A New Porous Cadmium(II) Complex Based on 1,10-Phenanthroline Derivative and Dicarboxylate:Syntheses, Structure, Thermal Behavior and Luminescence①
GUO Sheng-Nan HU Yong-Jiao LIU Hong-Jie AN Xue ZOU Ting KONG Zhi-Guo ②
a (Key Laboratory of Preparation and Applications of Environmental Friendly Materials, Jilin Normal University, Ministry of Education, Siping 136000, China)
b (Department of Chemistry, Jilin Normal University, Siping 136000, China)
1 INTRODUCTION
In recent years, the construction of coordination compounds has developed rapidly because of their interesting crystal packing motifs and potential applications as functional materials[1-6]. In this regard, coordination compounds with porous structures are an interesting target for chemical synthesis and crystal engineering owing to their great potential applications in gas adsorption and environment processes[7-10]. Usually, these porous materials were constructed by various organic ligands containing pyridine rings and dicarboxylate as spacers and metal cores as connectors[11-13].
It is well-known that a carboxylate group can bridge metal ions to give rise to a wide variety of complexes ranging from discrete entities to threedimensional (3D) systems[14-16]. In these complexes,a carboxylate group can show various types of bridging conformations[17-19]. In this regard, the versatile 1,1΄-biphenyl-2,2΄-dicarboxylic acid(H2bpdc) is a good ligand for the construction of helical and porous coordination compounds. The H2bpdc ligand possesses the following characteristics. Firstly, upon coordination as a bridge, the freedom of the ligand to rotate around the C–C single bond is restrained and the ligand can be locked in a twisted chiral conformation. Secondly, the two phenyl rings are severely twisted across the C–C single bond, and the skew coordination orientation of carboxylate groups provides the potential of the formation of a helix and porous motifs.
Based on the above consideration, a new porous cadmium(II) complex was synthesized based on H2bpdc and 1,10-phenanthroline derivative 11-fluoro-dipyrido[3,2-a:2΄,3΄-c]phenazine (L), namely, 1.Its structure has been determined by single-crystal X-ray diffraction analysis. In addition, its thermal behavior and luminescent property are also studied.
2 EXPERIMENTAL
2.1 Generals
All the materials were of analytical reagent grade and used as received without further purification.Thermal stability experiment was performed on a TG SDT2960 thermal analyzer from room temperature to 800 ℃ under a nitrogen atmosphere at a heating rate of 10 ℃/min. Elemental analysis was carried out with a Perkin-Elmer 240C analyzer. IR spectrum was performed on a Perkin-Elmer Spectrum One FTIR spectrometer. The photoluminescent properties were measured on a FLSP920 Edinburgh Fluorescence Spectrometer. The powder X-ray diffraction (PXRD) data of the samples were collected on a Rigaku Dmax 2000 X-ray diffractometer equipped with graphite-monochromatized CuKα radiation (λ = 0.154 nm) in the 5≤2θ≤50° range.
2.2 Synthesis and crystal growth
1 was synthesized hydrothermally in a 23 mL Teflon-lined autoclave by heating a mixture of H2bpdc (1 mmol), Cd(NO3)2·4H2O (1 mmol) and L(1 mmol) in 10 mL of water at 170 ℃ for 6 days.After the mixture had been cooled to room temperature at a rate of 10 ℃·h-1, crystals of 1 were collected in 29% yield based on cadmium. Anal.Calcd. for C50H29F2N8O7Cd (%): C, 59.80; H, 2.91;N, 11.16. Found (%): C, 59.24; H, 2.78; N, 10.95. IR(KBr, cm-1): ν = 3414(s), 1621(m), 1572(m),1493(w), 1397(m), 1358(w), 1223(w), 1128(s),1003(w), 962(w), 859(w), 830(w), 753(w), 737(w),619(m), 540(w).
