In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit
Nizam Uddin, Md. Rakib Hasan, Md. Monir Hossain, Arjyabrata Sarker, A.H.M. Nazmul Hasan, A.F.M. Mahmudul Islam, Mohd. Motaher H. Chowdhury, Md. Sohel Rana
Laboratory of Natural Products Research, Department of Pharmacy, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh
In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit
Nizam Uddin*, Md. Rakib Hasan, Md. Monir Hossain, Arjyabrata Sarker, A.H.M. Nazmul Hasan, A.F.M. Mahmudul Islam, Mohd. Motaher H. Chowdhury, Md. Sohel Rana
Laboratory of Natural Products Research, Department of Pharmacy, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh
PEER REVIEW
Peer reviewer
Dr. Ibrahim Khalil, Associate Professor, Department of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh.
Tel: +88-7791045-51 (Ext.1379)
E-mail: drmikhalil@yahoo.com
Comments
This research is an excellent piece of work where hypoglycemic effect of a fruit extract from Citrus macroptera observed and this is a unique work of this fruit in the world as far as I know. Author coherently discussed therapeutic properties of phytochemical constituents to support the hypoglycemic potential of the fruit extract. α-Amylase inhibition property and OGTT was performed for this research and the result was significant; also there was a set up of standard methodology.
Details on Page 478
Objective:To investigate the therapeutic effects of methanol extract of Citrus macroptera Montr. fruit in α-amylase inhibitory activity (in vitro) and hypoglycemic activity in normal and glucose induced hyperglycemic rats (in vivo).
Diabetes mellitus, Hypoglycemic, Citrus macroptera, α-Amylase, OGTT, Glibenclamide
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by both postprandial and fasting hyperglycemia with disturbances in carbohydrate, fat and protein metabolism. Hyperglycemia in diabetes results either from an absolute deficiency in insulin secretion (type 1 DM) or insulin action (type 2 DM) or both. The incidence of diabetes has increased worldwide in recent years. The estimated number of people with diabetes was 30 million in 1985, 150 million in 2000 and then 246 million in 2007, according to the International Diabetes Federation. Itexpects this number to hit 380 million by 2025[1].
Treatment of diabetes include: enhancement of the action of insulin at the target tissues, with the use of sensitizers (biguanides, thiozolidinediones); stimulation of endogenous insulin secretion with the use of sulfonylureas (glibenclamide, glimepiride), and reduction of the demand for insulin using specific enzyme inhibitors (acarbose, miglitol)[2]. However, there is a burden of unwanted side effects like diarrhea, nausea, dyspepsia, myocardial infarction, peripheral edema and dizziness with the use of these drugs. Plants have been an exemplary source of drugs that have been derived directly or indirectly from them. It is reported that about 800 plants may possess anti-diabetic potential[3]. Hypoglycemic activity of medicinal plants is due to their ability to restore the function of pancreatic tissues by causing an increase in insulin output, inhibiting the intestinal absorption of glucose or facilitating metabolites in insulin dependent processes[4,5].
Citrus macropteraMontr. (family-Rutaceae) (C. macroptera) commonly called Sat Kara (wild orange), is a semi-wild species ofCitrusnative in Malesia and Melanesia. The tree, which has thorns, can reach 5 m in height. Its fruit is about 6-7 cm in diameter, has a fairly smooth, moderately thick rind, and turns yellow when it is ripe. The pulp of the fruit is greenish yellow and dry (does not produce much juice). The juice is very sour, and somewhat bitter. In Bangladesh the rind of theC. macropterais eaten as a vegetable. This plant is medicinally used locally in Assam. Physicians always suggest diabetic patient to eatCitrusfruits to control their blood glucose level. Researchers also concluded thatCitrusfruit extracts represent an excellent alternative for nutraceuticals and functional foods geared towards the management of diabetes. Essential oil of fruit ofCitrusmaximally showed significant reduction of fasting blood glucose and hepatic glucose levels while hepatic glycogen significantly increased when compared to diabetic control animals[6]. According to a 2006 animal study,Citrusextracts not only slow glucose uptake, but also inhibit the movement or transport of glucose through the intestines and liver[7]. A 2009 study examining extracts from a KoreanCitrusfruit called Dangyuja (Citrus grandis) found that it holds great potential for controlling blood glucose levels in diabetic patients[8].Citrus limettafruit peel demonstrated a potential anti-hyperglycemic effect in streptozotocin induced diabetic rats[9]. There are also many other research reports about this therapeutic effect ofCitrusfruits. Investigation onC. macropterafor hypoglycemic property has not been performed yet. That’s why we have designed our research work to explore possible mechanism of hypoglycemic activity of this fruit extract.
