Hydrothermal Reaction, Structure and Magnetic Properties of a Binuclear Cobalt Complex with N-donor Ligand:2-Methyldipyrido[3,2-f:2΄,3΄-h]quinoxaline①
HUANG Yn-Ju YAN Yong-Sheng ZHANG Hong-Yn
a (Department of Chemistry, Tonghua Normal University, Tonghua 134002, China)
b (School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China)
1 INTRODUCTION
In recent years, low-dimensional complexes have received much attention owing to their intriguing structural features and potential as functional materials different from those of 3D complexes[1]. Often in supramolecular architecture of crystal engineering,as seen throughout many of the papers, interactions between molecules are employed in terms of hydrogen bonds, π-π stacking and other directional noncovalent interactions, and there is no doubt that the hydrogen bond plays a fundamental role in this area[2–4]. These materials have attracted much interest due to their strong potential for a variety of applications including gas storage[5–7], catalytic properties[8,9], nonlinear optics[10,11], and magnetism[12,13].
Transition metal complexes can also form extended network structures through additional supramolecular interactions, such as hydrogen bonding from having donor or acceptor groups appended to the ligands[14–18], π-π stacking interactions, or combinations of interactions. The π-stacking arrangements of transition metal complexes of 1,10-phenanthroline (phen) have been well studied. 2-Methyldipyrido[3,2-f:2΄,3΄-h]quinoxaline (Medpq) as a derivative of 1,10-phenanthroline and possessing an extended aromatic system is a planar rigid bidentate chelating reagent which can not only act as a terminal ligand but also potentially provide supramolecular interactions such as aromatic stacking to construct intriguing structures. For the past several years, our group has worked on the synthesis of Medpq ligand complexes[19]. However, the investigation for Medpq ligands is not enough, especially Medpq and cinnamic acid constructed ligands complexes have not been reported. Based on the above reasons, we designed and synthesized complex[Co1.5(C6H5CHCHCOO)3(Medpq)]·H2O 1 and the research shows it is a magnetism of material.
2 EXPERIMENTAL
2.1 Materials
The Medpq ligand was synthesized according to the literature method[19]. Co(Ac)2·4H2O, cinnamic acid and NaOH were purchased commercially and used without further purification.
2.2 Instruments and measurements
Elemental analysis was carried out with a Perkin-Elmer 240C analyzer; TG measurements were performed on a NETZSCH STA 449C analyzer. The Infrared (IR) spectrum was recorded from KBr pellets in the range of 400~4000 cm-1on a Nicolet FT-IR 170SX spectrometer.
2.3 Synthesis and measurement
Complex 1 was prepared from a mixture of Co(Ac)2·4H2O (0.13 g), cinnamic acid (0.16 g),Medpq (0.1 g) and H2O (10 mL) under stirring at room temperature. When the pH value of the mixture was adjusted to about 6.0 with NaOH, the cloudy solution was put into a 15 mL Teflon-lined autoclave under autogenous pressure at 165 ℃ for six days. After cooling to room temperature, red block crystals of 1 were collected by filtration and washed with distilled water in 61% yield (based on Co). Anal. Calcd. (%) for C42H31.25Co1.50N4O6.10: C,64.78; H, 4.01; N, 7.19. Found (%): C, 64.77; H,3.97; N, 7.21. IR (KBr, cm-1): 3392m (respectively ascribed to the stretching vibrations of H2O, which indicates the existence of water molecules), 3055m,1639s, 1578s, 1543m, 1465m, 1398s, 1302m, 1258w,1107m, 983m, 827m, 710m.
