Evaluation of leptin, interleukin-1 beta and tumor necrosis factor alpha in serum of malaria patients as prognostic markers of treatment outcome
Mariam Abdulrhman Al-Fadhli, Mohammad Ahmed Saraya,2, Jafar Abdulrida Qasem
1Department of Medicine, Infectious Disease Hospital, Ministry of Health, Kuwait
2Department of Tropical Medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt
3Department of Applied Medical Sciences, College of Health Sciences, Public authority for Applied Education and Training, Kuwait
Evaluation of leptin, interleukin-1 beta and tumor necrosis factor alpha in serum of malaria patients as prognostic markers of treatment outcome
Mariam Abdulrhman Al-Fadhli1, Mohammad Ahmed Saraya1,2, Jafar Abdulrida Qasem3*
1Department of Medicine, Infectious Disease Hospital, Ministry of Health, Kuwait
2Department of Tropical Medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt
3Department of Applied Medical Sciences, College of Health Sciences, Public authority for Applied Education and Training, Kuwait
PEER REVIEW
Peer reviewer
Prof. Jiwan S. Sidhu, Ph.D, Director, Master’s Program, Department of Food Science & Nutrition, College of Life Sciences, Kuwait University, P.O. Box 5969, Safat-13060, Kuwait.
Tel: (965)-463-3324
Fax: (965)-2251-3929
E-mail: jiwan.sidhu@yahoo.com
Comments
This study has shown that 7 d after discharge, a significant decline in serum leptin levels, TNFα and IL1 in the patients group was noticed when compared between the time of admission and the time of discharge, with a positive correlation between serum leptin levels and TNFα & IL1. Thus, the leptin may play an important role in the development and outcome of malaria infection.
Details on Page 444
Objective:To analyze serum leptin levels in patients with malaria falciparum and compare them with healthy controls and correlate with development and outcome of malaria infection.
Malaria, Leptin, IL-1, Tumor necrosis factor alpha (TNFα), Kuwait
1. Introduction
Leptin is the 16-kDa non-glycosylated protein product of the ob gene[1]. It is a hormone synthesized mainly in adipose cells and it regulates body weight in a central manner, via its receptor in the hypothalamus[2]. Interestingly, there is increasing evidence that leptin has systemic effects apart from those related to energy homeostasis, including regulation of neuroendocrine, reproductive, haematopoietic and immune functions[3]. It is known that leptin secretion can be regulated by inflammatory mediators such as interleukin-1 (IL1) and tumor necrosis factor alpha (TNFα)[4]. Furthermore, leptin exerts central effects on hypothalamic-pituitaryfunction and these effects might affect the severity of malaria disease. Disturbance in hypothalamic-pituitaryadrenal axis duringPlasmodium falciparuminfection have been implicated in the pathogenic mechanism of severe malaria[5].
Leptin, which is involved in a range of physiological processes, could be an important factor in the pathogenesis of malaria. The aim of the present study is to analyze serum leptin levels in patients infected with malaria falciparum and compare them with healthy controls. We try to provide evidence that leptin could be an important factor in development and outcome of malaria infection.
2. Materials and methods
2.1. Study design and the participants
This study was conducted at the Infectious Disease Hospital, Kuwait, from October, 2010 to April, 2012. A total of 60 male cases of malaria falciparum were included in this study as patients group. Among them, 52 patients were Indian and eight were Bangladesh. In addition, 30 healthy male individuals of comparable age, race and body mass index (BMI) were taken as controls. All malaria patients were diagnosed by clinical picture and their confirmations were based on the presence of malaria parasites in blood film. Management was done as per standard guidelines for the management ofPlasmodium falciparum. And also its complications in the form of given quinine and vibramycin for 7 d in proper dose within 1 h of admission to the hospital.
2.2. Exclusion criteria
The patients were excluded from the study due to advanced age, gender (females), higher or lower BMI, diabetic state, advanced stage of the malaria disease such as cerebral malaria, disseminated intravascular coagulopathy, organ failure. Those with history of chronic liver disease, immunocompromized (HIV/drugs), positive viral hepatitis profile, and active alcohol consumers were also excluded from the study.
2.3. Data collection
The controls as well as malaria cases were submitted to full history taking, comprehensive clinical examination, BMI (kg/m2), liver function test, kidney function test, complete blood count, fasting blood sugar, fasting serum insulin, estimation of pro-inflammatory cytokines TNFα and IL1 (Biomedix medical group, Synlab, German) and estimation of morning serum leptin (Diagnostic Systems Laboratories, USA) using ELISA on the day of admission (before starting treatment), on the day of discharge and after 7 d of discharge from the hospital.
2.4. Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS) version 8.0 software. The significance of differences between mean values of the study variables was evaluated by usingt-test. The significance of differences between proportions was performed using theChi-square test. Correlation coefficient (r) between quantitative variables was calculated.Pvalue less than 0.05 was considered significant.
