Posterior quadrantic disconnection maintains the activity of isolated temporal-parietal-occipital nerve tissue: neuroprotective measures in the surgical treatment of epilepsy
Posterior quadrantic disconnection maintains the activity of isolated temporal-parietal-occipital nerve tissue: neuroprotective measures in the surgical treatment of epilepsy
Extensive lesions involving the posterior quadrant of the cerebral hemisphere (temporal, parietal, and occipital lobes) induce intractable epilepsy. These patients are potential candidates for surgical treatment[1]. Maintenance of isolated nerve tissue activity after surgery plays a crucial role in the neuroprotective effects of neurosurgery treatment. Disconnection surgery of the posterior quadrant is used to completely isolate nerve fi bers, while blood supply at the isolated lobes is maintained. Subsequently, cavities caused by cystic or necrotic nerve tissues should be reduced as much as possible, to maintain the activity of isolated nerve tissue[1-4]. In this study, we selected two patients with temporal-parietal-occipital intractable epilepsy, from the Department of Neurosurgery, Tianjin Huanhu Hospital and Tianjin Neurological Institute (Tianjin, China), between February and May in 2012. The two cases underwent post quadrant disconnection. After surgery, activity of temporal-parietal-occipital nerve tissue and the control of epileptic seizures were observed.
Case 1: An 11-year-old male, was admitted to Tianjin Huanhu Hospital in February, 2012, and received surgery 1 week later. Postoperative follow-up was performed every 6 months. The medical history showed mild head injury when he was 5 years old, resulting from a scalp laceration without loss of consciousness. The first seizure occurred 1 year later, with the head turning towards the left side, without limb and trunk spasms. He was conscious, and the seizure stopped after 2 minutes. After the second attack, he was diagnosed and treated for epilepsy. Control of seizures failed after 3 anticonvulsant treatments, became refractory, and he still had one seizure per week, while being treated with a combination of 3 anticonvulsants. Preoperative physical examination showed left homonymous hemianopsia. Video-electroencephalography monitoring showed interictal spikes and slow wave complexes in the right central occipital region (Figure 1A1). Magnetic resonance imaging (MRI) of the brain revealed right occipital congenital porencephaly (Figure 1A2). Based upon these fi ndings, this case was diagnosed as simple partial epilepsy. The left hemisphere was not involved during the seizure, because language function was not affected. Video-electroencephalography monitoring and brain imaging results, as well as the clinical features, identi fi ed the epileptogenic region as the right post quadrant. A right post quadrant disconnection was therefore performed.
After surgery, the patient orally received 300 mg of oxcarbazepine, twice a day, for an extended period. Follow-up was performed every 6 months. Six month postoperative electroencephalogram showed no epileptic waves (Figure 1B1). MRI revealed complete resection of the right posterior temporal-parietal-occipital lobes, and the majority of isolated brain tissue produced normal signals (Figure 1B2). Case 1 was followed-up for 22 months, during which time no aura or seizure was observed.
Case 2: A 28-year-old female was admitted to Tianjin Huanhu Hospital in April, 2012, and received surgery 1 week later. Medical history showed no remarkable features such as brain injury or infection. The first attack occurred 6 months before admission, involving generalized tonic-clonic seizures and disturbance of consciousness, which continued for 1–2 minutes, and then stopped. She was diagnosed with epilepsy and received anticonvulsant medication after a secondary seizure 2 months before admission. However, the seizures were not controlled, and a brain CT scan at a local hospital revealed suspicious “gray matter heterotopia”. Preoperative physical examination showed no seizure attacks during 3 days of video-electroencephalography monitoring. Interictal electroencephalogram showed interrupted short-range low and middle amplitude waves in the right hemisphere. There were also some interictal spikes and slow wave complexes, especially in the temporal and parietal regions (Figure 2A1). MRI of the brain showed an irregular and abnormal signal isodensity with gry matter subendymal at the atrium of the right ventricle. Similar signals were also observed in the temporal horn of the ipsilateral ventricle under the ventricular ependyma (Figure 2A2). The neuroimaging and electroencephalogram therefore showed an epileptogenic focus at the posterior right hemisphere, and posterior quadrant disconnection was performed with the same surgical procedures as case 1.
After surgery, the patient orally received 500 g of sodium valproate, twice a day, for an extended period. A 6 month postoperative electroencephalogram showed no epileptic waves (Figure 2B1). MRI revealed complete resection of the right posterior quadrant, but nerve tissue still survived (Figure 2B2). Case 2 was followed up for 20 months, during which time no seizures were observed.
The involved two patients were informed of the experimental scheme and risk prior to treatment, and both patients signed informed consents.
Disconnection surgery of the posterior quadrant is used to cut nerve fi bers between the posterior quadrant and the resting region in the hemisphere, isolating the epileptogenic zone at the posterior quadrant. In this study, we only partially removed the superior temporal gyrus (tissue width < 5 mm), so that the tissue could be isolated with less incision. A careful macroscopic examination was made to preserve arteries and veins while the incision was made, therefore the isolated lobe maintained a blood supply and survived. MRI scans of the two epileptic patients showed that the disconnected brain tissue still survived, the activity of temporal-parietal occipital nerve tissue was maintained, and nerve fi bers were completely disconnected. The involved patients were followed-up for over 1 year after surgery, during which time the epilepsy was completely controlled.