2.4 Crystallographic data collection and structure determination
A single crystal with dimensions of 0.23mm ×0.22mm × 0.22mm was collected at 293(2) K on a Bruker Smart Apex2 CCD diffractometer equipped with a graphite-monochromatic MoKa radiation (λ =0.71069 Å). Cell parameters were refined on all observed reflections by using the program Crystal Clear (Rigaku and MSc, Ver. 1.3.5, 2002). A total of 13534 reflections were collected in the range of 6.08≤2θ≤50º, of which 6495 were unique with Rint=0.0706, and 3900 observed reflections (I > 2σ(I))were used in the succeeding refinements. The structure of compound 1 was solved by direct methods with SHELXS-97 program[20]and refined by SHELXL 97[21]using full-matrix least-squares techniques on F2. All non-hydrogen atoms were refined anisotropically. All the hydrogen atoms were first found in difference Fourier maps, and then placed in the calculated sites and included in the final refinement in the riding model approximation with displacement parameters derived from the parent atoms to which they were bonded. The final refinement by full-matrix least-squares techniques based on 455 variables gave R = 0.0754 and wR =0.1332 (w = 1/[σ2(Fo2) + (0.0793P)2+ 4.3684P],where P = (Fo2+ 2Fc2)/3), (Δ/σ)max= 0.001 and goodness-of-fit indicator on F2is 1.115. The maximum peak in the final difference Fourier map is 0.701 and the minimum peak –0.458 e/Å3.
3 RESULTS AND DISCUSSION
3.1 Description of the crystal structure
Single-crystal X-ray diffraction analysis reveals that the binuclear complex [Co1.5(C6H5CHCHCOO)3(Medpq)]·H2O crystallizes in P1 space group and consists of a zero-dimensional structure. There are one and a half Co(II) ions, three cinnamic acid ligands, one Medpq ligand and one free water molecule in the local coordination environment of Co(II) (Fig. 1). The Co(1) ion is hexa-coordinated with six atoms (O(2), O(4), O(5), O(2A), O(4A),O(5A)) from three different cinnamic acid ligands and the symmetric three different cinnamic acid ligands, assuming a slightly distorted octahedral geometry. The Co(2) center adopts a coordination environment with two nitrogen atoms (N(1) and N(2)) from Medpq ligand and four oxygen atoms(O(1), O(2), O(3) and O(5)) from two different cinnamic ligands. This coordination configuration forms a CoO4N2unit which can be described as a slightly distorted octahedral geometry. The feature of the complex is that the distance between the dual cores is about 3.445 Å. The bond lengths are 2.101(5)and 2.149(6) Å for Co–N and 2.003(5)~2.332(4) Å for Co–O, comparable to those of 2.166(3) and 2.168(3) Å for Co–N in the complex of [Co-(dicnq)2Br2] (dicnq = 6,7-dicyanodipyridoquinoxaline)[22]. The Co–O bond distances vary from 2.0409(19) to 2.355(2) Å. The normal Co–N bond is 2.1255(5) Å and Co–O 2.1019(9) Å, close to the normal Co–N and Co–O bonds in 2.13 and 2.09 Å[23],respectively. The N(O)–Co–O(N) bond angles range from 59.2(16) to 180.0(1)º. The selected important bond parameters are given in Table 1.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Complex 1
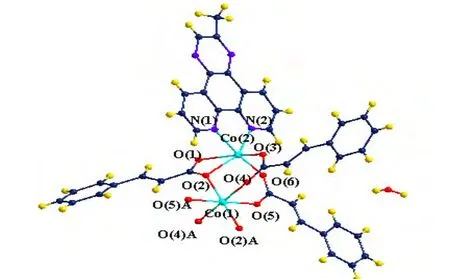
Fig. 1. ORTEP drawing of 1 showing the structure motif of complex 1 (symmetry codA: –x, 2 – y, 1 – z)
Hydrogen bonding interactions are usually important in the synthesis of supramolecular architectures.There are no classic hydrogen bonds in the compound, but, the C–H···O hydrogen bonding interactions complex exist between the cinnamic acid ligands and Medpq ligands (Table 2), which play an important role in stabilizing the network structure and controlling the orientation of inta ligands.Furthermore, the complex is extended into a twodimensional layer structure (Fig. 2). At the same time, there are π-π interactions in the complex. And the centroid-to-centroid distances of two adjacent and equivalent aromatic rings of Medpq ligand ranges from 3.682(5) to 3.953(5) Å (Table 3). These adjacent aromatic columns are piled up, and enhance the stability of the complex.