2. Materials and methods
2.1. Drugs, chemicals and apparatus
Methanol was bought from SIGMA® (Sigma-Aldrich®, St Louis, USA), while acarbose tablet was purchased from local market, manufactured by Pacific Pharmaceuticals Ltd., Bangladesh. Starch was purchased from local scientific market, Motijheel, Dhaka. Heparin injection was purchased from Rotex Medica, Germany. Amylase was obtained from Merck, Germany. All the chemicals and reagents were analytical grade. Match® glucometer with strips were purchased from Mohammadpur, Dhaka. Humalyzer 3500 was obtained from Human Inc., Germany.
2.2. Plant material
Fruits ofC. macropterawere collected from Sylhet, Bangladesh and authenticated by Md. Abdur Rahim, Technical officer, Department of Botany, Jahangirnagar University. A voucher specimen (Acc. No. 38619) was deposited in the herbarium for future reference. The peels were removed and the fruits were dried and used for further processing.
2.3. Preparation of plant extract
Fruits without rind were treated with sufficient amount of pure methanol for one week at room temperature with occasional shaking. The extract was filtered through a cotton plug followed by Whatman No. 1 filter paper. The filtrate was then evaporated under reduced pressure to give a dark green viscous mass and stored at 4 °C until use.
2.4. Animals and experimental set-up
Sprague-Dawley female rats of 100-200 g were collected from Pharmacology Laboratory, Department of Pharmacy, Jahangirnagar University and were acclimatized to normal laboratory conditions for one week prior to study and were assessed to pellet diet and waterad libitum. Temperature of facility was (22±3) °C and light/darkness alternated 12 h apart. The animals were divided into four groups of five animals each.
2.5. Phytochemical screening
The crude methanol extract ofC. macropterafruits underwent phytochemical screening to detect presence of potential phytochemical constituents like alkaloid, flavonoid, saponin, tannin, carbohydrate, glycoside, glucoside, fat and fixed oil, steroid and terpenoid[10].
2.6. In vitroα-amylase inhibitory activity
This study was performed by a modified starch iodine protocol[11]. In short, 1 mL of plant extract or standard of different concentration (2, 1, 0.5 mg/mL) was taken in prelabeled test tubes. A volume of 20 µL of α-amylase was added to each test tube and incubated for 10 min at 37 °C. After the incubation 200 µL of 1% starch solution was added to each test tube and the mixture was re-incubated for 1 h at 37 °C. Then 200 µL of 1% iodine solution was added to each test tube and after that, 10 mL distilled water was added. Absorbance of the mixture was taken at 565 nm. Sample, substrate and α-amylase blank were undertaken under the same conditions. Each experiment was done in triplicate. IC50value was calculated by using regression analysis.
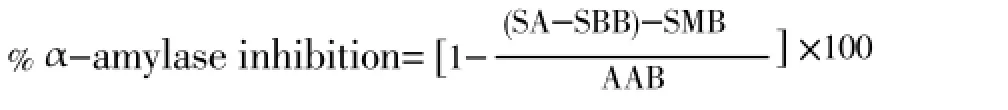
SA=Sample absorbance, SMB=Sample blank, SBB=Substrate blank, AAB=α-Amylase blank
2.7. Acute toxicity study
According to the method of Walum,et al. rats were divided into four groups of five animals each[12]. Different doses (1 000 mg/kg, 2 000 mg/kg, 3 000 mg/kg and 4 000 mg/kg) of methanol extracts were administered by stomach tube. Then the animals were observed for general toxicity signs.