3. Results
The age, sex and BMI of patients in both groups were compared; no statistically significant differences in the means between the groups were observed (Table 1). This was intended to exclude factors that affected serum leptin levels. At time of admission, leptin levels were significantly higher in patients group than in control group (mean serum leptin levels of 24.3 ng/mLv.s.7.9 ng/mL,P<0.001). While the fasting serum insulin levels were not significantly different between the two groups (Table 1). It has been further observed that there were significant increases as regard pro-inflammatory cytokine TNFα and IL1 in malaria patients when compared with controls (Table 1). There were significant differences between the studied groups as regard liver enzymes alanine transaminase and aspartate transaminase, hemoglobin and platelet.

Table 1 Comparison between studied groups at time of admission.
At time of discharge, we noted a decline in serum leptin levels and pro-inflammatory cytokine TNFα and IL1 in the patients group, but the same significant differences as regard of these parameters were observed between two groups (Table 2). Platelet was still significant low in patients group as compared with controls.

Table 2 Comparison between studied groups at time of discharge.
After 7 d of discharge, we observed a significant decline in serum leptin levels and pro-inflammatory cytokine TNFα and IL1 in the patients group as compared with time of admission and time of discharge. There were no significant differences between the patients group and the control group as regard of these parameters (Table 3). There were no significant differences between the studied groups as regard liver enzymes (alanine transaminase & aspartate transaminase), hemoglobin and platelet.
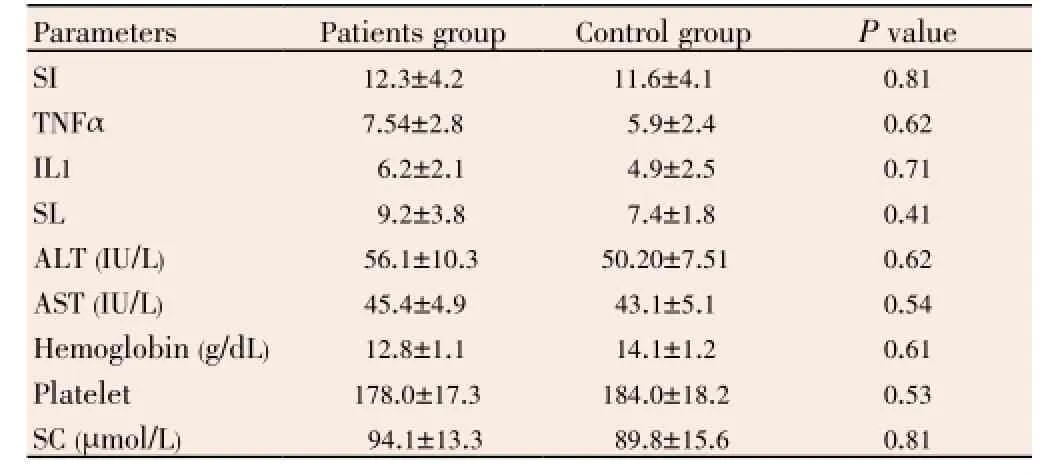
Table 3 Comparison between studied groups after 7 d of discharge.
In this study, we observed a positive correlation between serum leptin levels and pro-inflammatory cytokines TNFα & IL1 in patients group (Table 4). Similarly, we also found a positive correlation between serum leptin levels and serum insulin levels (Table 4). It has been further observed a positive correlation between serum insulin levels and TNFα.
4. Discussion
The exact nature of the association between leptin hormone and malaria, has not yet been clearly elucidated up to this date[1,5]. Since leptin is involved in a range of physiological processes, it could be an important factor in malaria infection development and outcome. In this study, we found a highly significant increase in serum leptin levels in patients group as compared with control group at the time of admission and time of discharge. At least two possible explanations may be given for this elevation of serum leptin levels. First, in this study, fasting serum insulin levels were high in patients group, but they were not significantly different as compared with controls. Furthermore, the serum leptin concentration was positively correlated with the concentration of serum insulin. Our results were similar to other studies[6], which described an important role for insulin in the state of hyperleptinemia. Hyperinsulinemia stimulates the adipocytes to produce leptin[7].
Secondly, we observed that pro-inflammatory cytokines TNFα and IL1 levels were significantly higher in patients group than in control group at time of admission and time of discharge. This matches what has earlier been reported by Lykeet al., who found the production of pro-inflammatory cytokines TNFα and IL1 is increased during malaria blood stage infection[8]. This suggests predominant Th1 response during the acute phase of the malaria infection. Th1 cells secreting interferon-γ andinterleukin-2 would activate macrophages to produce proinflammatory cytokines such as TNFα and IL1. Recently, IL1 and TNFα have been implicated in leptin secretion regulation where they induce the production of leptin from adipocytes[9].