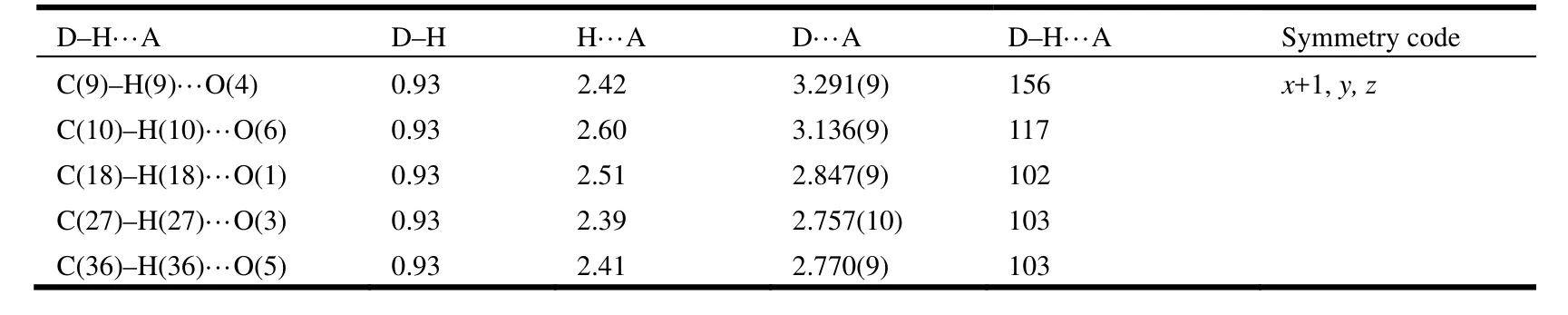
Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°) for Complex 1
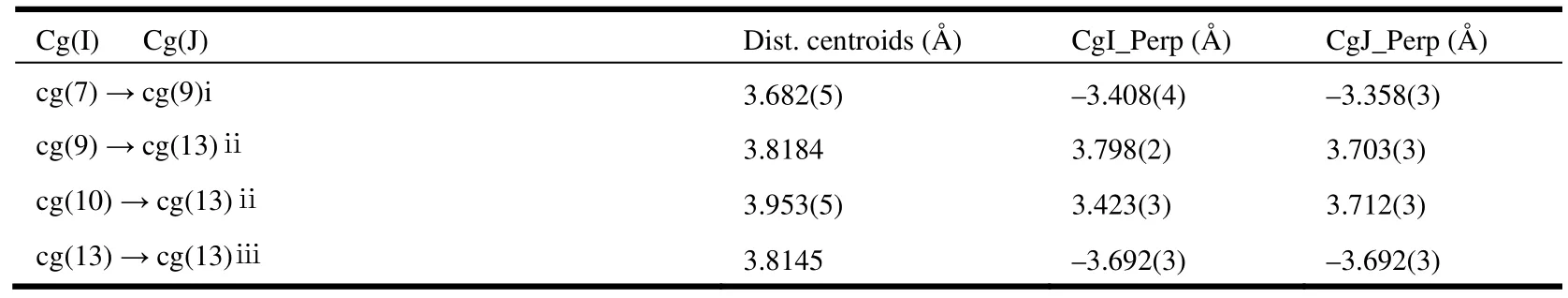
Table 3. Relative Parameters of Intermolecular π-π Interactions in Complex 1
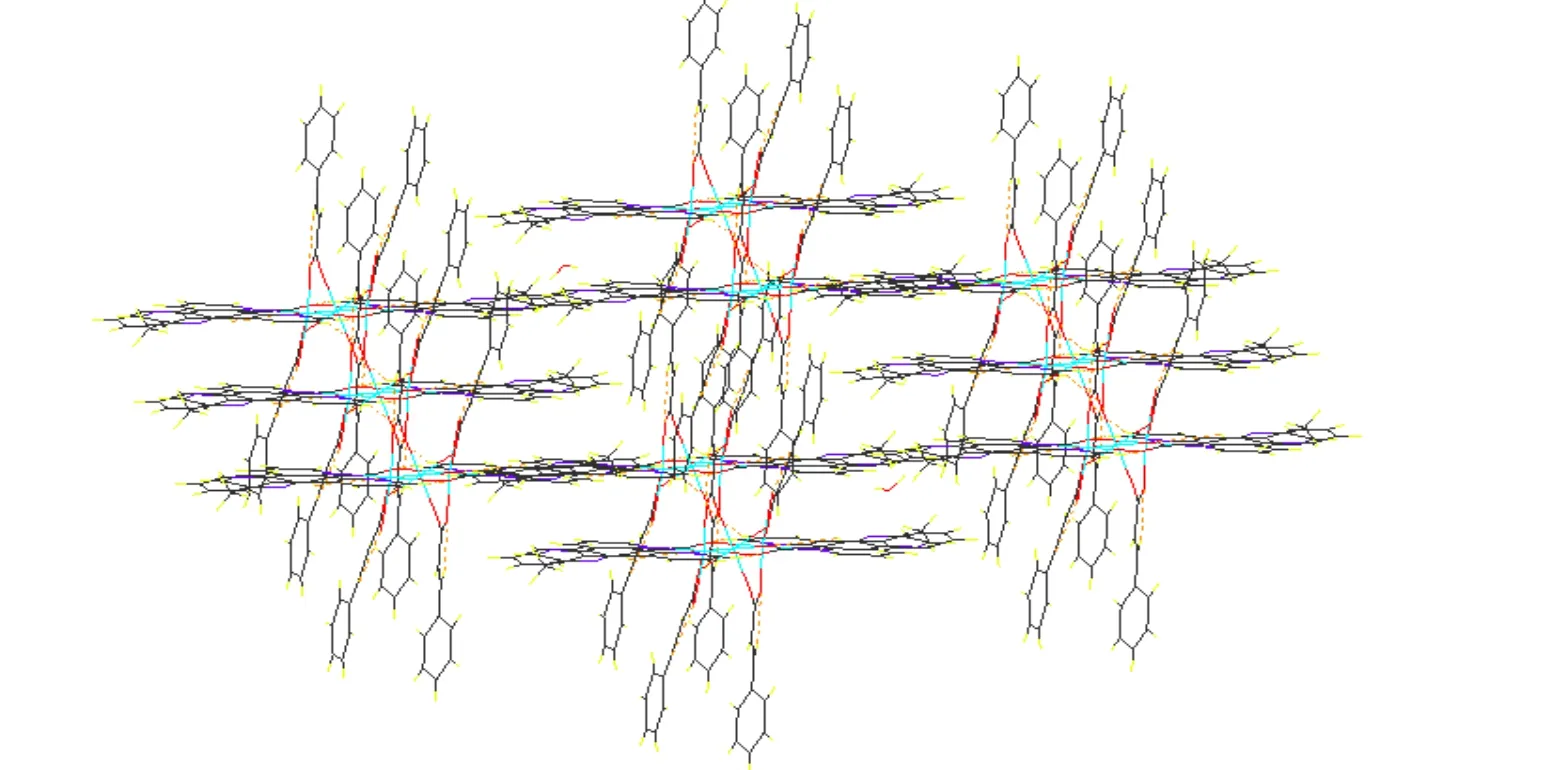
Fig. 2. View of the two-dimensional layer structure of complex 1
3.2 Thermal analysis
The stability of complex [Co1.5(C6H5CHCHCOO)3(Medpq)]·H2O was investigated by thermogravimetric analysis (Fig. 3). The first weight loss of 2.32% is in the range of 30.6~315.4 ℃,corresponding to the removal of H2O (calcd.: 2.31%).Upon further heating, an obvious weight loss(87.98%) occurs from 315.4 to 560.5 ℃ due to the release of cinnamic acid ligand and Medpq ligand(calcd.: 88.04%). Above 560.5 ℃, no weight loss is observed, indicating the complete decomposition of the complex. The residual weight of 9.70% (calcd.:9.65%) corresponds to CoO.