2.8. Experimental protocol for in vivo hypoglycemic activity
2.8.1. Hypoglycemic effect in normal rats
Rats were kept fasting overnight with free access to water. Group I was treated as control group, Group II was treated with glibenclamide (5 mg/kg body weight), Group III and IV was treated with 500 mg/kg and 1 000 mg/kg body weight respectively. Before administration of drug and extract solutions fasting blood glucose levels were estimated by glucose oxidase method[13]. Then blood glucose levels were again estimated after 2 h of administration of drug and extract solutions. Glucose levels were measured by blood Humalyzer instrument. The maximum hypoglycemic effect of glibenclamide was found after 2 h of its administration.
2.8.2. Hypoglycemic effect in glucose induced hyperglycemic rats (OGTT)
Oral glucose tolerance test (OGTT) was performed according to the standard method[14]. Group I was treated as normal control group, Group II treated with glibenclamide (5 mg/ kg body weight), Group III and IV treated with 500 mg/kg and 1 000 mg/kg body weight respectively. Glucose solution (1 g/kg body weight) was administered at first. Then drug and extract solutions were administered to the glucose fed. Serum glucose level of blood sample from tail vein was estimated by using glucometer at 0, 1, 2 and 3 h.
2.9. Statistical analysis
The results were expressed as the mean±SEM. The results were statistically analyzed using repeated measures analysis of variance with Dunnett’s multiple comparison when compared against control in OGTT. Pairedttest was performed to show significant variation between before and after blood glucose level. Student’sttest was performed between IC50values. Regression analysis was performed to calculate IC50values.P<0.05,P<0.01 andP<0.001 were considered as statistically significant. Statistical programs used were GRAPHPAD PRISM®(version 6.00; GraphPad Software Inc., San Diego, CA, USA), SIGMAPLOT (version 12.0, Systat Software Inc., San Jose, California, USA), and Microsoft Excel, 2007.
3. Results
3.1. Phytochemical screening
The active components found in the extract include; saponin, steroid and terpenoid. Results are shown in Table 1.

Table1 Phytochemical constituents identified in methanol extract of C. macroptera fruit.
3.2. In vitroα-amylase inhibitory activity
Methanol extract showed IC50value (3.638±0.190) mg/mL whereas standard acarbose showed (0.912±0.015) mg/mL. Methanol extract significantly inhibited α-amylase activity in a dose dependent manner like acarbose. Therefore we can conclude that this fruit extract have moderate α-amylase inhibitory activity. All results are shown in Table 2.
3.3. Acute toxicity study
The extract administered up to high dose (4 000 mg/kg) produced no mortality. The animals did not manifest any sign of restlessness, respiratory distress, general irritation, coma or convulsion. Hence this extract was considered safe for rats.

Table 2 IC50values (mg/mL) for C. macroptera methanolic fruit extract and acarbose in α-amylase inhibitory assay.
3.4. In vivo hypoglycemic activity
3.4.1. Hypoglycemic effect in normal rats
Both doses of methanol extract and glibenclamide significantly reduced fasting blood glucose level. Glibenclamide showed significant reduction at level ofP<0.01. Dose of 500 mg/kg and 1 000mg/kg methanolic fruit extract showed significant reduction at level ofP<0.05 andP<0.01 respectively. These results suggest that hypoglycemic activity of 1 000 mg/kg dose and glibenclamide has similar significance level. All results are presented in Table 3.

Table 3 Effect of C. macroptera methanolic fruit extract on fasting blood glucose level (mmol/L) in normal rats.
3.4.2. Hypoglycemic effect in glucose induced hyperglycemic rats (OGTT)
Experimental induction of hyperglycemia resulted in increased glucose level in blood (comparing the bar diagram of control of 0 h and 1 hour, Figure 1). Both dose of fruit extract did not manifest any significant reduction in 1st hour after administration. Most significant reduction (P<0.05) was observed for 500 mg/kg dose of methanolic fruit extract at 2 h but maximum reduction of 1 000 mg/kg dose occurred at 3 h showing a significance level ofP<0.01. At 2 h this dose also showed significant reduction (P<0.05). Standard glibenclamide (5 mg/kg) showed significant reduction in 1, 2 and 3 h. These findings suggest that evidently 1 000 mg/kg dose is more potent than 500 mg/kg dose. Time interaction with each specific hour in this experiment was also found extremely significant (P<0.000 1) with an F value 23.83 (Table 4).

