Circadian fl uctuations in three types of sensory modules in healthy subjects
Yong Hyun Kwon, Ki Seok Nam
Circadian fl uctuations in three types of sensory modules in healthy subjects
Yong Hyun Kwon, Ki Seok Nam
Department of Physical Therapy, Yeungnam University College of Science and Technology, Hyunchung-ro, Nam-gu, Daegu, Republic of Korea
This study was designed to observe and compare the circadian fluctuations in tactile sense, joint reposition sense and two-point discrimination in healthy subjects. Twenty-one healthy adult subjects received perceptual ability tests through these three different sensory modules at approximately 9:00, 13:00 and 18:00 in a day. The distribution of ranking for perceptual ability was signi fi cantly different among the three different time points in each individual, with highest perceptual ability in the evening compared with noon and morning, in terms of tactile sense and two-point discrimination. These fi ndings suggest that the perceptual ability of healthy subjects fl uctuates according to the time points in a day.
nerve regeneration; circadian pattern; biological rhythm; circadian fluctuations; perceptual ability; tactile sensory; joint reposition sense; two-point discrimination; Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology; neural regeneration
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012R1A1B4003477.
Kwon YH, Nam KD. Circadian fluctuations in three types of sensory modules in healthy subjects. Neural Regen Res. 2014;9(4):436-439.
Introduction
Sensory perception enables human beings to gather information on the external environment and is an essential component in protection of the body, acquisition of new knowledge and motor skill, and enrichment of everyday life[1-4]. Sensation is classically categorized according to superficial sense (i.e., pain, temperature, light touch, pressure which receiving stimulus from cutaneous receptors), deep sense (i.e., awareness of joint position and movement which receiving stimulus from proprioceptive receptors in tendon, ligament, and muscle), and combined cortical sense (i.e., two-point discrimination, tactile localization, graphesthesia, which are perceived as a combination of multimodal sensory modules)[5-6]. Internal process of these senses is transferred from each sensory receptor to multiple brain structures via the anteriolateral spinothalamic tract or the dorsal column-lemin
scal pathway[3,5]. It plays an important role in performance
of coordinated and delicate movement and in learning new motor skills by association with many aspects of the motor system. On account of prominent issues related to human life, an understanding of the sensory processing mechanism has been constantly highlighted in the fi eld of neuroscience. In addition, various clinical tests have been implemented for evaluation of sensory perceptual ability and degree of sensory damage. Recent studies have reported that physical and neurological functions in humans are physiologically modulated by circadian rhythm[7-9]. During a 24-hour time period, circadian system controls various physiologic functions in terms of cognitive ability, physical function, and metabolic process[5,10]. Critical time periods to reach optimal functional level exist throughout the circadian cycle depending on various human functions, although there is rhythmic deviation of individual characteristics. In particular, abundant features of the sensorimotor system fl uctuate according to biological rhythm. Many previous chronobiological studies have reported on detection of circadian fluctuation in performing simple motor tasks, fi ne skilled movement, and anaerobic exercise[11-16]. However, to the best of our knowledge, literature concerning variation of sensory function according to the circadian cycle is lacking. Therefore, we attempted to investigate the question that whether the function of three different sensory modules (i.e., tactile sense, joint reposition sense, and two-point discrimination) fluctuated according to the effect of the time-of-day in healthy subjects.
Results
Tactile sense
In tactile sense, the threshold of sensory perception for electro-tactile stimulation provoked at 18:00 was lower than at 9:00 and 13:00, although no statistical signi fi cance was observed (F(2,60)= 2.628, P = 0.081). However, the distribution of ranking for perception ability in each individual was signi fi cantly higher at 18:00 than at the other two time points (χ2= 34.857, P < 0.05; Table 1).
Joint reposition sense
In the joint reposition test, no signi fi cant differences in raw value (F(2,60)= 0.420, P = 0.651) and distribution of ranking for angular error of joint position (χ2= 4.286, P > 0.05) were observed among the three different time points, although performance at 18:00 showed the lowest angular error (Table 1).