Fig. 3. TG of complex 1
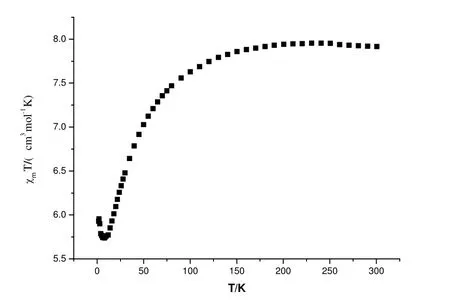
Fig. 4. Temperature dependence of magnetic susceptibilities in the form of χmT vs. T of complex 1
3.3 Magnetic susceptibility measurements
Magnetic measurements for complex [Co1.5(C6H5-CHCHCOO)3(Medpq)]·H2O were performed on a quantum design SQUID magnetometer. Variable temperature magnetic susceptibility data for χmT and l/χmversus T plots were collected for complex 1 in the temperature range of 1.8~300 K, as shown in Figs. 4 and 5. At 300 K, the value of χmT for 1 (7.92 cm3·mol-1·K) is as expected for a magnetically isolated spin quartet (7.92 cm3·mol-1·K). As the temperature is lowered, χmT decreases very slowly down to 100 K, then more and more rapidly, reaches a value of 5.93 cm3·mol-1·K at 1.8 K.
We have tried to fit the data based on the Curie-Weiss law

The values of parameters C and θ were obtained from the slope and intercept of the Curie-Weiss plot(l/χmversus T) over the entire temperature range, l/C= 0.12469, C = 8.02, θ/C = -0.5962 and θ = -4.78.
The negative value of the Weiss constant θ<0 indicates antiferromagnetic exchange interactions between the Co ions.
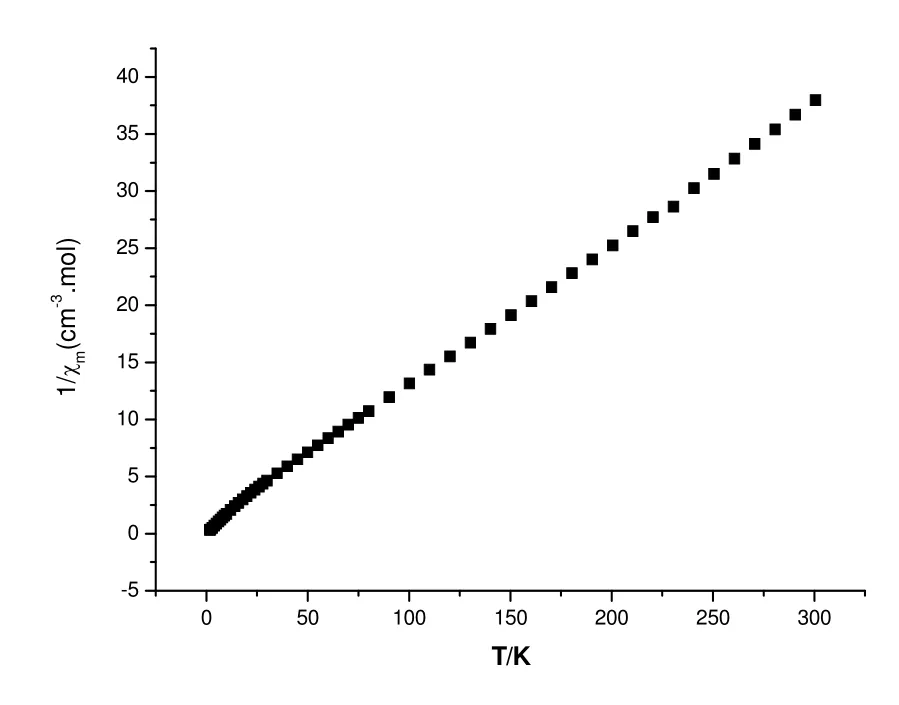
Fig. 5. 1/χm~T plot of complex 1
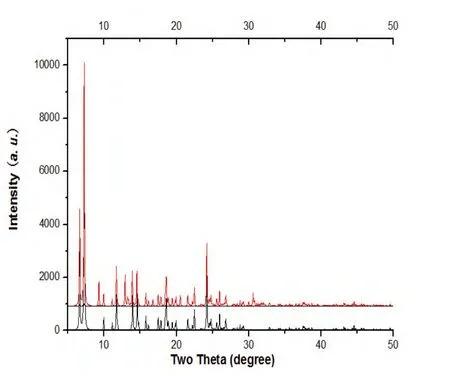
Fig. 6. PXRD analysis of the title complex:bottom-experimental, top-simulated
3.4 X-ray powder diffraction analysis
To investigate whether the analyzed crystal structure is truly representative of the bulk materials,X-ray powder diffraction (PXRD) technology has been performed for the complex at room temperature(Fig. 6). The main peak positions observed are in good agreement with the simulated ones. Although minor differences can be found in the positions,widths, and intensities of some peaks, it still can be considered that the bulk synthesized materials and the analyzed crystal are homogeneous. The differences may be due to the preferred orientation of the powder samples.