Table 4 Effect of C. macroptera methanolic fruit extract on glucose induced hyperglycemia (mmol/L) in normal rats.
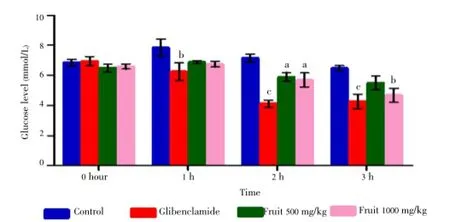
Figure 1. Effect of different doses of fruit extract of C. macroptera and glibenclamide on oral glucose tolerance test. Results are expressed as mean± SEM. Values with different superscripts are significantly different from control.
4. Discussion
4.1.α-Amylase inhibitory activity
α-Amylase is one of the main enzymes in human body that is responsible for the breakdown of starch to more simplesugars. α-Amylases hydrolyze complex polysaccharides to produce oligosaccharides and disaccharides which are then hydrolyzed by α-glycosidase to monosaccharide which are absorbed through the small intestines into the hepatic portal vein and increase postprandial glucose levels[15,16]. Amylase inhibitors are also known as starch blockers because they prevent dietary starch from being absorbed by the body and thereby lower postprandial glucose levels. Slowing the digestion and breakdown of starch may have beneficial effects on insulin resistance and glycemic index control in people with diabetes[17-19]. In our investigation we found that methanolic fruit extract moderately inhibited α-amylase. From phytochemical screening we can see that presence of saponin, steroid and terpenoid which may be responsible for this therapeutic activity. Natural polyphenols have been reported to inhibit the activity of carbohydrate hydrolyzing enzymes like α-amylase, α-glucosidase[18]. Terpenoids represent a promising source for biologically active natural compounds which have potential for research and development of new substances with pharmacologic activity. α-Amylase inhibitory activity was related only for oleanane, ursane and lupane type terpenoids[20]. In a previous study saponins have also been found to be a probable α-amylase inhibitor[21,22]. Lupeol, a terpenoid compound has been isolated from stem bark ofC. macropterawhich could be α-amylase inhibitor[23]. There are previous findings about the potentiality of this compound. Lupeol have been shown to inhibit α-amylase reported by Hasenah Ali,et al[24]. The mechanism by which this fruit extract exerted this effect may be due to its action on carbohydrate binding regions of α-amylase enzymes that catalyze hydrolysis of the internal α-1,4 glucosidic linkages in starch and other related polysaccharides have also been targeted for the suppression of postprandial hyperglycemia. Therefore, this study buttress the claim that natural inhibitors from dietary plants have α-amylase inhibitory activity and could be used as effective therapy for the management of postprandial hyperglycemia with minimal side effects.
4.2. In vivo hypoglycemic activity
In this study methanolic fruit extract ofC. macropteraexerted significant hypoglycemic activity in both fasting glucose level reduction in normal rats and oral glucose tolerance test in glucose induced hyperglycemic rats. To our best knowledge this is the first study about hypoglycemic activity ofC. macroptera. That’s why the precise mode of action is not determined yet. However this fruit extract contains some potent phytochemical constituents like saponin, steroid and terpenoid which may be responsible for this action. Earlier investigations found saponins to be bioactive against diabetes[25,26]. Saponins can influence transport systems that are situated in the brush-border. For example, Sidhu,et al.gave rats soyabean saponins (2 g/L) directly into the small intestine and this led to a reduced glucose uptake[27]. Glycemic decrease that may also occur due to the stimulatory effect on beta cells to promote insulin release and intracellular glycogen deposition. Total saponins identified from traditional medicinal plant in China dramatically reduced fasted blood glucose and serum insulin levels and alleviated hyperglycemia associated oxidative stress in experimental type 2 DM rats[28]. Terpenoids identified in primary screening may contribute to this property. Terpenoids isolated from the some anti-diabetic medicinal plants has been found to stimulate secretion or possess an insulin like-effect[29]. Glycogen concentration is directly proportional to insulin level[30]. Insulin promotes intracellular glycogen deposition by stimulating glycogen synthesis and inhibiting glycogen phosphorylase[30]. Such as insulin, terpenoids type component or monoterpenes may cause a restoration to normal glycogen metabolism when hepatic glycogen concentration is