symmetric stretching appearing at 1602 cm–1(v(OCO)assym) and 1406 cm–1(v(OCO)sym)[11], respectively. The Δν (ν(OCO)assym–ν(OCO)sym) is 196 cm–1(< 200), so bidentate linkages of carboxylates are present in the dianions.Therefore, the carboxylates coordinate to the metal as bidentate ligands via the carboxylate groups[12].
In addition, X-ray diffraction analysis further indicates the monodentate coordination manners of carboxylate groups in 1 and bidentate coordination manners in 2.
3.2 Description of the structure
Complex 1 crystallizes in P21/n space group. The coordination environment of Co(II) in 1 is shown in Fig. 1. There are half Co(II) ion, one Heoba ligand and half bib ligand in the asymmetric unit. Each Co(II) ion is six-coordinated by four carboxylate oxygen atoms (O(2), O(2A), O(6B) and O(6C)) from four different Heoba ligands and two nitrogen donors(N(1) and N(1A)) from two flexible bib molecules to furnish a slightly distorted octahedral coordination architecture. The bond distances of Co–O in compound 1 fall in the 2.0249(14)~2.3987(17) Å range, and Co–N bond length is 2.0831(17) Å,which are in the normal ranges[13]and the coordination angles around the Co atom vary from 86.68(6)to 180.00(8)º. In the coordination environment, the four carboxylate oxygen atoms (O(2), O(2A), O(6B)and O(6C)) are located in the basal plane, whereas two imidazole nitrogen atoms (N(1), N(1A)) occupy the axial positions from the opposite directions. In the crystal structure of complex 1, the bib ligand adopts a trans-conformation bridging mode with a dihedral angle between the two imidazole rings of 0º.The protonated Heoba ligands display one kind of coordination mode, namely monodentate bridging mode, and link the Co(II) ions to form a one-dimensioal chain. The distance of neighboring Co(II)ions is about 17.619 Å as illustrated in Fig. 2. The neighboring chains are bridged by bib ligands to afford a 2D network with (4,4) topology (Fig. 3).The Co···Co distances separated by the bib ligands are 12.053 Å.

Fig. 1. Coordination environment of Co(II) in complex 1

Fig. 2. 1D chain-like structure viewed along the a axis in 1
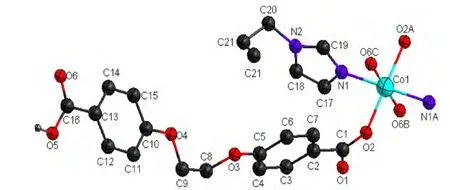
Fig. 3. View of the two-dimensioal network construced from [Co4(Heoba)2(bib)2] units
Complex 2: Single-crystal X-ray diffraction analysis reveals that complex [Cd(eoba)(phen)]n2 crystallizes in Fdd2 space group. The coordination environment of Cd(II) is shown in Fig. 4. There are one Cd(II) ion, one eoba ligand and one phen ligand in the asymmetric unit. The Cd(1) ion is six-coordinated by four carboxylate oxygen atoms (O(1),O(2), O(5A), O(6B)) from three different eoba ligands and two nitrogen atoms (N(1), N(2)) from the phen ligand, showing a distorted octahedral geometry. The N(1) and O(5A) atoms locate at the apex sites, while three carboxylate oxygen atoms and the other nitrogen atom (O(1), O(2), O(6B),N(2)) define an equatorial plane. The bond distances of Cd–O in complex 2 fall in the range of 2.247(3)~2.384(2) Å, and those of Cd–N in 2.373(3)~2.406(3)Å. The coordination angles around the Cd atom are in the 55.29(9)~160.93(11)º range.
Two coordination modes of the eoba ligand are present in the structure of compound 2, namely bidentate bridging mode and bidentate chelating mode. Based on this, two Cd(II) ions are linked by eoba ligands to form binuclear subunits, which are connected into a three-dimensional network structure (Fig. 5) by eoba ligands again.
Moreover, there are π-π interactions in complex 2 between phen ligands. The centroid-to-centroid distance between the adjacent sphen is 3.770 Å with the perpendicular distances to be 3.622 Å and the dihedral angle of 1.66°. These supramolecular interactions together with the coordinate-covalent interactions between metal ions and organic ligands strengthen the stability of the network structure.
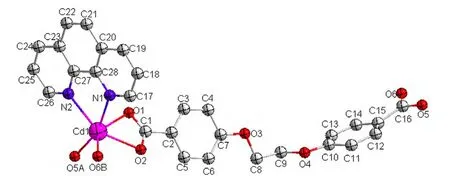
Fig. 4. Coordination environment of Cd(II) in complex 2

Fig. 5. Perspective view of the 3D network along the c axis
3.3 Thermal analysis
TG curve of 1 (Fig. 6) shows that the first weight loss of 68.9% from 215 to 365 ℃ corresponds to the removal of Heoba ligands (calcd: 70.7%). Upon further heating, an obvious weight loss (23.1%)occurs in the temperature range of 365~650 ℃,corresponding to the release of bib ligands (calcd.:22.3%). Above 650 ℃, no weight loss is observed,which means the complete decomposition of 1. The residual weight should be CoO.
