A Luminescent Zn(II) Supramolecular Coordination Polymer Constructed from 3,4-Thiophenedicarboxylic Acid and Flexible Benzimidazole-based Connector①
DU Xi-Gang MI Gang LIU Ji-Chun ZHANG Jun
(School of Chemical Engineering and Pharmaceutics,Henan University of Science and Technology, Luoyang 471003, China)
1 INTRODUCTION
An especially active research area in recent years has involved the preparation of coordination polymers (CPs) due to their intriguing architectures in structure and potential properties[1-3]. Among them,self-assembly supramolecular CPs can be well tuned by hydrogen bonding, π-π stacking interaction and other weak intermolecular interactions, and mainly affected by the combination of some factors including the central metal ion, organic ligands, reaction conditions and other possible influences[4-6].
It is known that rigid multicarboxylate ligands are good candidates in assembling CPs with peculiar structures and new functionalities, because multicarboxylate groups can exhibit a rich variety of coordination modes[7-10]. Recently, thiophene-based CPs have received increasing attention due to their improved luminescence intensity and interesting topologies[11-19]. To the best of our knowledge, the introduction of N-donor ancillary ligands to the synthetic systems is of great potential to construct new CPs. N-donor ancillary ligands bearing alkyl spacers are especially preferable because the flexible nature of the spacers allows the ligands to bend and rotate when they coordinate to the metal centers, and this often causes such CPs with chain, layered, or 3D structures. For instance, the combination of di(1H-imidazol-1-yl)butane and m-phthalic acid generates porous supramolecular architecture[20], while di(1H-imidazol-1-yl)methane mixed together with mphthalic acid results in a 2D layer structure[21]. In contrast to 1H-imidazol-1-yl-containing ligands, the pbim ligand has been employed far less frequently in the construction of CPs.
In this paper, we report the hydrothermal synthesis, crystal structure, and thermal and photoluminescent properties of a new znic(II) coordination polymer [Zn(tdc)(pbim)]n. As far as we know, this is the first example of a CP based on pbim and H2tdc mixed ligands. X-ray diffraction analysis indicates the polymer is a 1D double chain, which is further connected into a 2D supramolecular structure by π-π stacking interactions.
2 EXPERIMENTAL
All chemicals were purchased from commercial sources and used without further purification.Elemental analyses for H elements were performed with a Model 240 Perkin-Elmer elemental analyzer.IR spectra were recorded as KBr pellets on a Nicolet Avatar-360 spectrometer in the range of 4000~400 cm-1. Thermogravimetric analyses (TGA) were carried out on a SDTQ600 thermogravimetric analyzer (DTA Instruments, New Castle, DE) in N2atmosphere at a heating rate of 10 ℃ min-1from 30 to 800 ℃. Fluorescence measurements were recorded with a Hitachi F4500 fluorescence spectrophotometer.
2.1 Synthesis of complex [Zn(tdc)(pbim)]n
A mixture of 3,4-thiophenedicarboxylic acid (17.2 mg, 0.1 mmol), Zn(OAc)2·2H2O (22.0 mg, 0.1 mmol), pbim (27.6 mg, 0.1 mmol), and 8 mL deionized water was sealed in a 25 mL Teflon-lined autoclave and heated at 140 ℃ for 5 days, followed by cooling to room temperature at a rate of 5 ℃ h-1.Yellow prismatic crystals of 1 were collected (yield:41% based on Zn). Elemental analysis calcd. (%) for C23H18ZnN4O4S: C, 53.92; H, 3.52; N, 10.94. Found(%): C, 53.89; H, 3.54; N, 10.91. IR data (KBr, cm-1):3076(w), 1606(s), 1511(m), 1463(m), 1389(w),1354(m), 1319(m), 1295(m), 1264(m), 1204(w),1195(w), 1088(w), 1009(w), 928(m), 834(m),788(m), 768(m), 744(s), 676(w).