decreased[31]. Lupeol and stigmasterol isolated from stem bark ofC. macropteramay have hypoglycemic potential[23]. Lupeol is a pharmacologically active terpenoid. One study has also found some activity as a dipeptidyl peptidase-4 inhibitor. Dipeptidyl peptidase-4 plays a major role in glucose metabolism. It is responsible for the degradation of incretins such as glucagon-like peptide-1[32]. Lupeol may also have amylase inhibition property which we told in previous section. We found the presence of steroid in phytochemical screening test which could also contribute to hypoglycemic action. In an earlier investigation Daisy,et al.isolated a novel steroid demonstrated a significant antidiabetic activity by reducing the elevated blood glucose levels and restoring the insulin levels in streptozotocininduced diabetic rats[33]. Stigmasterol, an steroid molecule, also possess hypoglycemic property[34]. Lemonene isolated from peel extract ofC. macropteramay be a potential alternative[35]. In a previous study its anti-hyperglycemic activity has been confirmed in streptozotocin-induced diabetic rats by increasing glucokinase activity along with liver glycogen synthesis and decreasing plasma glucose and glycosylated hemoglobin levels[36]. Besides phytochemical constituents found in this extract may also have antiα-amylase activity (discussed in previous section) and contribute to thisin vivohypoglycemic action.
We are still not sure about how this fruit extract can exertpotent hypoglycemic activity. It is a logical inference that this extract may decrease the activity of α-amylase in the digestive canal, improve the metabolism of glucose and increase insulin secretion by stimulating beta cells. It is possibe to suggest that the bioactive compounds present in the fruit extract may be responsible for multifaceted effects. However further co-ordinated and well-structured studies would be required to isolate the bioactive compounds and determine their underlying molecular mechanism of action on diabetes induced rat model.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are thankful to Pharmacology Laboratory, Department of Pharmacy, Jahangirnagar University for providing sufficient number of rats and wish to thank Laboratory of Natural Products Research, Jahangirnagar University for providing financial support and necessary reagents to conduct this research project.
Comments
Background
C. macropterais commonly called ‘wild orange’. It is socalled name because of the large ‘wings’ (-ptera) on the petiole, which is as large as the blade of the leaf. The juice is very sour, and somewhat bitter. It has a unique taste and aroma. According to the literature search, there is no work that has been done on the fruit of this plant for hypoglycemic activity.
Research frontiers
The hypoglycemic effect in OGTT and α-amylase inhibitory activity was evaluated. The results support significant activity of this fruit extract which was the cutting age in the field of the research in this paper.
Related reports
Lupeol and stigmasterol were isolated from the crude extracts of the stem bark ofC. macroptera(family: Rutaceae) by Chowdhury,et al(2008). The essential oil ofC. macropteraMontr. contained limonene, beta-caryophyllene and geranial as main compounds reported by Rana,et al(2012).
Traditionally this fruit is used as vegetable and curries cooked with Satkara and beef or mutton is now served in many restaurants in Bangladesh.
Innovations and breakthroughs
This is the first research work onC. macropterafruit for hypoglycemic effect. In future these findings will help researchers to find out potential antidiabetic agents in this plant.
Applications
C. macropterafruit is safe to eat. This study supports the claim that diabetes patients can eat this fruit because there is no restriction from physicians to takeCitrusfruits. So this plant could be further studied for both drug development and establishment of the ethno medicinal use from this.
Peer review
This research is an excellent piece of work where hypoglycemic effect of a fruit extract fromC. macropteraobserved and this is a unique work of this fruit in the world as far as I know. Author coherently discussed therapeutic properties of phytochemical constituents to support the hypoglycemic potential of the fruit extract. α-Amylase inhibition property and OGTT was performed for this research and the result was significant; also there was a set up of standard methodology.
[1] Riaz S. Diabetes mellitus. Sci Res Essay 2009; 4(5): 367-373.
