Macrosomia in non-gestational diabetes pregnancy: glucose tolerance test characteristics and feto-maternal complications in tropical Asia Pacific Australia
Algenes Aranha, Usman H Malabu, Venkat Vangaveti, Elham Saleh Reda, Yong Mong Tan, Kunwarjit Singh Sangla
1Mater Private Hospital, 21-37 Fulham Road, Townsville, Queensland 4812, Australia
2Townsville Hospital, Department of Diabetes & Endocrinology, 100 Angus Smith Drive, Townsville, Queensland, Australia 4814
3School of Medicine & Dentistry, James Cook University, School of Medicine,1 James Cook Drive Townsville, Queensland, Australia 4811
4Gold Coast Hospital, Diabetes & Endocrinology, 1 Hospital Boulevard, Southport, Gold Coast, Queensland, Australia 4215
Macrosomia in non-gestational diabetes pregnancy: glucose tolerance test characteristics and feto-maternal complications in tropical Asia Pacific Australia
Algenes Aranha1*, Usman H Malabu2, Venkat Vangaveti3, Elham Saleh Reda4, Yong Mong Tan2, Kunwarjit Singh Sangla2
1Mater Private Hospital, 21-37 Fulham Road, Townsville, Queensland 4812, Australia
2Townsville Hospital, Department of Diabetes & Endocrinology, 100 Angus Smith Drive, Townsville, Queensland, Australia 4814
3School of Medicine & Dentistry, James Cook University, School of Medicine,1 James Cook Drive Townsville, Queensland, Australia 4811
4Gold Coast Hospital, Diabetes & Endocrinology, 1 Hospital Boulevard, Southport, Gold Coast, Queensland, Australia 4215
PEER REVIEW
Peer reviewer
Dr. Nilanjana Suthaharan, Consultant Physician and Endocrinologist, Hervey Bay and Maryborough Hospitals, Cnr Nissen Street and Urraween Road, Pialba, QLD 4655, Australia.
Tel: +61743256666
E-mail: Emershia_Suthaharan@ health.qld.gov.au
Comments
This is a valuable research in which authors have demonstrated the pattern of blood glucose tests in non-GDM macrosomic subjects in the Asia Pacific region. Such study is relevant in the region noted to have the highest prevalence of gestational diabetes in the world.
Details on Page 439
Objective:To look into the glucose tolerance test characteristics and determine complications in non-gestational diabetes pregnant subjects.
Non-gestational diabetes, Macrosomia, Glucose tolerance test characteristics, Pregnancy
1. Introduction
Macrosomia defined as weight of a full-term infant greater than 90th percentile for gestational age or higher than 4 000 g occurs in 6%-10% of all deliveries[1,2]. Foetal macrosomia is associated with a higher frequency of operative deliveries, post partum haemorrhages, birth injury during vaginal delivery, and neonatal hypoglycaemia. Known maternal risk factors are only identified in 40% of women who deliver macrosomic babies. For instance, in North America, African Americans have been reported to have higher risk of delivering macrosomic babies[3]. InAustralasia where obesity and metabolic syndrome has been a growing concern, recent studies have shown higher rate of macrosomia in the population sub-group[4].
Diagnosing gestational diabetes mellitus (GDM) may involve an initial screening of all pregnant women between 24 to 28 weeks gestation with a 50 g oral glucose challenge test (GCT). A venous glucose >7.7 mmol/L at 1 h is then followed up by a 75 g oral glucose tolerance test (OGTT). A fasting glucose level of >5.5 mmol/L, or a 2 h level >8.0 mmol/L confirmed GDM in Australia and >9.0 mmol/L, in New Zealand[5]. However, recently the validity of using diagnostic criteria to diagnose GDM is questioned due to amongst other factors poor predictive value of macrosomia in subjects with non-GDM[6,7]. Nevertheless various tests have been used with different threshold aiming at predicting macrosomia which is noted for having high feto-maternal complications. Although glucose tolerance studies have been conducted to diagnose GDM, few studies have investigated its pattern in subjects with normoglycemia in pregnant women with macrosomia. In view of the need to identify macrosomia in order to prevent peri-natal complications, usefulness of blood glucose profile in non-diabetic pregnant women might be helpful. The aim of the study was to determine blood glucose tolerance test characteristics and associated perinatal complications in women with non-GDM macrosomic babies at delivery.