4 CONCLUSION
In summary, we have prepared a metal-organic complex by the combination of cinnamic acid and 2-methyldipyrido[3,2-f:2΄,3΄-h]quinoxaline ligand.The complex has been hydrothermally synthesized and structurally characterized by elemental analysis,IR spectrum, TG, single-crystal X-ray diffraction and magnetic susceptibility measurements. Moreover,the negative value of the Weiss constant indicates that there are antiferromagnetic exchange interactions between the Co ions.
(1) Han, Z. B.; Cheng, X. N.; Chen, X. M. Effect of the size of aromatic chelate ligands on the frameworks of metal dicarboxylate polymers: from helical chains to 2D networks. J. Cryst. Growth Des. 2005, 5, 695-700.
(2) Brunsveld, L.; Folmer, B. J. B.; Meijer, E. W.; Sijbesma, R. P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071-4098.
(3) Fyfe, M. C. T.; Stoddart, J. F. Synthetic supramolecular chemistry. J. Acc. Chem. Res. 1997, 30, 393-401.
(4) Caulder, D. L.; Raymond, R. N. Supermolecules by design. J. Acc. Chem. Res. 1999, 32, 975-982.
(5) Pan L.; Liu, H.; Lei, X.; Huang, X.; Olson, D. H.; Turro, N. J.; Li, J. PM-1: a recyclable nanoporous material suitable for ship-in-bottle synthesis and large hydrocarbon sorption. Angew. Chem. Int. Ed. 2003, 42, 542-546.
(6) Rowsell, J. L. C.; Millward, A. R.; Park, K. S.; Yaghi, O. M. Hydrogen sorption in functionalized metal-organic frameworks. J. Am. Chem. Soc. 2004, 126, 5666-5667.
(7) Chae, H. K.; Siberio-Perez, D. Y.; Kim, J.; Go, Y. B.; Eddaoudi, M. A.; Matzger, J.; O’Keeffe, M.; Yaghi, O. M. A route to high surface area,porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523-527.
(8) Aoyama, Y. Functional organic zeolite analogues. M. Design of Organic Solids. Springer Berlin Heidelberg 1998, 165, 131-161.