Two-point discrimination
In the sense of two-point discrimination, minimal distance for the ability to perceive two points was shorter at 18:00, compared to at 9:00 and 13:00, although no statistical significance was observed (F(2,60)= 2.513, P = 0.090). However, the distribution of ranking for accuracy in perceiving distance between two points was signi fi cantly higher at 18:00 than at the other two time points (χ2= 34.286, P < 0.05; Table 1).
Discussion
This current study was conducted in order to investigate the question that whether optimized threshold of three different sensory modules (i.e., tactile sense, joint reposition sense, and two-point discrimination) fl uctuated with the course of time in the day. According to the results, sensory thresholds of all modules were generally more sensitive at 18:00, compared with at 9:00 and 13:00, although no signi fi cant statistical differences were observed. This nonsigni fi cance is likely attributed to the diverse individual differences of receptive threshold in each sensory module. However, the distribution of order of priority for perceptual ability among three time points (i.e., at 9:00, at 13:00, and at 18:00) in each individual showed a signi fi cant difference in tactile sense and two-point discrimination. For example, the distribution of optimized perceptual ability at 18:00 was higher than at 9:00 and 13:00 in the two sensory modules. Accordingly, we con fi rmed that sensory perceptual ability such as somatosensory, proprioception, and discriminative sensation could fl uctuate according to the effect of time of day, and that sensory threshold was optimally more sensitive in the early evening, compared with morning and afternoon.
As these results of our study, many previous investigations have reported that various physiologic functions in the human body fl uctuated according to diurnal pattern in terms of metabolism, cognitive ability, and motor behavioral function[5,8-9,17-18]. In particular, according to experiments concerning the diurnal effect of motor performances, Bessot et al.[19]reported on the chronobiological effect on muscle activity and ef fi cient force production during cycling at different pedal rates, and Dosseville et al.[20]reported that spontaneous motor tempo fl uctuated throughout the day in parallel with diurnal variation in heart rate. In addition, time-dependent changes in muscle torque under isometric condition and neuromuscular ef fi ciency in the contractile state of muscle by the in fl uence of time of day was observed[11-12,21-23]. As a possible explanation for these fl uctuations throughout the day, it might be assumed to be physiological changes of diurnal pattern, such as body temperature and hormone secretion. Many previous studies have suggested a close association of body temperature with muscle performance and cognitive abilities[7,12,23-25]. Therefore, our results were in accordance with converging evidence, indicating the existence of circadian fl uctuation in human physiological components from basic biologic function to complex motor ability.
In comparison among three time periods, the results showed that proportion of optimized perceptual performance was signi fi cantly higher in the early evening than at other time points in each of the sensory modules. To the best of our knowledge, no evidence concerning time of day effect of sensory function has been published. Therefore, at present, it is impossible to perform a direct comparison of our findings with those of previous studies. However, through studies regarding time of day effect on postural control ability, we can infer that threshold of human sensory receptors also fl uctuates throughout the day as a diurnal pattern. Successful execution of postural control is the product of dynamic sensorimotor processes by the interaction of various sensory modules in terms of vision, somatosensory, and proprioception. Many previous studies have reported that optimal performance of postural balance was changed by the effect of time of day throughout the day. In addition, Bougard et al.[26]reported that postural ability is the best at 18:00, whereas Forsman et al.[27]reported that it was low around midday. These previous results showed correspondence with our findings, indicating that optimized time period of the day for perceptual ability was approximately at evening time. Possibility of circadian fluctuation might be suggested as a rhythm that is close to that of hormone secretion, body temperature, and/or vigilance[25,28-31]. To the best of our knowledge, beside this explanation, no mechanismshave been clearly clarified to date. Accordingly, circadian fl uctuation of postural balance might be accompanied by diurnal pattern of somatosensory and proprioception, because it is closely related to these two sensory modules.