2.3 X-ray structure determination
A single crystal with dimensions of 0.28mm ×0.22mm × 0.18mm was chosen and mounted on a Bruker-AXS Smart CCD diffractometer equipped with a graphite-monochromatized MoKα (λ =0.71073 Å) radiation by using an ω-φ scan method at 293(2) K. Out of the 24694 total reflections collected in the 1.77≤θ≤26.08º range, 4426 were independent with Rint= 0.0423, of which 3829 were considered to be observed (I > 2σ(I)) and used in the succeeding refinement. The structure was solved by direct methods with SHELXS-97 program[20]and refined with SHELXL 97[21]by full-matrix leastsquares techniques on F2. All H atoms were positioned geometrically (C–H = 0.93 Å) and refined as riding, with Uiso(H) = 1.2Ueq(carrier). The water H atoms were not included in the model. The final R =0.0566 and wR = 0.1585 (w = 1/[σ2(Fo2) +(0.0931P)2+ 2.7238P], where P = (Fo2+ 2Fc2)/3). S= 1.130, (Δρ)max= 0.642, (Δρ)min= –0.577 e/Å3and(Δ/σ)max= 0.000.
3 RESULTS AND DISCUSSION
3.1 Description of the crystal structure
Selected bond lengths and bond angles for the compound are given in Table 1. X-ray crystallographic analysis reveals the asymmetric unit of 1 is composed up of one Cd(II) atom, one L ligand, half a bpdc anion, and two halves and two fourth water molecules (Fig. 1). Each Cd(II) atom is six-coordinated by two carboxylate oxygen atoms (O(1) and O(1i)) from two different bpdc anions, and four nitrogen atoms (N(1), N(2), N(1i) and N(2i)) from two distinct L ligands in a distorted