Table 4 Correlation between serum leptin levels, TNFα, IL1 and other parameters in studied groups.
It is also noted that the kinetics of leptin production during inflammation and infection is similar to that of cytokine IL1 and TNFα production. These matches what has earlier been reported by Bracho-Riquelme and Reyes-Romero[10]. The observations suggest that increased leptin production could be found as a normal component of inflammatory response in malaria infection. It could also contribute to the development and outcomes of malaria. Increased leptin levels could be linked with anorexia induced by parasite infection[11]. In this context, increased leptin levels could be contribute to the pathological effects, through the influence of leptin on the wasting syndrome and through its role in causing a positive feedback loop in the inflammatory process[12].
Santos-Alvarazet al. have demonstrated that human leptin stimulates the production of cytokines such as TNFα and interleukin-6[13]. TNFα has long been recognized to promote malaria parasite killing, but also to contribute to the development of severe malaria disease. The precise molecular mechanisms that influence these different outcomes in malaria patients are not well understood, but the virulence and drug-resistance phenotype of malaria parasites and the genetic background or age of patients are likely to be important determinants. The low levels of the inflammatory cytokine (TNFα) are anti-parasitic working together with interferon-γ to induce production of nitric oxide and other toxic radicals[8]. On the other hand, the high levels of this cytokine (and other inflammatory cytokines such as IL-1 and interleukin-6) have been associated with malarial pathology such as fever[14], and cerebral malaria[15].
What was observed in this study that liver enzymes were significant high in patients group as compared with control. It was documented that increased leptin levels have been associated with necro-inflammatory activity of liver[16]. We also noted thrombocytopenia and anemia in patients group as compared with controls. Recent studies suggested that leptin could play an important role in hematopoiesis and immune function[17].
We can conclude that hyperleptinemia in malaria patients is due to hyperinsulinemia and overproduction proinflammatory cytokines such as TNF-α and IL1.
Leptin hormone may possibly play an important role in development and outcome of malaria infection. To our knowledge, this is the first clinical study to show the possible association of serum leptin level with malaria.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to thank Kuwait Ministry of Health for the support in this study, also we would like to thank Department of Tropical Medicine, Faculty of Medicine, Zagazig University, Egypt for the excellent statistical work. The study was partially supported by College of Health Sciences (Grant No. HS-10-01).
Comments
Background
Malaria is a common disease in all the topical areas. Leptin is involved in a range of physiological processes, especially as an important factor in the pathogenesis of malaria. Disturbance in hypothalamic-pituitary-adrenal axis duringPlasmodium falciparuminfection have been implicated in the pathogenic mechanism of severe malaria. The authors have reviewed the background literature quite well.
Research frontiers
This is the first article that tackles the role of leptin in the development and outcome of malaria infection. They reported that hyperleptinimia in malaria patients is due to hyperinsulinemia and overproductoion of proinflammatory cytokines such as TNFα and IL1.
Related reports
This is one of the first studies in this area of research. A few studies have reported other aspects, such as the secretion of leptin is affected by pro-inflammatorymediators like TNFα and IL1. The role of TNFα and IL1 in malaria pathogenesis has been reported, but no study is available so far on the role of leptin.
Innovations and breakthroughs
The level of leptin in blood can be used as an indicator for the treatment outcomes and pathogenesis of malaria patients.
Applications
The level of leptin in blood of patients can used as a biomarker for diagnosis of malaria.
Peer review
This study has shown that 7 d after discharge, a significant decline in serum Leptin levels, TNFα and IL1 in the patients group was noticed when compared between the time of admission and the time of discharge, with a positive correlation between serum leptin levels and TNFα & IL1. Thus, the leptin may play an important role in the development and outcome of malaria infection.
[1] Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology-emerging clinical applications. Nat Clin Pract Endocrinol Metab 2006; 2(6): 318-327.
[2] Sánchez-Pozo C, Rodriguez-Baño J, Domínguez-Castellano A, Muniain MA, Goberna R, Sánchez-Margalet V. Leptin stimulates the oxidative burst in control monocytes but attenuates the oxidative burst in monocytes from HIV-infected patients. Clin Exp Immunol 2003; 134: 464-469.
[3] Fernández-Riejos P, Najib S, Santos-Alvarez J, Martín-Romero C, Pérez-Pérez A, González-Yanes C, et al. Role of leptin in the activation of immune cells. Mediators Inflamm 2010; 2010: 568343. doi: 10.1155/2010/568343.
[4] Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci U S A 2000; 97(5): 2367-2372.