(9) Bassani, D. M.; Darcos, V.; Mahony, S.; Desvergne, J. P. Supramolecular catalysis of olefin [2+ 2] photodimerization.J. Am. Chem. Soc. 2000, 122, 8795-8796.
(10) Muthuraman, M.; Masse, R.; Nicold, J. F.; Desiraju, G. R. Molecular complexation as a design tool in the crystal engineering of noncentrosymmetric structures. ideal orientation of chromophores linked by O–H···O and C–H···O hydrogen bonds for nonlinear optics.Chem. Mater. 2001, 13, 1473-1479.
(11) Ko¨nig, O.; Bu¨rgi, H. B.; Armbruster, T.; Hulliger, J.; Weber, T. A study in crystal engineering: structure, crystal growth, and physical properties of a polar perhydrotriphenylene inclusion compound. J. Am. Chem. Soc. 1997, 119, 10632-10640.
(12) Kahn, O. Chemistry and physics of supramolecular magnetic materials. Acc. Chem. Res. 2000, 33, 647-657.
(13) Miller, J. S. Organometallic- and organic-based magnets: new chemistry and new materials for the new millennium.Inorg. Chem. 2000, 39, 4392-4408.
(14) Tynan, E.; Jensen, P.; Kruger, P. E.; Lees, A. C.; Nieuwenhuyzen, M. Solvent templated synthesis of metal-organic frameworks: structural characterisation and properties of the 3D network isomers {[Mn(dcbp)]·½DMF}nand {[Mn(dcbp)]·2H2O}n.Chem. Commun. 2004, 7, 776-777.
(15) Kurth, D. G.; Fromm, K. M.; Lehn, J. M. Hydrogen-bonding and metal-ion-mediated self-assembly of a nanoporous crystal lattice. J. Inorg. Chem.2001, 1523-1526.
(16) Aakeroy, C. B.; Beatty, A. M. Supramolecular style: design and hydrogen bond directed assembly of ternary supermolecules. Angew. Chem. Int. Ed.2001, 40, 3240-3242.
(17) Braga, D.; Grepioni, F. Intermolecular interactions in nonorganic crystal engineering. Acc. Chem. Res. 2000, 33, 601-608.
(18) Tadokoro, M.; Nakasuji, K. Hydrogen bonded 2,2′-biimidazolate transition metal complexes as a tool of crystal engineering. Coord. Chem. Rev.2000, 198, 205-218.
(19) Huang, Y. J.; Ni, L. Hydrothermal syntheses and crystal structures of two coordination polymers with terephthalic acid and n-donor ligand:2-methyldipyrido[3,2-f:2΄,3΄-h]quinoxaline. J. Inorg. Organomet. Polym. 2011, 21, 97-102.
(20) Sheldrick, G. M.SHELXS 97, Program for the Solution of Crystal Structure. University of Göttingen 1997.
(21) Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure. University of Göttingen 1997.
(22) Stephenson, M. D.; Hardie, M. Extended structures of transition metal complexes of 6,7-dicyanodipyridoquinoxaline: π-stacking, weak hydrogen bonding, and CN···π interactions. J. Cryst. Growth Des. 2006, 6, 423-432.
(23) Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. J. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. Chem. Soc. Perkin Trans. 1987, 2, 1–19.
- 结构化学的其它文章
- Synthesis and Crystal Structure of(Z)-2-Methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one O-Prop-2-yn-1-yl Oxime Derivatives①
- Synthesis and Crystal Structure of Cobalt(II) and Copper(II) Complexes Involving L-Aamino Alcohols
- Synthesis, Crystal Structure and Photoluminescence of a Three-coordinate Ag(I) Complex①
- Synthesis, Crystal Structure and Antimicrobial Activity of Ethyl 2-(1-cyclohexyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2-oxoacetate①
- Synthesis, X-ray Crystallographic Analysis and Bioactivities of α-Aminophosphonates Featuring Pyrazole and Fluorine Moieties①
- Syntheses and Structural Characterizations of a Series of Capped Keggin Derivatives①