octahedral coordination sphere. The atoms N(2), N(1i), N(2i) and O(1i) constitute the basal plane of the octahedron,while N(1) and O(1) are located at the axial positions. The Cd(1)–O(1) bond distance is 2.221(5)Å, and the Cd–N distances vary from 2.363(4) to 2.369(5) Å. Each bpdc anion chelates with one Cd(II)atom in a bidentate mode. The dihedral angle is ca.56.9obetween phenyl rings C(20)~C(25) and C(20i)~C(25i). It is noteworthy that there exist π-π interactions among L ligands of neighboring discrete molecules [Cd(bpdc)(L)2]·3H2O (1), resulting in an interesting 1D supramolecular chain (Fig. 2). Moreover, adjacent 1D supramolecular chains are packed with each other to give a porous structure, in which an infinite channel is filled with water molecules(Fig. 3). Calculations with PLATON showed that the effective solvent-accessible volume in 1 is 743.2 Å3per unit cell, being 16.68% of the crystal volume.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
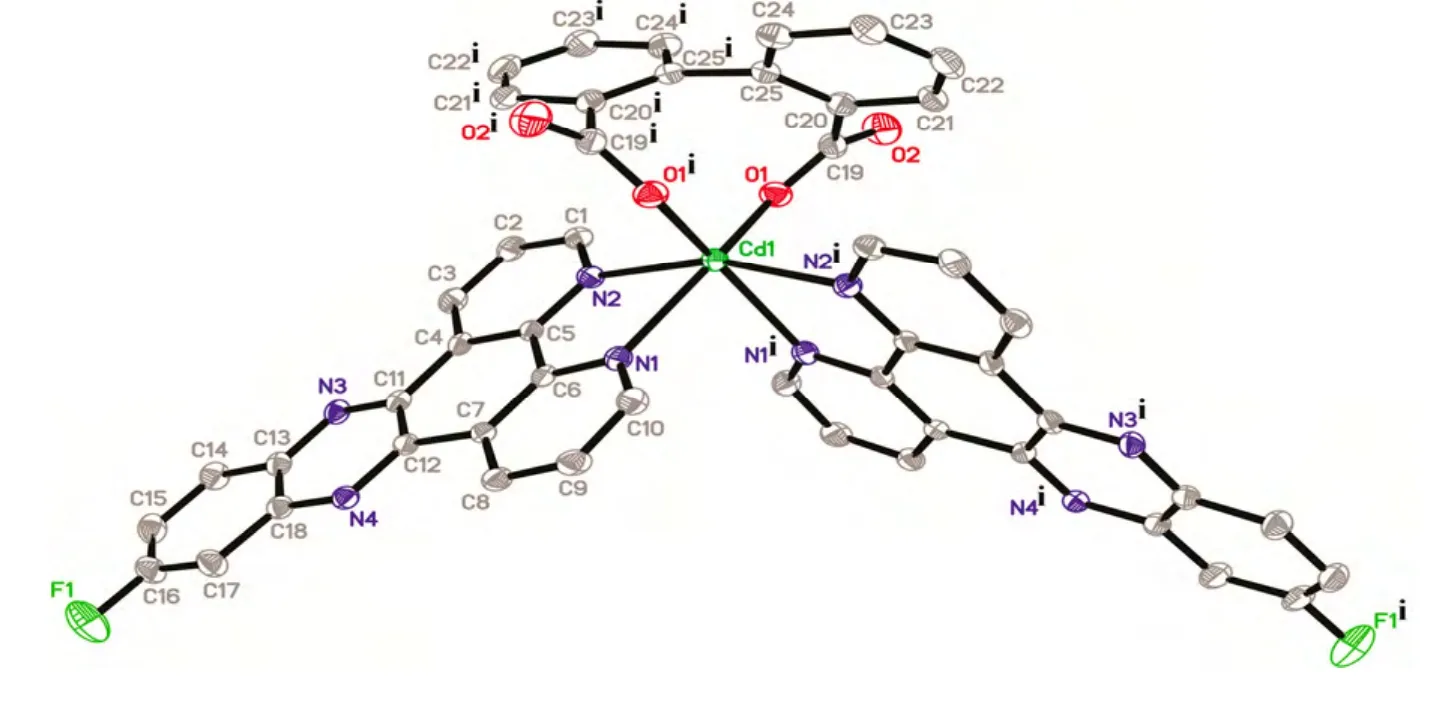
Fig. 1. View of the coordination environments of Cd(II) atoms in complex 1
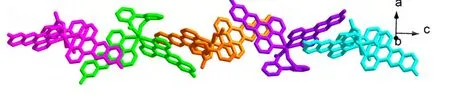
Fig. 2. View of the one-dimensional supramolecular chain of the title complex
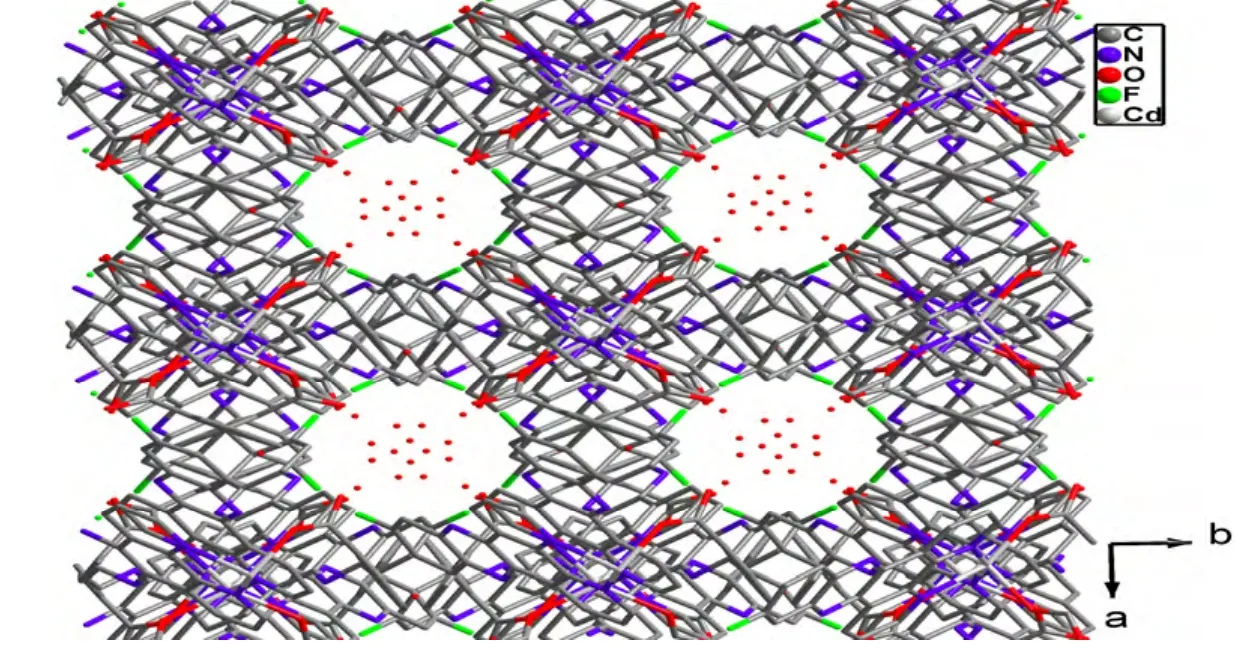
Fig. 3. View of the porous packing structure of the title complex
3.2 IR analysis