2.2 X-ray structure determination
A light yellow block-like single crystal with dimensions of 0.36mm × 0.25mm × 0.19mm was mounted at 293(2) K by using a graphite-monochromated MoKα (λ = 0.71073 Å) radiation with an ω and φ scan mode (2.41<θ<28.32º). A total of 18549 reflections were collected and 5143 were independent with Rint= 0.0327, of which 3656 were observed with I > 2σ(I). Data reduction and absorption correction were made with SADABS software[22]. The structure was solved by direct methods with SHELXS-97 program[23]and refined by fullmatrix least-squares techniques using SHELXL-97[24]. All non-hydrogen atoms were refined anisotropically and all hydrogen atoms except those of aqua molecule were determined with theoretical calculations and refined isotropically. The final R =0.0361, wR = 0.0824 (w = 1/[σ2(Fo2) + (0.0384P)2+0.8338P], where P = (Fo2+ 2Fc2)/3) and S = 1.036.The selected bond lengths and bond angles are listed in Table 1.
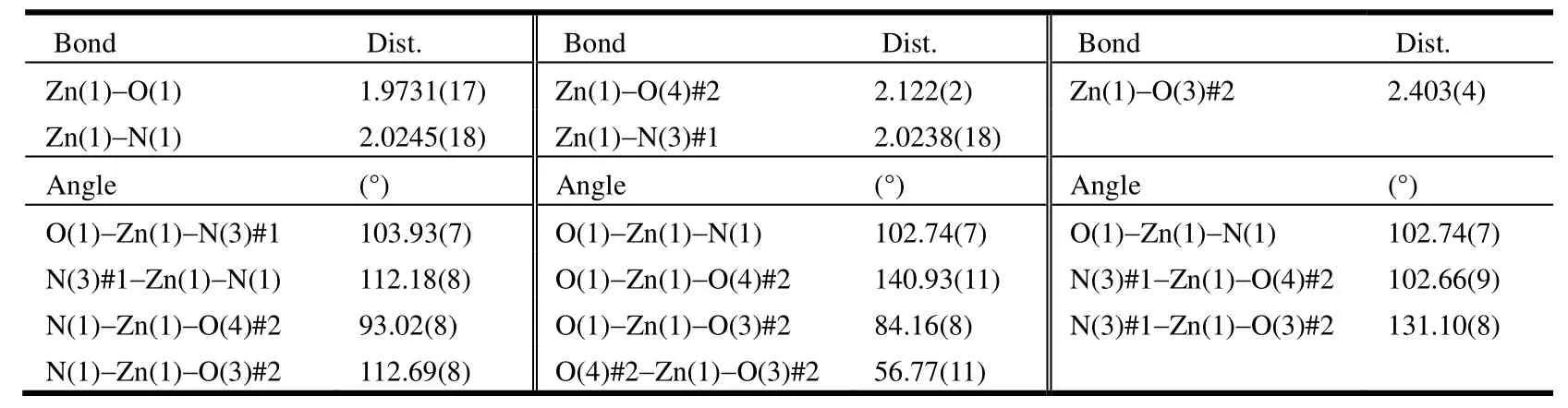
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
3 RESULTS AND DISCUSSION
Polymer 1 crystallizes in the monoclinic space group P21/c and exhibits a 1D double chain. As shown in Fig. 1, the asymmetric unit of 1 contains one crystallographically unique Zn2+ion, one deprotonated tdc2-anion, and one pbim ligand. The Zn center adopts a slightly distorted tetrahedral geometry by coordinating to two N atoms N(1),N(3#1) (symmetry codes for #1: x, 0.5 - y, 0.5 + z)from two pbim ligands and two O atoms O(1),O(4#2) (symmetry codes for #2: x, 0.5 - y, -0.5 + z)from two tdc2-anions. The bond angles around the Zn center range from 93.02(8) to 140.93(11)°. The bond lengths of Zn-N are 2.0238(18), 2.0245(18) Å and Zn-O distances are 1.9731(17), 2.122(2) Å,respectively, which are typical and comparable with those of other related Zn2+polymers[25,26]. The maximum and minimum bond angles for the Zn centers are 140.93(11) and 93.02(8)°, respectively,with an average of 109.24(3)°, which slightly deviates from the angle of 109.47° in a perfect tetrahedron.