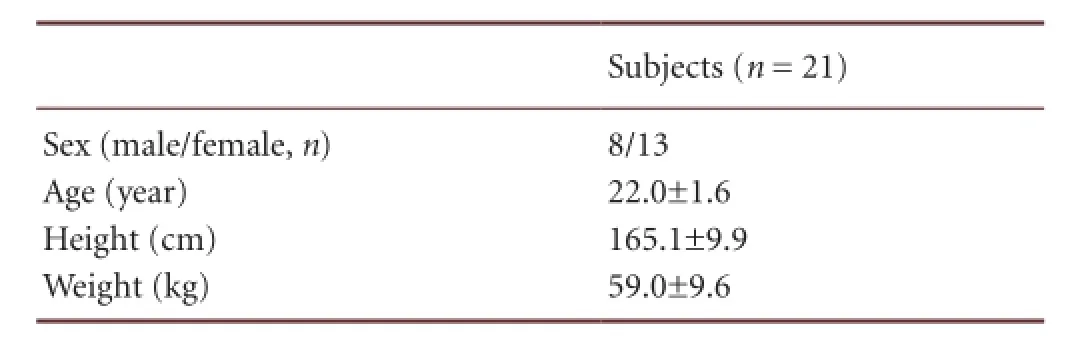
Table 2 Demographic information of subjects
In summary, we have provided evidence indicating that a circadian rhythmic pattern existed in the human sensory system in sensory modules in terms of somatosensory, proprioception, and discriminative sensation, along with other physiologic functions. In particular, all of the sensory modules were modulated into an optimized threshold in the early evening at approximately 18:00. A better understanding of the circadian pattern of the sensory system has clinical implications for clinicians in performance of a more detailed evaluation of sensory function and planning therapeutic intervention in patients with neurological dysfunction. This study has limitations due to small sample size and just one day of observation of circadian rhythm. We suggest that further studies including a large sample size and repetitive measurements for several days will be required in order to solve these limitations. These procedures will lead to high generalization and statistical power for elucidation of the effect of time of day.
Subjects and Methods
Subjects
Twenty healthy subjects voluntarily participated in this study. We recruited them through advertisements posted in Yeungnam University College News. The demographic information is shown in Table 2. They were recruited according to the following criteria: (1) no history of neurological impairment in brain and spinal cord; (2) no history of musculoskeletal and peripheral nerve injury in the right upper limb within the past 3 years; (3) no medication uptake for any medical problems within 1 month; (4) no neuropsychological or psychiatric symptoms. Participants who did not have adequate sleep, for at least 7 hours, or who consumed alcohol on the day before the experiment, were excluded. After attaining a full understanding of the purpose of this study, all participants were asked to sign a written informed consent form prior to conduct of the experiment. The study was approved by the Institutional Review Board of the local ethics committee, in accordance with the ethical standards of the Declaration of Helsinki.
Methods
Participants were asked to sit comfortably on a chair with a back rest in a calm laboratory room. All measurements of sensory ability were performed at three time points, approximately 9:00, 13:00, and 18:00. Measurements of three different types of sensory modules were repeated in an order that was counterbalanced across the participants for prevention of learning effect. Each one-third of participants was allocated into one of three different experimental schedules according to time sequence, such as schedule I (i.e., 9:00, 13:00, and 18:00), schedule II (i.e., 13:00, 18:00, and 9:00 of the next day), and schedule III (18:00, 9:00 and 13:00 of the next day). All experimental procedures were performed in the same laboratory room at the condition of under 55 dB noise level. On the day of measurements, all participants were asked to perform general daily activity of life without heavy physical performance.
Assessments
Tactile sensory stimulation was generated using a Biopac STM100C stimulator module with a STMISOC isolation unit (Biopac Systems, Inc., Goleta, CA, USA). The STMISOC unit modulated the current voltage output from the electrodes, with constant square pulse. The stimulator module was connected through a serial-interface to a laptop that controlled the constant current voltage. Electrical stimulation was delivered to the dorsum of the right hand through Ag-AgCl disposable electrodes placed 2−4 cm apart, which was modulated by intensity of current voltage with a gradual increase. Participants were asked to notify the experimenter of the time point when they felt stimulation of the dorsal skin of the hand. The experimenter recorded intensity of current voltage (mV) when the participant initially perceived the electrical stimulation.