As shown in Fig. 4, the solid-state IR spectrum of 1 was performed in the region of 4000~400 cm–1.The broad band at 3414 cm-1corresponds to the vibrations of water molecules of compound 1. The peaks at 1397 and 1128 cm-1can be attributed to the C–N and C=N stretching vibrations of L ligand[19].The asymmetric and symmetric vibrations of carboxylate groups of the bpdc anions were observed at 1621 and 1572 cm–1, respectively.
3.3 PXRD patterns and thermal behavior
As shown in Fig. 5, the experimental PXRD patterns of 1 correspond well to the simulated ones,indicating the phrase purity. For the water-con-
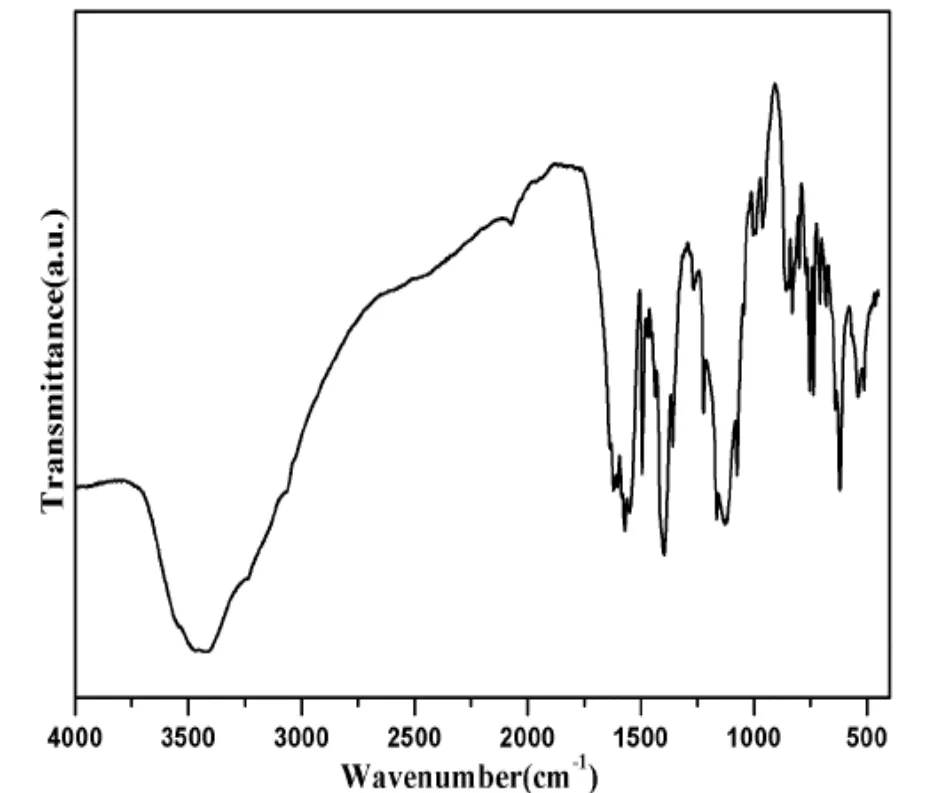
Fig. 4. IR spectrum of complex 1
3.4 Luminescent property
It is well-known that Cd-containing coordination compounds show luminescent properties, and have potential applications in chemical sensors, photochemistry and electroluminescent (EL) display[22]. In this work, the luminescent properties of the free organic ligands H2bpdc, L and compound 1 have been studied in the solid state at room temperature.As shown in Fig. 7, the free H2bpdc and L display emissions at 505 and 491 nm, respectively (λex=325 nm), which can be assigned to the π*-n or π*-π transitions. Compound 1 shows an emission centered at about 499 nm (λex= 325 nm). The resemblance of the emission spectrum of compound 1 with those of free H2bpdc and L indicates that the luminescence of taining compound 1, its thermal behavior was carried out under N2atmosphere with a heating rate of 10 ℃/min in temperature range of 24~800 ℃.As illustrated in Fig. 6, the first weight loss of 5.2%between 38 and 224 ℃ can be assigned to the removal of free water molecule (calcd. 