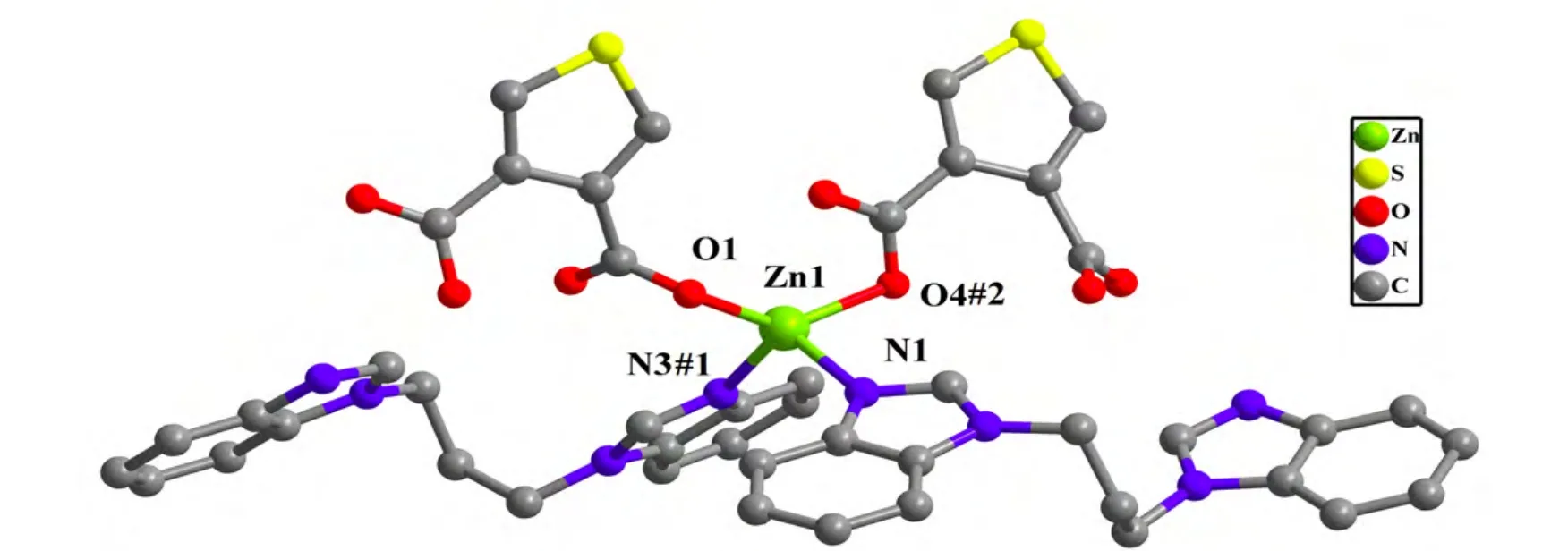
Fig. 1. View of the coordination environment of Zn2+ ion in 1.Symmetry codes: (#1) x, 0.5 - y, 0.5 + z; (#2) x, 0.5 - y, -0.5 + z
Unlike other found CPs in which each carboxylate group from tdc2-anion adopts a chelating mode,the tdc2-anion in 1 is connected to two Zn2+ions through its two monodentate carboxylate moieties,acting as a μ2-bridge ligand. It should be noted that the pbim ligand adopts a twisted cis conformation with the dihedral angles between the pairs of benzimidazole rings being 5.199(82)°. As viewed in Fig. 2, two Zn2+ions are bridged by one pbim and one tdc2-anion to form a 17-membered metallomacrocycle unit with a Zn···Zn separation of 7.4625(9)Å, then the units are further connected to each other by sharing the Zn2+ions to give a 1D infinite double chain-like structure along the c axis. Moreover, the 1D infinite double chains are interconnected to produce a 2D supramolecular framework by faceto-face π-π interaction of benzimidazole with interplane separation of 3.6812~3.7935 Å (Fig. 3).Obviously, the architecture of 1 is different from the documented CPs based on N-donor ancillary ligands and H2tdc mixed ligands[17].