2. Materials and methods
A retrospective observational analysis was performed on 91 clinical case notes of non-GDM subjects, who had given birth to macrosomic infants defined as birth weights exceeding 90th percentile for a given gestational week. A total of 41 subjects with normal birth weights served as controls. The patients were randomly selected on the first 91 available case notes received, between 2006 and 2009.
Subjects were assessed for maternal age, height, body mass index (BMI), parity, previous history of macrosomic birth, lifestyle and mode of delivery. Complications at birth assessed for included post partum haemorrhage, neonatal birth injuries, neonatal hypoglycaemia and neonatal respiratory distress. Post partum haemorrhage was defined as the loss of 500 mL or more of blood after vaginal delivery, and the loss of more than 1 000 mL of blood after a caesarean section delivery[8].
BMI was calculated from the booking in or pre-pregnancy weight, as recorded on the maternal hand held charts. Paternal BMI was unavailable. There was insufficient documentation of weight gain with respect to non-GDM large for gestational age subjects and thus it was not included in the analysis.
We assessed the total number of our study subjects who had given birth to macrosomic infants despite normal 50 g GCT and/or a normal 75 g OGTT. An abnormal GCT was defined as a 1 h venous glucose value equal to or greater than >7.7 mmol/L. An abnormal OGTT was defined as a fasting glucose of ≥5.5, or a 2 h value ≥8.0 mmol/L. Subjects diagnosed to have GDM were excluded from the study. Data were represented as absolute numbers and percentages whileChi-square test was performed to test for association between two categorical factors. For continuous data that were not normally distributed, Mann-Whitney test was performed using SPSS version 12.0 (Chicago, IL, USA). Results were considered statistically significant at aPvalue less than 0.05.
3. Results
One hundred and thirty two subjects were reviewed comprising 91 with non-GDM macrosomic babies while 41 delivered non-macrosomic babies (Table 1). An overwhelming majority of our study population was Caucasian 85.6% (113/132) compared to 9.8% (13/132) of Indigenous Australians with relative risk. About 13.1% (12/91) of our study participants with non-GDM macrosomia were of ATSI ethnicity compared to 84.6% (77/91) of the Caucasian subjects. Others 5.5% (5/91) comprising two each of Maori of New Zealand and Pacific Islander descent, and one Asian. There were no significant differences in the Caucasian population at risk of macrosomia. About 20.9% (19/91) of subjects with non-GDM macrosomia gave a history of being smokers during the course of the pregnancy, 16.5% (15/91) consumed unspecified amounts of alcohol, and 4.4% (4/91) gave a history of recreational drug usage.
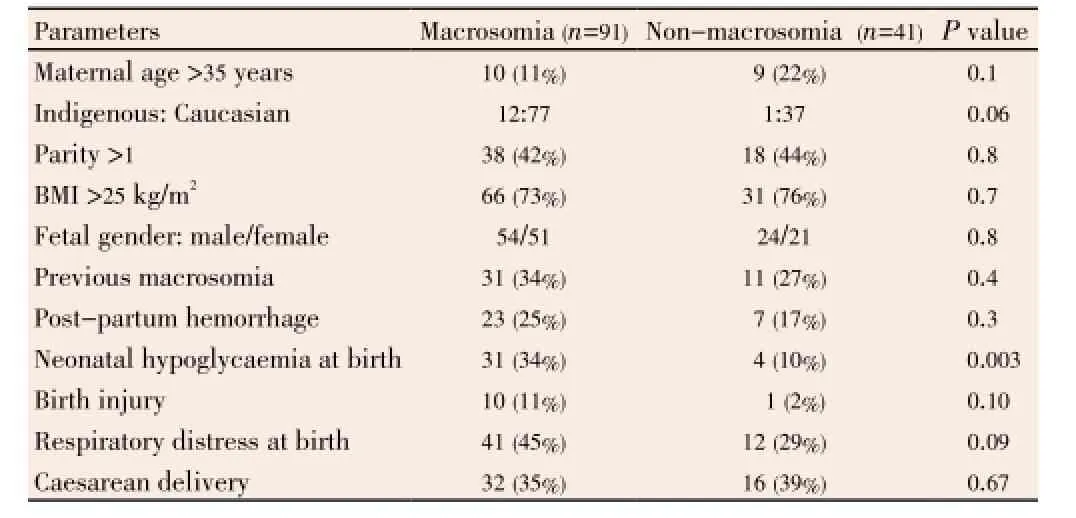
Table 1 Characteristics and foeto-maternal complications of non-GDM macrosomia subjects compared with non-macrosomic controls.