TG curve of 2 (Fig. 7) shows that the first weight loss of 50.1% from 34 to 334 ℃ corresponds to the removal of eoba ligands (calcd.: 50.6%). Upon further heating, an obvious weight loss (28.6%) occurs in the temperature range of 334~1045 ℃ due to the release of phen ligand (calcd.: 30.4%). After 1045 ℃ no weight loss is observed, which means the complete decomposition of 2. The residual weight should be CdO.
3.4 Luminescence property
The solid-state photoluminescence spectra of 2,free H2eoba and phen ligands were investigated at room temperature. Excited by 273 nm, coordination polymer 1 gives wide purple emission with the maximum peak at 420 nm (Fig. 8). However, no obvious emission bands are observed for the free H2eoba and phen ligand in the range of 400~800 nm under the same experimental conditions. The significant phenomenon of the fluorescenc emission of 2 here could be tentatively assigned to the ligandto-metal charge transfer (LMCT)[14]. As it possesses strong fluorescent intensity, complex 2 appears to be good candidates for novel hybrid inorganic-organic photoactive materials.
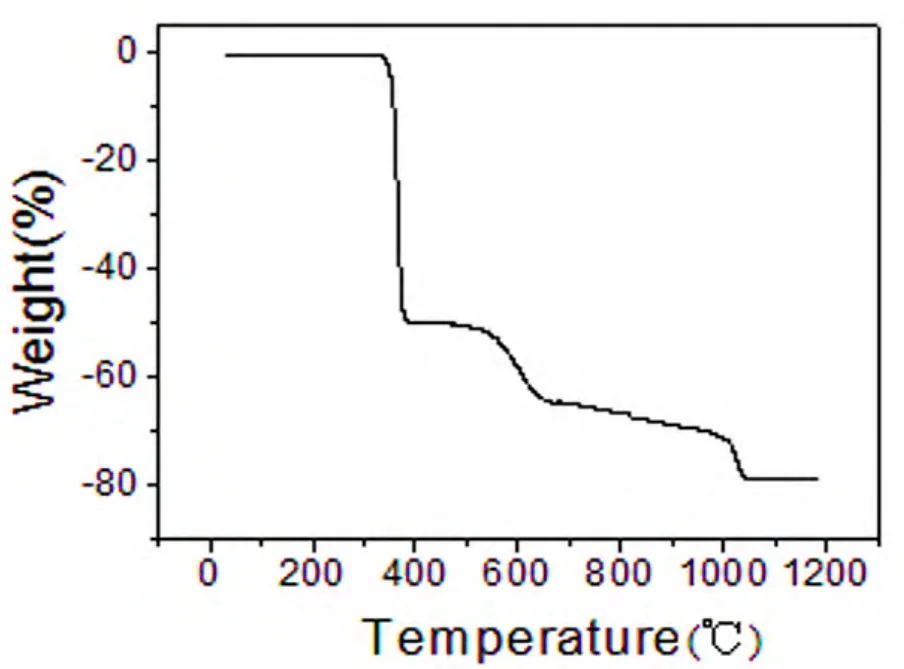
Fig. 7. TG curve of the title complex 2
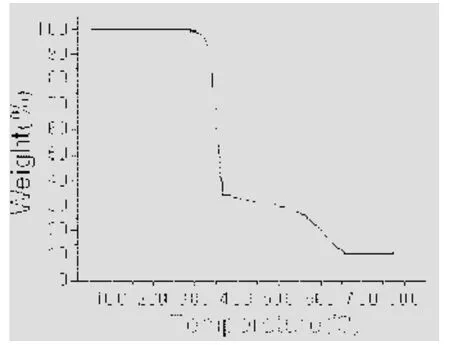
Fig. 6. TG curve of the title complex 1
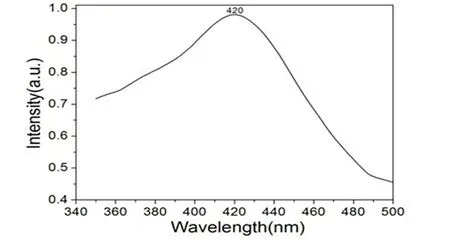
Fig. 8. Solid-state emission spectrum of 2 at room temperature
(1) (a) Moulton, B.; Zaworotko, M. J. From molecules to crystal engineering:supramolecular isomerism and polymorphism in network solids.Chem. Rev. 2001, 101, 1629–1658.(b)Eddaoudi, M.; Moler, D. B.; Li, H.; Chen, B.; Reineke, T. M.; O’Keeffe, M.; Yaghi, O. M. Modular chemistry:secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330.