Fig. 2. 1D double chain-like structure
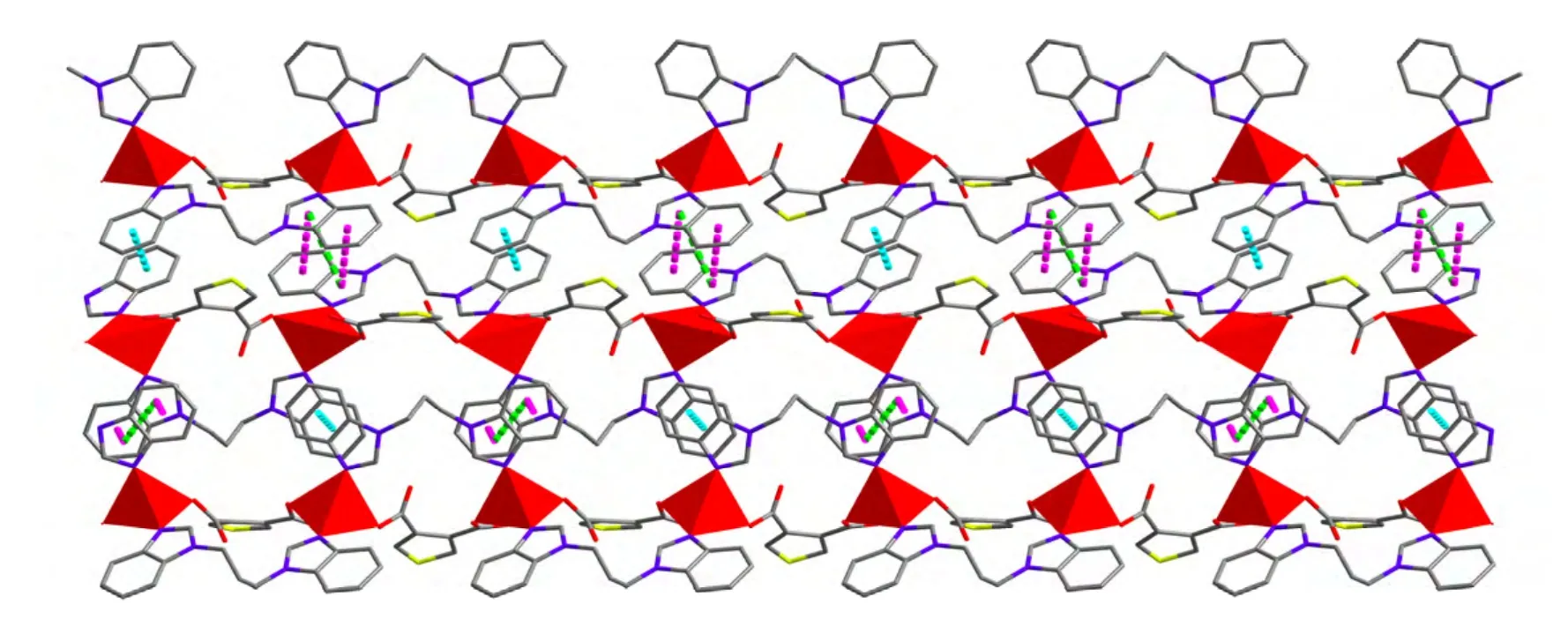
Fig. 3. 2D supramolecular framework constructed from π-π interaction
Polymer 1 was insoluble in water and common organic solvents such as methanol, ethanol,acetonitrile, toluene and N,N-dimethylformamide.In the IR spectra of 1, the absorbance peaks in the 1606 and 1319~1463 cm-1are attributed to the asymmetric stretching and symmetric stretching vibrations of the carboxylate groups, respectively.The absence of bands at the 1690~1730 cm-1region suggests the complete deprotonation of carboxylate groups in H2tdc, which are in agreement with the crystallographic structural analysis[27]. In order to get more information about the thermal stability, 1 was examined by the thermogravimetric analyses (TGA) in N2atmosphere with a heating rate of 10 ℃ min-1from 30 to 800 ℃. The TG curve exhibits 1 can be relatively stable to 300 ℃and then the framework begins to collapse, accompanied by the release of pbim and tdc ligands (Fig.4). As is well known, CPs with d10metal centers are usually investigated for their luminescent properties and potential applications. The lumine-scent properties