[2] Groop L, Forsblom C, Lehtovirta M. Characterization of the prediabetic state. Am J Hypertens 1997; 10: 172S-180S.
[3] Grover JK, Yadav S, Vats V. Medicinal plants of India with antidiabetes potential. J Ethnopharmacol 2002; 81(1): 81-100.
[4] Algariri K, Meng KY, Atangwho IJ, Asmawi MZ, Sadikun A, Murugaiyah V, et al. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pac J Trop Biomed 2013; 3(5): 358-366.
[5] Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm 2010; 67(2): 113-118.
[6] Peng CH, Ker YB, Weng CF, Peng CC, Huang CN, Lin LY, et al. Insulin secretagogue bioactivity of finger citron fruit (Citrus medica L. var. Sarcodactylis Hort, Rutaceae). J Agric Food Chem 2009; 57(19): 8812-8819.
[7] Li JM, Che CT, Lau CBS, Leung PS, Cheng CHK. Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. Int J Biochem Cell Biol 2006; 38: 985-995.
[8] Kim GS, Shin JG, Jang HD. Antioxidant and antidiabetic activityof Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem 2009; 117(1): 35-41.
[9] Kundusen S, Haldar PK, Gupta M, Mazumder UK, Saha P, Bala A, et al. Evaluation of antihyperglycemic activity of Citrus limetta fruit peel in streptozotocin-induced diabetic rats. ISRN Endocrinol 2011; doi: 10.5402/2011/869273.
[10] Kujur RS, Singh V, Ram M, Yadava HN, Singh KK, Kumari S, et al. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharmacogn Res 2010; 2(4): 258-263.
[11] Hossan SJ, El-Sayed M, Aoshima H. Antioxidative and anti α-amylase activities of four wild plants consumed by nomads in Egypt. Orient Pharm Exp Med 2009; 9(3): 217-224.
[12] Walum E. Acute oral toxicity. Environ Health Perspect 1998; 106(Suppl 2): 497-503.
[13] Barham D, Trinder P. An improved color reagent for the determination of blood glucose by oxidase system. Analyst 1972; 97: 142-145.
[14] Vigneaud DU, Karr WG.Carbohydrate utilization: I. rate of disappearance of d-glucose from the blood. J Biol Chem 1925; 66: 281-300.
[15] Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol 2010; 101(12): 4676-4689.
[16] El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev 2011; 7(6): 392-405.
[17] Barrett ML, Udani JK. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): a review of clinical studies on weight loss and glycemic control. Nutr J 2011; 10: 24.
[18] Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycemic potential in the treatment of diabetes: an update. Mini Rev Med Chem 2010; 10(4): 315-331.
[19] Nair SS, Kavrekar V, Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol 2013; 3(1): 128-132.
[20] Sales PM, Souza PM, Simeoni LA, Silveira D. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci 2012; 15(1): 141-183.
[21] Ponnusamy S, Ravindran R, Zinjarde S, Bhargava S, Ravi Kumar A. Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid Based Complement Alternat Med 2011; doi: 10.1155/2011/515647.
[22] Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian ayurvedic medicinal plants. BMC Complement Altern Med 2011; doi: 10.1155/2011/515647.
[23] Chowdhury SA, Sohrab MH, Datta BK, Hasan CM. Chemical and antioxidant studies of Citrus macroptera. Bangladesh J Sci Ind Res 2008; 43(4): 449-454.
[24] Ali H, Houghton PJ, Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2006; 107(3): 449-455.
[25] Hamden K, Jaouadi B, Salami T, Carreau S, Bejar S, Elfeki A. Modulatory effect of fenugreek saponins on the activities of intestinal and hepatic disaccharidase and glycogen and liver function of diabetic rats. Biotechnol Bioprocess Eng 2010; 15(5): 745-753.
[26] Chen ZH, Li J, Liu J, Zhao Y, Zhang P, Zhang MX, et al. Saponins isolated from the root of Panax notoginseng showed significant anti-diabetic effects in KK-Ay mice. Am J Chin Med 2008; 36(5): 939-951.