(2) Sun, D. F.; Cao, R.; Liang, Y. C.; Shi, Q.; Su, W. P.; Hong, M. C. Hydrothermal syntheses, structures and properties of terephthalate-bridged polymeric complexes with zig-zag chain and channel structures. Chem. Soc., Dalton Trans. 2001, 2335–2340.
(3) Kobel, W.; Hanack, M. Bis axially coordinated (phthalocyaninato)ruthenium(II) compounds. Inorg. Chem. 1986, 25, 103–107.
(4) Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O'Keeffe, M.; Yaghi, O. M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472.
(5) Noro, S. I.; Kitagawa, S.; Kondo, M.; Seki, K. A new, methane adsorbent, porous coordination polymer [{CuSiF6(4,4΄-bipyridine)2}n]. Angew.Chem., Int. Ed. 2000, 39, 2081–2084.
(6) Chen, B. L.; Eddaoudi, M.; Hyde, S. T.; O'Keeffe, M.; Yaghi, O. M. Interwoven metal-organic framework on a periodic minimal surface with extra-large pores. Science 2001, 291, 1021–1023.
(7) Seo, J. S.; Whang, D.; Lee, H.; Sung, I. J.; Jinho, O. A homochiral metal-organic porous material for enantioselective separation and catalysis.Nature 2000, 404, 982–986.
(8) (a) Li, X. M.; Wang, Q. W.; Liu, B.; Wang, Z. T.; Zhou, S. Hydrothermal synthesis and crystal structure of a zinc(II) polymer with a two-dimensional layer structure: [Zn(pzdc)(phen)]n·nH2O. Chin. J. Struct. Chem. 2009, 10, 1257–1260.(b) Li, X. M.; Wang, Q. W.; Cui, Y. C.; Li, C. B.; Liu, B.; Sun, L. J. Hydrothermal synthesis and crystal structure of a new manganese(II) complex with 2,4-pyridine-dicarboxylic acid and 2,2´-bipyridine ligands. Chin. J. Struct. Chem. 2009, 10, 1317–1320.(c) Li, X. M.; Wang, Q. W.; Liu, B.; Wang, Z. T. Hydrothermal synthesis and crystal structure of a Mn(II) coordination polymer assembled by 5-nitroisophthalic acid and 1,10-phenanthroline. Chin. J. Inorg. Chem. 2011, 6, 1207–1211.(d) Li, X. M.; Ji, J. Y.; Niu, Y. L.; Wang, Q. W.; Liu, B.; Wang, Z. T. Hydrothermal synthesis and crystal structure of a 2D network Mn(II)coordination polymer based on oxalic acid and bis(imidazol) ligands. Chin. J. Inorg. Chem. 2013, 6, 1302–1306.(e) Liu, B.; Guo, J.; Zhou, S.; Wang, Q. W.; Li, X. M.; Li, C. B. Synthesis and crystal structure of a two-dimensional coordination polymer constructed by thiophene-2,5-dicarboxylic acid and 1,4-bis(imidazol-1-ylmethyl)-butane. Chin. J. Struct. Chem. 2013, 2, 199–204.
(9) Sheldrick, G. M. SHELXS 97, Program for the Solution of Crystal Structure. University of Göttingen, Germany 1997.
(10) Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure. University of Göttingen, Germany 1997.
(11) Devereux, M.; Shea, D. O.; Kellett, A.; McCann, M.; Walsh, M.; Egan, D.; Deegan, C.; Kędziora, K.; Rosair, G.; Müller-Bunz, H. Synthesis, X-ray crystal structures and biomimetic and anticancer activities of novel copper(II)benzoate complexes incorporating 2-(4΄-thiazolyl)benzimidazole(thiabendazole), 2-(2-pyridyl)benzimidazole and 1,10-phenanthroline as chelating nitrogen donor ligands. Inorg. Biochem. 2007, 101, 881-892.
(12) Bellamy, L. J. The Infrared Spectra of Complex Molecules. Wiley, New York 1958.
(13) Li, X. M.; Guo, J.; Wang, Q. W.; Ji, J. Y.; Wang, Z. T. Hydrothermal synthesis and crystal structure of a Co(II) coordination polymer assembled by 2,4-pyridinedicarboxylic acid and 1,4-bis(imidazol-1-ylmethyl)-benzene) ligands. Chin. J. Inorg. Chem. 2012, 7, 1520–1524.
(14) Fu, Z. Y.; Wu, X. T.; Dai, J. C.; Hu, S. M.; Du, W. X.; Zhang, H. H.; Sun, R. Q. The structure and fluorescence properties of two novel mixed-ligand supramolecular frameworks with different structural motifs. Eur. J. Inorg. Chem. 2002, 2002, 2730–2735.
- 结构化学的其它文章
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis and Crystal Structure of Cobalt(II) and Copper(II) Complexes Involving L-Aamino Alcohols
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①