5.4%). The second weight loss corresponds to the release of one dpdc ligand from 286 to 464 ℃ (obsd. 22.4%, calcd.23.9%). The final weight loss from 483 to 678 ℃can be attributed to the decomposition of two L ligands (obsd. 58.3%, calcd. 59.6%). The remaining weight may be attributed to the formation of CdO(obsd. 13.7%, calcd. 12.8%).compound 1 is ligand-based emission involved in both H2bpdc and L[22].

Fig. 5. Experimental and simulated PXRD patterns of 1
4 CONCLUSION
A new porous Cd(II) coordination compound[Cd(bpdc)(L)2]·3H2O (1) has been synthesized under hydrothermal conditions. One bpdc anion and two L ligands chelate with one Cd(II) atom to give a discrete molecule. Neighboring discrete molecules are stacked through π-π interactions, yielding an interesting 1D supramolecular chain. The 1D supramolecular chains are further packed with each other to afford the porous architecture. In addition,compound 1 shows intense luminescent property.
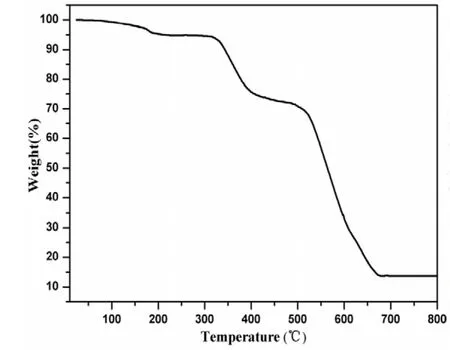
Fig. 6. TG curve of compound 1
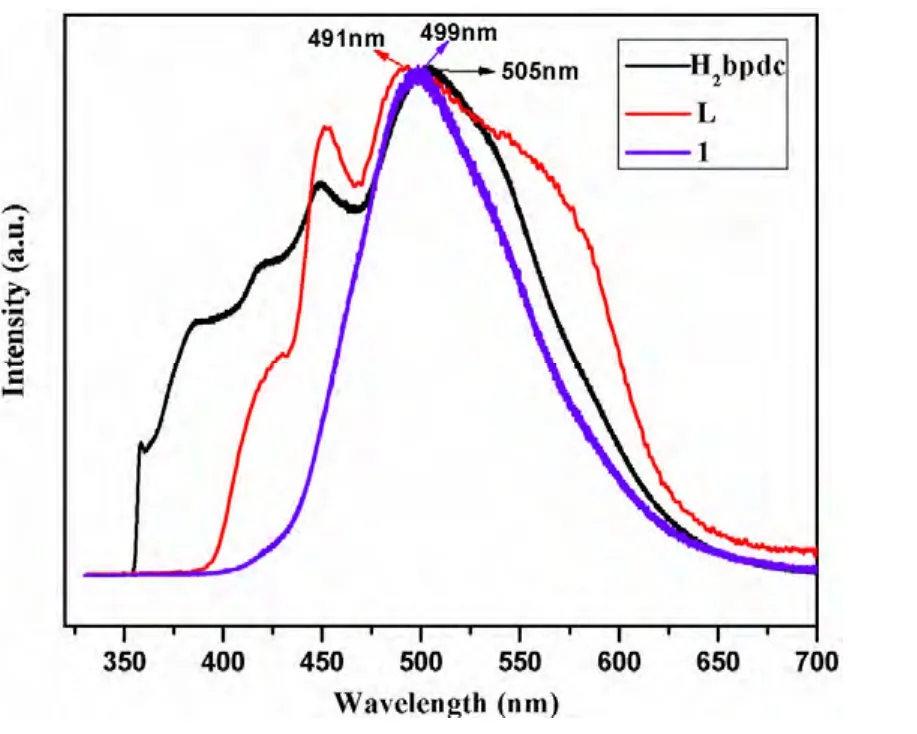
Fig. 7. Emission spectra of H2bpdc, L and compound 1 in the solid state at room temperature
(1) Chou, J. H.; Kosal, M. E.; Nakagaki, S.; Smithenry, D. W.; Wilson, S. R. Microporous porphyrin solids. Acc. Chem. Res. 2005, 38, 283–291.
(2) Moulton, B.; Zaworoko, M. J. From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem. Rev.2001, 101, 1629–1658.