Age distribution and anthropometric data were similar in both groups. Majority of the subjects in both groupswere aged less than 35 years. Two-third of the mothers with non-GDM macrosomic babies and normal controls had initial antenatal clinic booking in or pre-pregnancy BMI greater than 25. Almost all the subjects with non-GDM macrosomic babies 97.7% (88/91) of subjects gave birth at 37-42 weeks gestation. About one-third [35% (32/91)] of subjects underwent Caesarean section delivery while the remaining had normal vaginal delivery with or without an assisted birth. One-quarter of the subjects with non-GDM large for gestational age subjects and thus it was not included in the analysis (23/91) suffered from postpartum haemorrhage. Other complications included 34% (31/91) of our subjects’ neonates suffered from neonatal hypoglycaemia, defined as the first measured venous blood glucose of the infant less than 3 mmol/L compared to 10% in non-macrosomic neonates (P<0.01). All of the 31 neonates necessitated neonatal intensive care unit admission, with uneventful outcome. Birth injuries in the non-GDM macrosomic neonates occurred in 11.0% (10/91) of births, predominantly from shoulder dystocia. Respiratory distresses, defined as the slightest need of oxygen to assist breathing in the neonate, and including need for intubation, were present in 45% (41/91) and 29% (12/41) of cases in subjects with non-GDM mothers with macrosomia and non-macrosomic controls respectively (P>0.05). Only 23% (21/91) of neonate subjects had antenatal ultrasound features of macrosomia while the remaining 68 had nonmacrosomic findings.
Details of the glucose profile of the subjects with macrosomic babies revealed 50 g GCT as a screening test was performed in the first instance on 73% of cases (66/91 subjects), with 17% (11/66) recording 1 h post challenge glucose value of ≥7.7 mmol/L. Another 16 out of 91 subjects considered at risk for GDM, preceded directly to the 75 g OGTT, without undergoing the 50 g GCT. The remaining nine subjects were transferred from interstate, and did not have available GCT or OGTT information.
We identified four patterns of glucose test profiles in our non-GDM women with macrosomic babies:
(a) 45 subjects who had a normal 50 g GCT (<7.8 mmol/L) went on to deliver macrosomic infants.
(b) Eight subjects with an abnormal 50 g GCT (>7.7 mmol/ L), but a normal 75 g OGTT, delivered macrosomic babies.
(c) 14 out of the 16 subjects, who had their 75 g OGTT tests done in the first instance, owing to risk factors for a macrosomic infant, delivered macrosomic infants, despite having had normal biochemical 75 g OGTT response.
(d) Four subjects who underwent a 75 g OGTT despite a normal biochemical 50 g GCT response, delivered macrosomic infants, despite the normal 75 g OGTT result.
4. Discussion
We have reported poor predictive value of macrosomia in non-GDM mothers using glucose tests. Subjects with macrosomic babies had virtually normal screening and diagnostic values of glucose tolerance. Our report further highlighted the need to look for other risk factors beyond blood glucose profile. Although not the commonest, GDM is the strongest risk factor for macrosomia with a twofold increase in the incidence[6,9]. Many of the other risk factors (e.g., prolonged gestation, obesity and multiparity) are highly prevalent among parturient, thus limiting their utility as shown in our study population.