Joint reposition test was performed on the metacarpophalangeal joint of the right hand, using a plastic-made frame device with an embedded potentiometer. The potentiometer detected fl exion and extension motion generated in the metacarpophalangeal joint, and these quantities of the joint motion were transferred from the analog signal to a laptop computer. The signals were analyzed using analog-to-digital data acquisition software. The subjects were instructed to actively reproduce the position of the metacarpophalangeal joint with eyes blinded after 15 seconds, and then the joint position was held by the examiner for approximately 5 seconds. The passively positioned angle was located on approximately 50% fl exed angles of total range of motion in the metacarpophalangeal joint. Angular error (volt) of joint reposition between the passively positioned and the actively positioned angles was measured. Two assessments were performed repetitively, and the mean value of those trials was used.
Two-point discrimination test was performed using a Digimatic caliper (CD-15CPS, Mitutoyo, Kawasaki, Japan), in which the tips of two pointers were blunt in order to avoid provoking a pain sensation. The instrument was capable of digitally applying a gap of 0.01 mm between the two points. Two-point sense was touched simultaneously with equal pressure on the dorsum of the right hand just proximally below the third metacarpophalangeal joint. Distance between the two points was made close with the value gap of 0.2 mm, within 5 mm to 20 mm intervals. Minimal value for consistent discrimination of the two points by the participant was adopted. Adequate resting time was provided in each stim-ulus interval in order to minimize neural accommodation to the sensory stimulation. For test reliability, all procedures were performed by the same examiner.
Statistical analysis
Statistical analysis of all data was performed using PASW 18.0 statistical package (SPSS, Chicago, IL, USA). One-way analysis of variance was used for comparison of differences in raw values among the three different time points of the day, in terms of tactile sense, joint reposition sense, and twopoint discrimination. In addition, distribution of ranking for sensory perceptual ability in each sensory module was analyzed by chi-square test. The measurement data were expressed as mean ± SD. Criterion for statistical signi fi cance was set at the level of P < 0.05.
Author contributions:Kwon YH and Nam KS designed this study and wrote the paper. Nam KS was responsible for data acquisition and analysis. Kwon YH contributed to paper review and revision. Both authors approved the final version of this paper.Con fl icts of interest:None declared.
[1] Dijkerman HC, de Haan EH. Somatosensory processes subserving perception and action. Behav Brain Sci. 2007;30:189-201.
[2] Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1148-1156.
[3] Kendal E, Schwartz J, Jessell T. Principles of Neural Science. Mcgraw-Hill, Internation Edition. 2000.
[4] Porro CA, Lui F, Facchin P, et al. Percept-related activity in the human somatosensory system: functional magnetic resonance imaging studies. Magn Reson Imaging. 2004;22:1539-1548.
[5] Shibin K, Samuel AJ. The discrimination of two-point touch sense for the upper extremity in indian adults. Int J Health Rehabil Sci. 2013;2:38-43.
[6] Schimitz TJ. Sensory assessment. In: O’Sullivan SB, Schmitz TJ, eds. Physical Rehabilitation. F.A. Davis Company, New York. 2006.
[7] Akatsuka K, Noguchi Y, Harada T, et al. Neural codes for somatosensory two-point discrimination in inferior parietal lobule: an fMRI study. Neuroimage. 2008;40:852-858.
[8] Saunders D. Introduction to Biological Rhythms. Blackie, Glasgow, Scotland, England. 1977.
[9] Webb W. Biological Rhythms, Sleep, and Performance. John Wiley and Sons, Ltd. New York, NY. 1982.
[10] Winget CM, DeRoshia CW, Holley DC. Circadian rhythms and athletic performance. Med Sci Sports Exerc. 1985;17:498-516.
[11] Gauthier A, Davenne D, Martin A, et al. Time of day effects on isometric and isokinetic torque developed during elbow fl exion in humans. Eur J Appl Physiol. 2001;84:249-252.
[12] Guette M, Gondin J, Martin A. Time-of-day effect on the torque and neuromuscular properties of dominant and non-dominant quadriceps femoris. Chronobiol Int. 2005;22:541-558.
[13] Moussay S, Bessot N, Gauthier A, et al. Diurnal variations in cycling kinematics. Chronobiol Int. 2003;20:879-892.
[14] Moussay S, Dosseville F, Gauthier A, et al. Circadian rhythms during cycling exercise and finger-tapping task. Chronobiol Int. 2002;19:1137-1149.