(3) Carlucci, L.; Ciani, G.; Proserpio, D. M. Polycatenation, polythreading and polyknotting in coordination network chemistry. Coord. Chem. Rev.2003, 246, 247–289.
(4) Ockwig, N. W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O. M. Reticular chemistry: occurrence and taxonomy of nets and grammar for the design of frameworks. Acc. Chem. Res. 2005, 38, 176–182.
(5) Bradshaw, D.; Claridge, J. B.; Cussen, E. J.; Prior, T. J.; Rosseinsky, M. J. J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 2005, 38, 273–282.
(6) Hou, H. W.; Wei, Y. L.; Song, Y. L.; Mi, L. W.; Tang, M. S.; Li, L. K.; Fan, Y. T. Metal ions play different roles in the third-order nonlinear optical properties of d10metal-organic clusters. Angew. Chem., Int. Ed. 2005, 44, 6067–6074.
(7) Hong, M. C.; Zhao, Y. J.; Su, W. P.; Cao, R.; Fujita, M.; Zhou, Z. Y.; Chan, A. S. C. A silver(I) coordination polymer chain containing nanosized tubes with anionic and solvent molecule guests. Angew. Chem., Int. Ed. 2000, 39, 2468–2470.
(8) Zhao, B.; Yi, L.; Dai, Y.; Chen, X. Y.; Cheng, P.; Liao, D. Z.; Yan, S. P.; Jiang, Z. H. Systematic investigation of the hydrothermal syntheses of Pr(III)-PDA (PDA = pyridine-2,6-dicarboxylate anion) metal-organic frameworks. Inorg. Chem. 2005, 44, 911–920.
(9) Zang, S. Q.; Su, Y.; Song, Y.; Li, Y. Z.; Ni, Z. P.; Zhu, H. Z.; Meng, Q. J. Tuning the framework formation of Ni(II) complexes by controlling the hydrolysis of 2,2΄,3,3΄-thiodiphthalic dianhydride: syntheses, crystal structures, and physical properties. Cryst. Growth Des. 2006, 6, 2369–2375.