Interestingly, our study detected four distinct patterns of glucose test characteristics in non-GDM macrosomia using 50 g GCT and 75 g OGTT. Firstly, almost 50% of cases had a normal 50 g GCT, and did not undergo further tests. Secondly, eight other subjects had an abnormal response to GCT, but a normal response to OGTT. Thirdly, another 16 subjects, deemed to have risk factors for macrosomia, had a normal biochemical response to the 75 g OGTT in the first instance yet delivered macrosomic babies. Fourthly, four subjects had normal results for both tests but had macrosomic babies at delivery. Analysis from Huynhet al. indicates that the 75 g OGTT may be the best procedure, without prior GCT although about 10% of our cohort had abnormal GCT indicating sub-clinical dysglycemia[10]. Systematic review on prediction of macrosomic outcomes between using the International Association Of Diabetes And Pregnancy Study Groups criteria and World Health Organisation guidelines for diagnosis of GDM were shown not sensitive enough in predicting macrosomia in subjects at risk[11,12].
Previous history of macrosomia was the strongest predictor for non-GDM macrosomia in our cohort with normal OGTT/GCT. Indeed 87.5% of our subjects with past history of delivering macrosomic babies had normal OGTT. Our result is in support of Ogonowskiet al.and others reports in Caucasians, African Americans, Chinese and Asians where past history of macrosomia showed higher diagnostic value[1,9,13]. Koyanagiet al.recently reported increasing prevalence of non-GDM macrosomia worldwide suggesting that maternal hyperglycemia is not the only causative factor for macrosomic infants[14]. Apart from genetic predisposition, other independent predictors of macrosomia have been identified such as pre-pregnancy overweight or obesity, excessive weight gain during pregnancy, prior GDM, advanced age, family medical history of diabetes and prior pregnancy[14,15].
As previously reported, our study showed significantfoeto-maternal complications in subjects with non-GDM macrosomic babies. We recorded significant neonatal hypoglycemia at delivery in spite of documented normal maternal glycemic profile in keeping with possible pathogenetic role of glucose-insulin interaction in non-GDM macrosomia[16,17]. In contrast, Kewet al.demonstrated that non-GDM mothers who gave birth to macrosomic infants do not display the post partum metabolic dysfunction of women with established GDM, indicating dysglycemia, insulin resistance and beta cell dysfunction are not restricted to only GDM[18]. Our result however further supports having glucose profile as part of work up for pregnant women with macrosomia and to further follow up postnatally for diabetes[17]. Thus, a macrosomic delivery, in the absence of GDM, is not necessarily indicative of benign metabolic profile. Data from Wollschlaegeret al.that included 956 cases of macrosomia[19], detected increased umbilical vein insulin levels in a third consistent with previous studies showing elevated cord insulin and C-peptide levels[16,17], indicative of foetal hyperinsulinemia, and hence, support the possibility of undetected maternal glucose intolerance during the pregnancy.
The limitations of the study were that the glucose test profile was not repeated during the period of pregnancy and two subjects did not have glucose profile at entry though no report of elevated serum glucose was found in the chart and at delivery. Also the discrepancy in BMI-as weight derived from the first antenatal visit may not necessarily be the pre-pregnant body weight. The study was retrospective, therefore important variables, such as glycosylated haemoglobin and fixed durations of gestation for weight measurements in pregnancy were not always indicated. Lastly, it is important to note that there is a limitation to retrospective studies in general. Observations derived from such studies may contain some missing information and thus may serve as a stimulus to further prospective work to clarify findings. The present work must be interpreted in the knowledge of the defects inherent in such studies. Despite these our result is consistent with other reports[13,20].
In conclusion, we report history of previous macrosomia yielding higher pre-test probability of macrosomia in subjects with normal glucose tolerance tests during pregnancy. Macrocosmic adverse events observed in our series were similar to previously reported in literature. There is need to devise better ways of defining non-GDM macrosomia beyond GCT/OGTT. Further prospective studies on a larger population to characterise glucose homeostasis in non-GDM macrosomic mothers are needed to confirm our findings.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was supported in part by funds from Australia’s James Cook University research infrastructure block grant RIBG 09-2009. We recognise the contributions of Jenine Lawlor of Townsville Hospital research unit and our medical officers Su Min Wong and Ai Fern Han for their help in data collection.