of 1 and free H2tdc were investigated in the solid state and corresponding spectra are depicted in Fig. 5. It was observed that the emission peaks occurred at 430 nm (λex= 300 nm) for 1 and 436 nm(λex= 295 nm) for free H2tdc ligand, respectively.Considering that Zn(II) ions are difficult to oxidize or reduce, the emission is neither metal-to-ligand charge transfer (MLCT) nor ligand- to-metal charge transfer (LMCT) and may be as- signed to the π-π*transitions arising from the deprotonated H2tdc ligand, namely, ligand-to-ligand charge transfer(LLCT)[28].

Fig. 4. TGA curve of 1
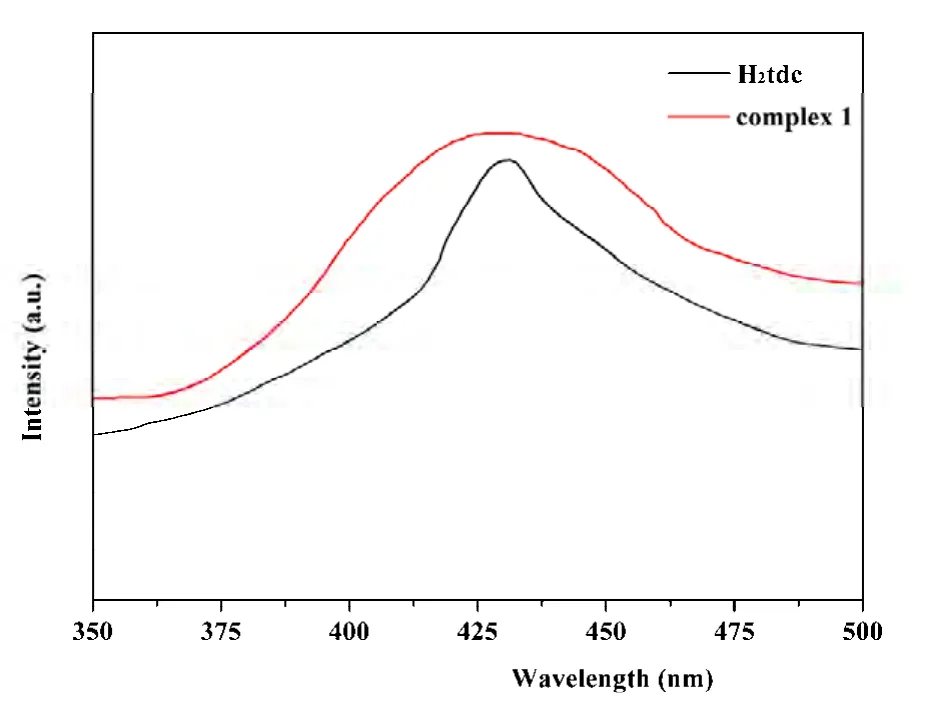
Fig. 5. Solid-state emission spectra for compound 1 and H2tdc
4 CONCLUSION
In summary, a luminescence Zn(II) coordination polymer was successfully synthesized using H2tdc and pbim mixed ligands by a hydrothermal approach.Structural analyses reveal that the polymer exhibits a 1D double chain structure, which is further connected into a 2D supramolecular structure by π-π stacking interactions. Furthermore, infrared spectroscopy, elemental analyses, and thermal and luminescence properties of the compound were inves- tigated in the solid state.