[5] Libonati RM, de Mendonça BB, Maués JA, Quaresma JA, de Souza JM. Some aspects of the behavior of the hypothalamus-pituitary-adrenal axis in patients with uncomplicated Plasmodium falciparum malaria: cortisol and dehydroepiandrosterone levels. Acta Trop 2006; 98(3): 270-276.
[6] Oncül O, Top C, Cavuslu T. Correlation of serum leptin levels with insulin sensitivity in patients with chronic hepatitis C infection. Diabetes Care 2002; 25: 937.
[7] Tsai M, Asakawa A, Amitani H, Inui A. Stimulation of leptin secretion by insulin. Indian J Endocrinol Metab 2012; 16(Suppl 3): S543-S548.
[8] Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 2004; 72: 5630-5637.
[9] Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor α and interleukin-1 treated human preadipocytes are potent leptin producers. Cytokine 2005; 32(2): 94-103.
[10] Bracho-Riquelme RL, Reyes-Romero MA. Leptin in sepsis: a well-suited biomarker in critically ill patients? Crit Care 2010; 14(2): 138.
[11] Roberts HC, Hardie LJ, Chappell LH, Mercer JG. Parasite induced anorexia: leptin, insulin and cortisone responses to infection with the nematode, Nippostrongylus brasiliensis. Parasitology 1999; 118: 117-123.
[12] Engineer DR, Garcia JM. Leptin in anorexia and cachexia syndrome. Int J Pept 2012; 2012: 287457. doi: 10.1155/2012/287457.
[13] Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol 1999; 194(1): 6-11.
[14] Skorokhod OA, Schwarzer E, Ceretto M, Arese P. Malarial pigment haemozoin, IFN-gamma, TNF-alpha, IL-1beta and LPS do not stimulate expression of inducible nitric oxide synthase and production of nitric oxide in immuno-purified human monocytes. Malar J 2007; 6: 73.
[15] Gimenez F, Barraud de Lagerie S, Fernandez C, Pino P, Mazier D. Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell Mol Life Sci 2003; 60(8): 1623-1635.
[16] Caner I, Selimoglu MA, Yazgi H, Ertekin V. Serum leptin levels in children with acute viral hepatitis A. West Indian Med J 2006; 55(6): 409-413.
[17] Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol 2007; 4(1): 1-13.
10.12980/APJTB.4.201414B11
*Corresponding author: Jafar Abdulrida Qasem, Ph.D, Public Authority for Applied Education and Training, College of Health Sciences, Department of Applied Medical Sciences, P. O. Box 9508, Al-Ahmadi City 61006, Kuwait.
Te: +(965)-24812710 ext 6713 (work), +(965)-66625554 (mobile)
Fax: +(965)-23906099
E-mail: Dr.Jafar.Qasem@gmail.com, Ja.qasem@paaet.edu.kw
Foundation Project: partially supported by College of Health Sciences (Grant No.
HS-10-01).
Article history:
Received 2 Apr 2014
Received in revised form 9 Apr, 2nd revised form 12 Apr, 3rd revised form 18 Apr 2014
Accepted 27 Apr 2014
Available online 28 Jun 2014
Methods:Sixty cases of malaria falciparum were included in this study as patients. Thirty healthy individuals of comparable age, racial and body mass index were taken as controls. All patients were diagnosed by clinical picture and the presence of malaria parasites in blood film. Estimation of liver function test, kidney function test, complete blood count, fasting blood sugar, fasting serum insulin, pro-inflammatory cytokine tumor necrosis factor alpha (TNFα) and interleukin 1 (IL1), estimation of morning serum leptin and calculation of body mass index (kg/m2) were done in both groups on the day of admission, on discharge and 7 d after discharge.
Results:At admission, leptin levels were significantly higher in patients group than in control while fasting serum insulin levels were not significantly different between the two groups. There were significant increases as regard to TNFα and IL1 in malaria patients. Significant differences were observed between the control and the patient group for leptin, TNFα and IL1 at the time of admission and discharge. After discharge for 7 d, a significant decline in serum leptin levels, TNFα and IL1 in the patients group was observed as compared with time of admission and time of discharge, a positive correlation between serum leptin levels and TNFα and IL1.
Conclusions:Leptin hormone level might play an important role in development and outcome of malaria infection.
 Asian Pacific Journal of Tropical Biomedicine2014年6期
Asian Pacific Journal of Tropical Biomedicine2014年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.)
- Macrosomia in non-gestational diabetes pregnancy: glucose tolerance test characteristics and feto-maternal complications in tropical Asia Pacific Australia
- Entamoeba histolytica acetyl-CoA synthetase: biomarker of acute amoebic liver abscess
- Traumatic myiasis agents in Iran with introducing of new dominant species, Wohlfahrtia magnifica (Diptera: Sarcophagidae)
- Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
- Antimicrobial activity of some essential oils against oral multidrugresistant Enterococcus faecalis in both planktonic and biofilm state