[27] Sidhu GS, Upson B, Malinow MR. Effects of soy saponins and tigogenin cellobioside on intestinal uptake of cholesterol, cholate and glucose. Nutr Rep Int 1987; 35(3): 615-623.
[28] Zheng T, Shu G, Yang Z, Mo S, Zhao Y, Mei Z. Antidiabetic effect of total saponins from Entada phaseoloides (L.) Merr. in type 2 diabetic rats. J Ethnopharmacol 2012; 139(3): 814-821.
[29] Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res 2010; doi: 10.1155/2010/483958.
[30] Jensen J, Lai YC. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch Physiol Biochem 2009; 115(1): 13-21.
[31] Muhammad NO, Soji-Omoniwa O, Usman LA, Omoniwa BP. Antihyperglycemic activity of leaf essential oil of Citrus sinensis (L.) Osbeck on alloxan-induced diabetic rats. Ann Rev Res Biol
2013; 3(4): 825-334.
[32] Marques MR, Stüker C, Kichik N, Tarragó T, Giralt E, Morel AF, et al. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia 2010; 81(6): 552-556.
[33] Daisy P, Jasmine R, Ignacimuthu S, Murugan E. A novel steroid from Elephantopus scaber L. an ethnomedicinal plant with antidiabetic activity. Phytomedicine 2009; 16: 252-257.
[34] Panda S, Jafri M, Kar A, Meheta BK. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009; 80(2): 123-126.
[35] Rana VS, Blazquez MA. Compositions of the volatile oils of Citrus macroptera and C. maxima. Nat Prod Commun 2012; 7(10): 1371-1372.
[36] Murali R, Saravanan R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed Prev Nutri 2012; 2(4): 269-275.
10.12980/APJTB.4.2014C1173
*Corresponding author: Nizam Uddin, Laboratory of Natural Products Research, Department of Pharmacy, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh.
Tel: +8801763148391
E-mail: sami.pharm22@gmail.com
Foundation Project: Supported by Laboratory of Natural Products Research, Jahangirnagar University, Dhaka, Bangladesh
Article history:
Received 13 Feb 2014
Received in revised form 1 Mar, 2nd revised form 4 Mar, 3rd revised form 14 Mar 2014
Accepted 12 Apr 2014
Available online 28 Jun 2014
Methods:Fruits of Citrus macroptera without rind was extracted with pure methanol following cold extraction and tested for presence of phytochemical constituents, α-amylase inhibitory activity, and hypoglycemic effect in normal rats and glucose induced hyperglycemic rats.
Results:Presence of saponin, steroid and terpenoid were identified in the extract. The results showed that fruit extract had moderate α-amylase inhibitory activity [IC50value=(3.638±0.190) mg/mL] as compared to acarbose. Moreover at 500 mg/kg and 1 000 mg/kg doses fruit extract significantly (P<0.05 and P<0.01 respectively) reduced fasting blood glucose level in normal rats as compared to glibenclamide (5 mg/kg). In oral glucose tolerance test, 500 mg/kg dose significantly reduced blood glucose level (P<0.05) at 2 h but 1 000 mg/kg dose significantly reduced blood glucose level at 2 h and 3 h (P<0.05 and P<0.01 respectively) whereas glibenclamide (5 mg/ kg) significantly reduced glucose level at every hour after administration. Overall time effect is also considered extremely significant with F value=23.83 and P value=0.0001 in oral glucose tolerance test.
Conclusion:These findings suggest that the plant may be a potential source for the development of new oral hypoglycemic agent.
 Asian Pacific Journal of Tropical Biomedicine2014年6期
Asian Pacific Journal of Tropical Biomedicine2014年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.)
- Macrosomia in non-gestational diabetes pregnancy: glucose tolerance test characteristics and feto-maternal complications in tropical Asia Pacific Australia
- Evaluation of leptin, interleukin-1 beta and tumor necrosis factor alpha in serum of malaria patients as prognostic markers of treatment outcome
- Entamoeba histolytica acetyl-CoA synthetase: biomarker of acute amoebic liver abscess
- Traumatic myiasis agents in Iran with introducing of new dominant species, Wohlfahrtia magnifica (Diptera: Sarcophagidae)
- Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