[15] Souissi N, Bessot N, Chamari K, et al. Effect of time of day on aerobic contribution to the 30-s Wingate test performance. Chronobiol Int. 2007;24:739-748.
[16] Souissi N, Gauthier A, Sesboüé B, et al. Circadian rhythms in two types of anaerobic cycle leg exercise: force-velocity and 30-s Wingate tests. Int J Sports Med. 2004;25:14-19.
[17] Seidel HM, Ball JW, Dains JE, et al. Neurologic system. In: HM Seidel, JW Ball, JE Dains, Benedict GW, eds. Mosby’s Guide to Physical Examination. Mosby Year Book, St Louis, USA. 1987.
[18] Swartz MH. The nervous system. In: MH Swartz, ed. Textbook of Physical Diagnosis: History and Examination. W.B.Saunders Company, Philadelphia. 1994.
[19] Bessot N, Nicolas A, Moussay S, et al. The effect of pedal rate and time of day on the time to exhaustion from high-intensity exercise. Chronobiol Int. 2006;23:1009-1024.
[20] Dosseville F, Moussay S, Larue J, et al. Physical exercise and time of day: in fl uences on spontaneous motor tempo. Percept Mot Skills. 2002;95:965-972.
[21] Callard D, Davenne D, Gauthier A, et al. Circadian rhythms in human muscular efficiency: continuous physical exercise versus continuous rest. A crossover study. Chronobiol Int. 2000;17:693-704.
[22] Gauthier A, Davenne D, Gentil C, et al. Circadian rhythm in the torque developed by elbow fl exors during isometric contraction. Effect of sampling schedules. Chronobiol Int. 1997;14:287-294.
[23] Gauthier A, Davenne D, Martin A, et al. Diurnal rhythm of the muscular performance of elbow fl exors during isometric contractions. Chronobiol Int. 1996;13:135-146.
[24] Rutkove SB, Kothari MJ, Shefner JM. Nerve, muscle, and neuromuscular junction electrophysiology at high temperature. Muscle Nerve. 1997;20:431-436.
[25] Wright KP Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370-1377.
[26] Bougard C, Lepelley MC, Davenne D. The influences of timeof-day and sleep deprivation on postural control. Exp Brain Res. 2011;209:109-115.
[27] Forsman P, Haeggström E, Wallin A, et al. Daytime changes in postural stability and repeatability of posturographic measurements. J Occup Environ Med. 2007;49:591-596.
[28] Naessen T, Lindmark B, Lagerström C, et al. Early postmenopausal hormone therapy improves postural balance. Menopause. 2007;14:14-19.
[29] Naessen T, Lindmark B, Larsen HC. Better postural balance in elderly women receiving estrogens. Am J Obstet Gynecol. 1997;177:412-416.
[30] Naessen T, Lindmark B, Larsen HC. Hormone therapy and postural balance in elderly women. Menopause. 2007;14:1020-1024.
[31] Tamura Y, Hoshiyama M, Inui K, et al. Cognitive processes in two-point discrimination: an ERP study. Clin Neurophysiol. 2004;115:1875-1884.
Copyedited by Fonken LK, Li CH, Song LP, Zhao M
10.4103/1673-5374.128256
Ki Seok Nam, M.S., Department of Physical Therapy, Yeungnam University College of Science and Technology, 170, Hyunchung-ro, Nam-gu, Daegu, 705-703, Republic of Korea, seokah@hanmail.net.
http://www.nrronline.org/
Accepted: 2013-11-02
- 中国神经再生研究(英文版)的其它文章
- Posterior quadrantic disconnection maintains the activity of isolated temporal-parietal-occipital nerve tissue: neuroprotective measures in the surgical treatment of epilepsy
- Examination of Huntington’s disease in a Chinese family
- 7.0T nuclear magnetic resonance evaluation of the amyloid beta (1–40) animal model of Alzheimer’s disease: comparison of cytology veri fi cation
- Local inhibition of GABA affects precedence effect in the inferior colliculus
- Compound Formula Rehmannia alleviates levodopainduced dyskinesia in Parkinson’s disease
- The Pael-R gene does not mediate the changes in rotenone-induced Parkinson’s disease model cells