Shaoya Yin1, Keke Feng1, Mei Feng2, Xueqing Zhang2, Yuqin Zhang3
1 Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin, China
2 Department of Electroneurophysiology, Tianjin Huanhu Hospital, Tianjin, China
3 Department of Pediatric Neurology, Tianjin Children’s Hospital, Tianjin, China
[1] Davis KL, Murro AM, Park YD, et al. Posterior quadrant epilepsy surgery: predictors of outcome. Seizure. 2012;21(9):722-728.
[2] Daniel RT, Meagher-Villemure K, Farmer JP, et al. Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy, and case series. Epilepsia. 2007;48(8):1429-1437.
[3] Dofer C, Czech T, Fahrngruber AM. Disconnective surgery in posterior quadrantic epilepsy: experience in a consecutive series of 10 patients. Neurosurg Focus. 2013;34(6):1-6.
[4] Mohamed AR, Freeman JL, Maixner W, et al. Temporoparietooccipital disconnection in children with intractable epilepsy. J Neurosurg Pediatr. 2011;7(6):660-670.
Copyedited by Takemoto D, Wang J, Yang Y, Li CH, Song LP, Zhao M
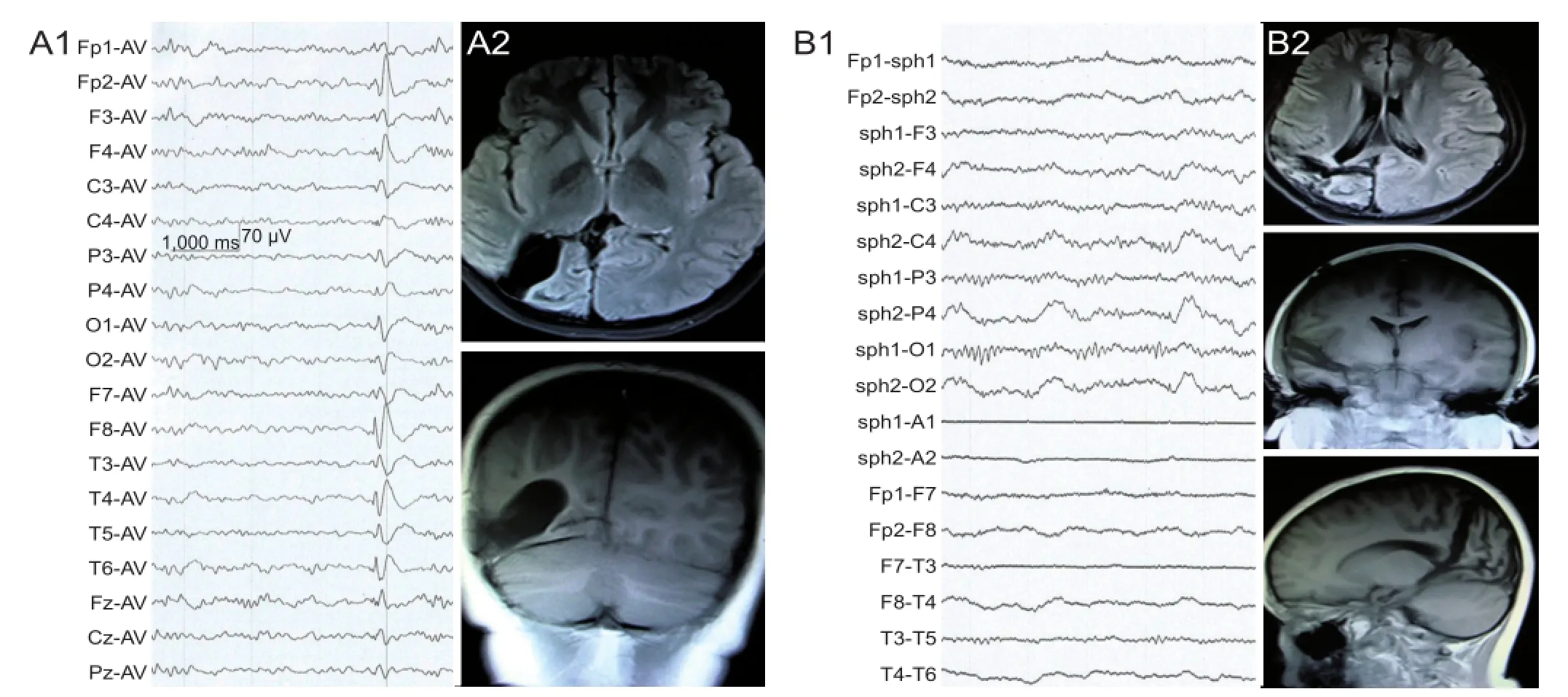
Figure 1 Electroencephalogram (EEG) and MRI changes of case 1, an 11-year-old male patient with temporal-parietal-occipital epilepsy, before and after posterior quadrant disconnection.