(1) Ayyappan, P.; Evans, O. R.; Cui, Y.; Wheeler, K. A.; Lin, W. B. Nonlinear optically active polymeric coordination networks based on metal m-pyridylphosphonates. Inorg. Chem. 2002, 41, 4978–4980.
(2) Kaye, S. S.; Dailly, A.; Yaghi, O. M.; Long, J. R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3(MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177.
(3) O’Keeffe, M.; Yaghi, O. M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 2011, 112, 675–702.
(4) Huang, X. C.; Zhang, J. P.; Lin, Y. Y.; Yu, X. L.; Chen, X. M. Two mixed-valence copper(I, II) imidazolate coordination polymers: metal-valence tuning approach for new topological structures. Chem. Commun. 2004, 1100–1101.
(5) Zhang, W. H.; Song, Y. L.; Zhang, Y.; Lang, J. P. Binuclear cluster-to-cluster-based supramolecular compounds: design, assembly, and enhanced third-order nonlinear optical performances of {[Et4N]2[MoOS3Cu2(μ-CN)]2·2aniline}nand {[Et4N]4[MoOS3Cu3CN(μ′-CN)]2(μ-CN)2}n. Cryst.Growth Des. 2008, 8, 253–258.
(6) Wang, Y. H.; Chu, K. L.; Chen, H. C.; Yeh, C. W.; Chan, Z. K.; Suen, M. C.; Chen, J. D.; Wang, J. C. Adjusting the frameworks of polymeric silver(I) complexes with 2-aminopyrimidyl ligands by changing the counterions. CrystEngComm. 2006, 8, 84–93.
(7) Chen, W.; Wang, J. Y.; Chen, C.; Yue, Q.; Yuan, H. M.; Chen, J. S.; Wang, S. N. Photoluminescent metal-organic polymer constructed from trimetallic clusters and mixed carboxylates. Inorg. Chem. 2003, 42, 944–946.
(8) Sun, J. Y.; Zhou, Y. M.; Fang, Q. R.; Chen, Z. X.; Weng, L. H.; Zhu, G. S.; Qiu, S. L.; Zhao, D. Y. Construction of 3D layer-pillared homoligand coordination polymers from a 2D layered precursor. Inorg. Chem. 2006, 45, 8677–8684.
(9) Lu, W. G.; Gu, J. Z.; Jiang, L.; Tan, M. Y.; Lu, T. B. Three achiral and one chiral coordination polymers containing helical chains: the chirality transfer between the helical chains. Cryst. Growth Des. 2008, 8, 192–195.
(10) Li, Z. G.; Wang, G. H.; Jia, H. Q.; Hu, N. H.; Xu, J. W. Constructing porous frameworks from one-dimensional cobalt-oxygen clusters and pyridinedicarboxylate ligands adopting two rare coordination modes. CrystEngComm. 2008, 10, 173–176.
(11) Chen, B. L.; Mok, K. F.; Ng, S. C.; Drew, M. G. B. Syntheses, structures and properties of copper(II) complexes with thiophene-2,5-dicarboxylic acid (H2Tda) and nitrogen-containing ligands. Polyhedron 1999, 18, 1211–1220.
(12) Demessence, A.; Rogez, G.; Welter, R.; Rabu, P. Structure and magnetic properties of a new cobalt(II) thiophenedicarboxylate coordination polymershowing unprecedented coordination. Inorg. Chem. 2007, 46, 3423–3425.
(13) Zhang, J.; Chen, S.; Wu, T.; Feng, P.; Bu, X. Homochiral crystallization of microporous framework materials from achiral precursors by chiral catalysis. J. Am. Chem. Soc. 2008, 130, 12882–12883.
(14) Takashima, Y.; Bonneau, C.; Furukawa, S.; Kondo, M.; Matsuda, R.; Kitagawa, S. Periodic molecular boxes in entangled enantiomorphic lcy nets.Chem. Commun. 2010, 46, 4142–4144.