(10) Mi, L. W.; Hou, H. W.; Song, Z. Y.; Han, H. Y.; Xu, H.; Fan, Y. T.; Ng, S. W. Rational construction of porous polymeric cadmium ferrocene-1,1΄-disulfonates for transition metal ion exchange and sorption. Cryst. Growth Des. 2007, 7, 2553–2561.
(11) Yang, P.; Li, M. X.; Shao, M.; Wang, M. S.; Cao, S. X.; Zhang, J. C.; Zhang, H. H. Porous and nanorod-nike coordination polymers assembled from a new V-shaped bis(1,2,4-triazolyl)tripyridine ligand. Cryst. Growth Des. 2013, 13, 4305-4314.
(12) Dey, R.; Haldar, R.; Maji, T. K.; Ghoshal, D. Three-dimensional robust porous coordination polymer with Schiff base site on the pore wall:synthesis, single-crystal-to-single-crystal reversibility, and selective CO2adsorption. Cryst. Growth Des. 2011, 11, 3905–3911.
(13) Wang, K. B.; Geng, Z. R.; Zheng, M. B.; Ma, L. Y.; Ma, X. Y.; Wang, Z. L. Controllable Fabrication of Coordination Polymer Particles (CPPs): A Bridge between Versatile Organic Building Blocks and Porous Copper-Based Inorganic Materials. Cryst. Growth Des. 2012, 12, 5606–5614.
(14) Zang, S. Q.; Su, Y.; Li, Y. Z.; Ni, Z. P.; Zhu, H. Z.; Meng, Q. J. Interweaving of triple-helical and extended metal-O-metal single-helical chains with the same helix axis in a 3D metal-organic framework. Inorg. Chem. 2006, 45, 3855–3857.
(15) Xu, Z. L.; Chen, H.; Zhang, Y. N.; Chen, Y.; Xu, N.; Wang, X. Y. Synthesis, structure and thermal stability of a new lead coordination polymer:[Pb(L1)(L2)]·1.25H2O. Chin. J. Struct. Chem. 2012, 31, 1056–1060.
(16) Wang, X. Y.; Zhang, Y. N.; Li, D. Synthesis, structure and characterization of a new iron coordination polymer: [Fe(L)(trans-1,4-chdc)]n. Chin. J.Struct. Chem. 2012, 31, 1589–1593.
(17) Kong, Z. G.; Sun, X. R.; Guo, S. N.; Xiao, Y. B.; Pang, G. J. Synthesis, structure and physical property of a new 3D Lead coordination polymer:[Pb2(L)2(dea)]n·nH2O. Chin. J. Struct. Chem. 2013, 32, 1031–1035.
(18) Kong, Z. G.; Zhang, Y. N.; Liu, F. Y. Synthesis, structure and photoluminescence of a new Cd(II) coordination polymer based on 4-(carboxymethoxy)-benzoic acid and 1,10-phenanthroline derivative. Chin. J. Struct. Chem. 2013, 32, 1175–1179.
(19) Kong, Z. G.; Sun, X. R.; Li, H.; Fan, Y. J.; Zhang, L. Synthesis, structure and characterization of PbⅡcoordination polymer constructed by 1,10-phenanthroline derivative and succinic acid. Chin. J. Inorg. Chem. 2013, 29, 1942–1946.
(20) Sheldrick, G. M. SHELXS 97, Program for the Solution of Crystal Structure. University of Göttingen 1997.
(21) Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure. University of Göttingen 1997.
(22) Zhu, C.; Xuan, W.; Cui, Y. Luminescent microporous metal-metallosalen frameworks with the primitive cubic net. Dalton Trans. 2012, 41,3928–3932.
- 结构化学的其它文章
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis and Crystal Structure of Cobalt(II) and Copper(II) Complexes Involving L-Aamino Alcohols
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①