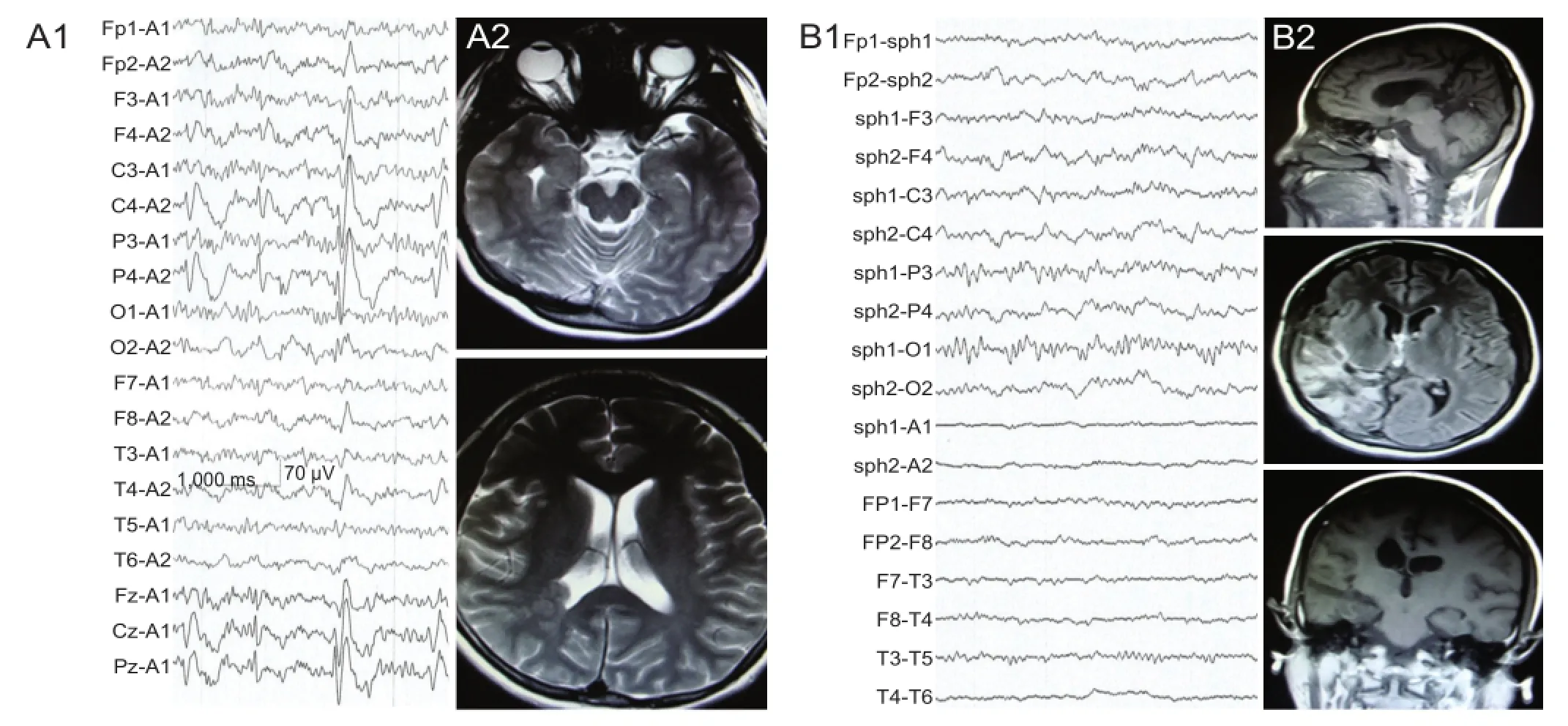
Figure 2 Electroencephalogram (EEG) and MRI changes of case 2, a 28-year-old female patient with temporal-parietal-occipital epilepsy, before and after posterior quadrant disconnection.
Shaoya Yin, M.D., Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin 300060, China, yinsy@163.com.
10.4103/1673-5374.128259 http://www.nrronline.org/
Accepted: 2014-01-09
Funding: The Science Fund of Health Bureau of Tianjin, No. 2013KZ046.
Yin SY, Feng KK, Feng M, Zhang XQ, Zhang YQ. Posterior quadrantic disconnection maintains the activity of isolated temporal-parietal-occipital nerve tissue: neuroprotective measures in the surgical treatment of epilepsy. Neural Regen Res. 2014;9(4):447-448.
- 中国神经再生研究(英文版)的其它文章
- Examination of Huntington’s disease in a Chinese family
- Circadian fl uctuations in three types of sensory modules in healthy subjects
- 7.0T nuclear magnetic resonance evaluation of the amyloid beta (1–40) animal model of Alzheimer’s disease: comparison of cytology veri fi cation
- Local inhibition of GABA affects precedence effect in the inferior colliculus
- Compound Formula Rehmannia alleviates levodopainduced dyskinesia in Parkinson’s disease
- The Pael-R gene does not mediate the changes in rotenone-induced Parkinson’s disease model cells