(15) Zou, H. H.; He, Y. P.; Gui, L. C.; Liang, F. P. A new 8-connected porous coordination polymer: crystal structure and selective adsorption properties.CrystEngComm. 2011, 13, 3325–3329.
(16) Osta, R. E.; Frigoli, M.; Marrot, J.; Medina, M. E.; Walton, R. I.; Millange, F. Synthesis, structure, and crystallization study of a layered lithium thiophene-dicarboxylate. Cryst. Growth Des. 2012, 12, 1531–1537.
(17) Li, Z. H.; Xue, L. P.; Li, S. H.; Wang, J. G.; Zhao, B. T.; Kan, J.; Su, W. P. Bis(pyridyl) ancillary ligands modulated uninodal 4-, 5- and 6-connected Cd(II) coordination polymers based on 3,4-thiophenedicarboxylate linker. CrystEngComm. 2013, 15, 2745–2752.
(18) Li, Z. H.; Zhang, T.; Xue, L. P.; Miao, S. B.; Zhao, B. T.; Kan, J.; Su, W. P. Three-dimensional pillared layered supramolecular networks comprising polymeric layers and tetrameric {H2O}4pillars. CrystEngComm. 2013, 15, 7423–7425.
(19) Xue, L. P.; Li, Z. H.; Li, S. H.; Wang, J. G. Synthesis, crystal structure and fluorescent property of a new one-dimensional cadmium(II)coordination polymer based on 3,4-thiophenedicarboxylic acid and 1,10-phenanthroline. Chin. J. Struct. Chem. 2013, 32, 704–708.
(20) Ma, J. F.; Yang, J.; Zheng, G. L.; Li, L.; Liu, J. F. A porous supramolecular architecture from a copper(II) coordination polymer with a 3D four-connected 86net. Inorg. Chem. 2003, 42, 7531–7534.
(21) Zhang, X. F.; Song, W. C.; Yang, Q.; Bu, X. H. Zn(II) and Cd(II) coordination polymers assembled by di(1H-imidazol-1-yl) methane and carboxylic acid ligands. Dalton Trans. 2012, 41, 4217–4223.
(22) Sheldrick, G. M. SADABS, University of Göttingen, Gottingen 1997.
(23) Sheldrick, G. M. SHELXS-97, Program for X-ray Crystal Structure Solution. University of Göttingen, Göttingen, Germany 1997.
(24) Sheldrick, G. M. SHELXL-97, Program for X-ray Crystal Structure Refinement. University of Göttingen, Göttingen, Germany 1997.
(25) Hao, H. J.; Sun, D.; Liu, F. J.; Huang, R. B.; Zheng, L. S. Discrete octamer water cluster and 1D T5(2) water tape trapped in two luminescent Zn(II)/1,2-bis(imidazol-1΄-yl)ethane/dicarboxylate hosts: from 2D (4,4) net to 3D 5-fold interpenetrated diamond network.Cryst. Growth Des. 2011, 11, 5475–5482.
(26) Liu, Y. Y.; Ma, J. F.; Yang, J.; Ma, J. C.; Ping, G. J. Structures of metal-organic networks based on flexible 1,1΄-(1,4-butanediyl) bis(imidazole-2-phenyl) ligand. CrystEngComm. 2008, 10, 565–572.
(27) Luo, J.; Hong, M.; Wang, R.; Cao, R.; Han, L.; Lin Z. Synthesis, crystal structure and fluorescence of two novel mixed-ligand cadmium coordination polymers with different structural motifs. Eur. J. Inorg. Chem. 2003, 14, 2705–2710.
(28) Zhao, J.; Wang, X. L.; Shi, X.; Yang, Q. H.; Li, C. Synthesis, structure, and photoluminescent properties of metal-organic coordination polymers assembled with bithiophenedicarboxylic acid. Inorg. Chem. 2011, 50, 3198–3205.
- 结构化学的其它文章
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis and Crystal Structure of Cobalt(II) and Copper(II) Complexes Involving L-Aamino Alcohols
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①