Comments
Background
Non-GDM is a growing concern in Asia Pacific region where diabetes prevalence is the highest in the world. Non-GDM macrosomia is a diagnosis of exclusion based on blood glucose profile in early part of pregnancy. In those diagnosed as having large for gestational age babies not due to GDM, there is need to further characterise glucose tolerance profile which may assist in predicting macrosomia with its associated peri-natal complications.
Research frontiers
Maternal hyperglycemia has been implicated as the major cause of neonatal macrosomia, yet clinicians frequently report large for gestational age infants in normoglycemic pregnancies. Review of glycemic profile in such population may assist in predicting macrosomia and outcomes in non-GDM. This study will lead to revision of the International Association Of Diabetes And Pregnancy Study Groups criteria and World Health Organisation guidelines for diagnosis of GDM as they were shown not sensitive enough in predicting macrosomia in subjects at risk insulin resistance particularly in the Asia Pacific region.
Related reports
The authors reported significant neonatal complications in subjects with non-GDM macrosomic babies confirming possible links in the pathogenesis of macrosomia in both diabetic and diabetic pregnancies.
Innovations and breakthroughs
This study is novel in the region and only very few studies of this nature worldwide conducted detailing blood glucose characteristics in non-GDM mothers with macrosomic babies.
Applications
The finding in this study is applicable in the high risk population of Asia Pacific region where the need to diagnose macrosomia is vital so as to prevent maternal and fetal complications.
Peer review
This is a valuable research in which authors have demonstrated the pattern of blood glucose tests in non-GDM macrosomic subjects in the Asia Pacific region. Such study is relevant in the region noted to have the highest prevalence of gestational diabetes in the world.
[1] Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep 2006; 55(1): 1-101.
[2] Mondestin MA, Ananth CV, Smulian JC, Vintzileos AM. Birth weight and fetal death in the United States: the effect of maternal diabetes during pregnancy. Am J Obstet Gynecol 2002; 187(4): 922-926.
[3] Auger N, Park AL, Zoungrana H, Fon Sing M, Lo E, Luo ZC. Widening inequality in extreme macrosomia between Indigenous and non-Indigenous populations of Québec, Canada. Aust N Z J Public Health 2013; 37(1): 58-62.
[4] Porter C, Skinner T, Ellis I. The current state of Indigenous and Aboriginal women with diabetes in pregnancy: a systematic review. Diabetes Res Clin Pract 2012; 98(2): 209-225.
[5] Martin FI. The diagnosis of gestational diabetes. Ad Hoc Working Party. Med J Aust 1991; 155: 112.
[6] Shang M, Lin L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J Perinatol 2014; 34(2): 100-104.
[7] Reyes-Muñoz E, Parra A, Castillo-Mora A, Ortega-González C. Effect of the diagnostic criteria of the International Association of Diabetes and Pregnancy Study Groups on the prevalence of gestational diabetes mellitus in urban Mexican women: a cross-sectional study. Endocr Pract 2012; 18(2): 146-151.
[8] Leveno K, Cunningham F, Alexander J, Bloom S, Casey B, Dashe J, et al. Williams manual of obstetrics: pregnancy complications. 22nd ed. New York: McGraw Hill Professional; 2007.
[9] Shi P, Yang W, Yu Q, Zhao Q, Li C, Ma X, et al. Overweight, gestational weight gain and elevated fasting plasma glucose and their association with macrosomia in Chinese pregnant women. Matern Child Health J 2014; 18(1): 10-15.
[10] Huynh J, Ratnaike S, Bartalotta C, Permezel M, Houlihan C. Challenging the glucose challenge test. Aust N Z J Obstet Gynaecol 2011; 51: 22-25.
[11] Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes - a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012; 12: 23.
[12] Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3): 676-682.
[13] Ogonowski J, Miazgowski T, Homa K, Celewicz Z, Kuczyńska M. Low predictive value of traditional risk factors in identifying women at risk for gestational diabetes. Acta Obstet Gynecol Scand 2007; 86(10): 1165-1170.
[14] Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP, et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet 2013; 381(9865): 476-483.
[15] Einerson BD, Huffman JK, Istwan NB, Rhea DJ, Joy SD. New gestational weight gain guidelines: an observational study of pregnancy outcomes in obese women. Obesity (Silver Spring) 2011; 19(12): 2361-2364.
[16] Mestan K, Ouyang F, Matoba N, Pearson C, Ortiz K, Wang X. Maternal obesity, diabetes mellitus and cord blood biomarkers in large-for-gestational age infants. J Pediatr Biochem 2010; 1(3): 217-224.
[17] Kaaja R, Rönnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. Rev Diabet Stud 2008; 5(4): 194-202.
[18] Kew S, Ye C, Sermer M, Connelly PW, Hanley AJ, Zinman B, et al. Postpartum metabolic function in women delivering a macrosomic infant in the absence of gestational diabetes mellitus. Diabetes Care 2011; 34(12): 2608-2613.
[19] Wollschlaeger K, Nieder J, Köppe I, Härtlein K. A study of fetal macrosomia. Arch Gynecol Obstet 1999; 263(1-2): 51-55.
[20] Tarim E, Cok T. Macrosomia prediction using different maternal and fetal parameters in women with 50 g glucose challenge test between 130 and 140 mg/dL. Arch Gynecol Obstet 2011; 284(5): 1081-1085.
10.12980/APJTB.4.2014APJTB-2013-0027
*Corresponding author: Dr. Algenes Aranha, Mater Private Hospital, 21-37 Fulham Road, Townsville, Queensland 4812, Australia.
Tel: +61415322037
Fax: +61747274019
E-mail: montecristo2009@gmail.com
Foundation Project: Supported in part by funds from Australia’s James Cook University research infrastructure block grant (Grant No. RIBG 09-2009).
Article history:
Received 1 Apr 2014
Received in revised form 8 Apr, 2nd revised form 12 Apr, 3rd revised form 18 Apr 2014
Accepted 28 Apr 2014
Available online 28 Jun 2014
Methods:From 2006 to 2009 all non-gestational diabetes mellitus (non-GDM) pregnant women who delivered macrosomia at the North Australia’s Townsville Hospital were retrospectively reviewed by extracting data from clinical record. Glucose tolerance tests results were analysed in the light of an earlier diagnosis of non-GDM.
Results:Ninety-one non-GDM mothers with macrosomia were studied and compared with 41 normoglycemic subjects without macrosomia. Of the subjects with non-GDM macrosomia, 45 (49.4%) had normal 50 g glucose challenge test (GCT) without further testing, another 8 (8.8%) had abnormal GCT but normal 75 g oral glucose tolerance test (OGTT). A total of 4 (4.4%) subjects had normal GCT and OGTT. Interestingly, 14 out of 16 (87.5%) subjects who were tested with OGTT owing to past history of macrosomia had normal results but delivered macrosomic babies. Only 12 subjects had both GCT and OGTT, the rest of the cohort had either of the two tests. Subjects with non-GDM macrosomia had higher frequency of neonatal hypoglycaemia 34% as compared to 10% in nonmacrosomic babies (P=0.003). Other feto-maternal complications were similar in both groups.
Conclusions:No significant pattern of glucose tolerance characteristics was identified in non-GDM mothers with macrosomic babies. In spite of being normoglycemic significant neonatal hypoglycaemia was recorded in non-GDM macrosomic babies. Further prospective studies on a larger population are needed to verify our findings.
 Asian Pacific Journal of Tropical Biomedicine2014年6期
Asian Pacific Journal of Tropical Biomedicine2014年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.)
- Evaluation of leptin, interleukin-1 beta and tumor necrosis factor alpha in serum of malaria patients as prognostic markers of treatment outcome
- Entamoeba histolytica acetyl-CoA synthetase: biomarker of acute amoebic liver abscess
- Traumatic myiasis agents in Iran with introducing of new dominant species, Wohlfahrtia magnifica (Diptera: Sarcophagidae)
- Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
- Antimicrobial activity of some essential oils against oral multidrugresistant Enterococcus faecalis in both planktonic and biofilm state
